Management of hypodermitis or lipodermatosclerosis: an up-to-date review

Lourdes Reina-Gutierrez, MD
Medical Doctor specialized in
Angiology and Vascular Surgery,
Head of the Angiology and
Vascular Department of University
Hospital Central de la Cruz Roja,
Madrid, Spain and Professor of
Medicine Grade in Alfonso X el
Sabio University
Alfonso
Sanjuanbenito-Reina, MD
Medical Doctor at University
Hospital Ramon y Cájal,
Madrid, Spain
ABSTRACT
Hypodermitis or lipodermatosclerosis (LDS) is a chronic inflammatory process of the dermis and subcutaneous layer in the legs of patients with advanced chronic venous disease (CVD). It is one of the clinical findings for class C4b in the CEAP classification (clinical, etiological, anatomical, and pathophysiological) of CVD. The cellular and molecular mechanisms responsible for progression of CVD and development of LDS remain unclear. Endothelial injury activates leukocytes, causing a perivascular inflammatory process that affects the dermis and hypodermis in the context of chronic venous hypertension. LDS presents 2 clinical stages: the acute phase and a chronic condition. The acute phase is characterized by the appearance of an erythematous, indurated, and warm plaque with intense local pain that is poorly demarcated in the inner surface of the lower leg. The chronic phase presents a demarcated, thickened, and indurated skin with a constriction of the distal leg that imparts the shape of an inverted champagne bottle. Ulcers may develop in this phase. The diagnosis is based on clinical findings and is frequently misdiagnosed with cellulitis and other panniculitides in the acute phase, delaying optimal treatment. Ultrasound examination with probes used in routine venous examination of lower limbs can easily identify the histopathologic changes that affect the dermis and subcutaneous layer in CEAP class C4b CVD. The treatment must focus on eliminating the ambulatory chronic venous hypertension through conservative and invasive measures and controlling the factors that determine the onset or progression of CVD.
Definition
Hypodermitis or lipodermatosclerosis (LDS) is a chronic inflammatory process characterized by the induration of the dermis, hypodermis, and sometimes the superficial fascia in the legs of patients with chronic venous disease (CVD).1-4
Various terms have been used through the years to delineate this condition. Huriez et al were the first to recognize and describe LDS in 1955. They called it hypodermitis sclerodermiformis and believed that the disease was caused by cellulitis in patients with venous insufficiency.5 Other terms such as sclerosing panniculitis and indurated cellulitis have been used to describe the same entity, whereas the term LDS has become the preferred nomenclature in the United States and the United Kingdom.2-4
LDS is a form of lobular panniculitis without vasculitis associated with CVD.6 The panniculitis or hypodermitis is defined as the focal inflammation of the cellular subcutaneous layer or hypodermis. It can be caused by multiple causes. The best way to classify the different types of panniculitis is from a histopathological point of view. Panniculitis can be lobular or septal, depending on where the initial inflammatory process originates, and each one of them can be classified as with or without vasculitis. These patterns can overlap, particularly in more advanced stages of the disease, comprising all areas of the subcutaneous layer, which is defined as mixed or diffused panniculitis (Table I).6,7
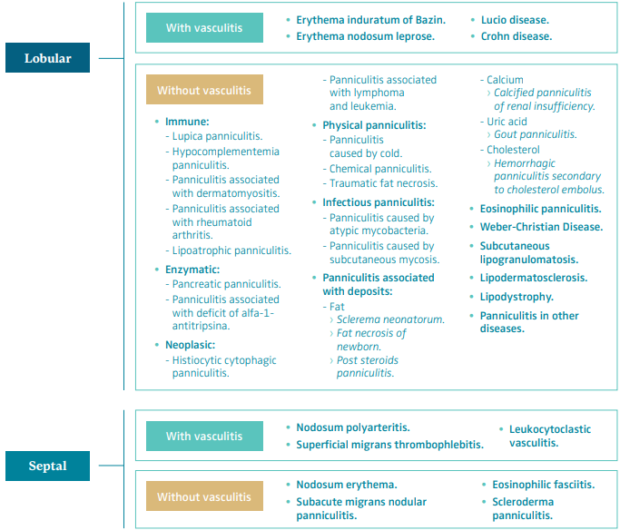
Table 1. Histopathologic classification of panniculitis.
Based on reference 6: Sánchez-Saldaña et al. Dermatol Peru. 2006;16(3):36-61.
Based on reference 7: Bondi et al. Paniculitis. In: Fitzpatrick TB ed. Dermatología en Medicina General. 5 ed. Éd.
Medica Panamericana; 2001:1341-1356.
The Vein Glossary8 has defined hypodermitis as one of the signs associated with chronic venous insufficiency (CVI) and one of the clinical findings in class C4b of the CEAP classification (clinical, etiological, anatomical, and pathophysiological; Table II).9 The Vein Glossary defined it as follows: “It consists of an inflammatory, edematous, fibrotic plaque of the medial lower third of the lower leg. It can be associated with stasis purpuric dermatitis and atrophie blanche. Often extremely painful, it can be the start of an ulcer.”8
Ulceration rate associated with LDS was estimated to be around 13% in a retrospective study.3 Kirsner et al proposed that the degree of thickening was associated with the appearance of a venous ulcer.1 The degree of skin thickening associated with LDS around the ulcer has been found to be predictive of a difficulty in healing.10 The larger the area of LDS, the greater the risk of re-ulceration in patients with CEAP class C5.11
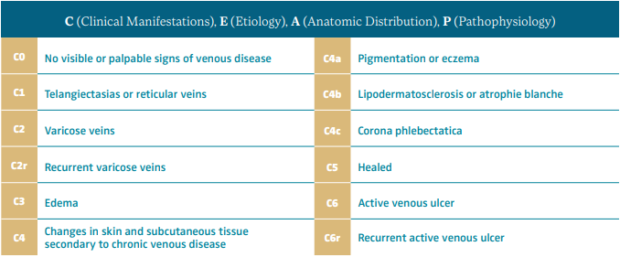
Table II. CEAP classification system and reporting standard revision 2020.
Hypodermitis or lipodermatosclerosis is one of the clinical findings for CEAP class C4b.
After reference 9: Lurie et al. J Vasc Surg Venous Lymphat Disord. 2020;8:342-352. © 2020, Society for Vascular Surgery
Prevalence
Based on epidemiological studies, the prevalence of class C4b in the general population varies from 2.1% to 13.2% depending on the country and the sex (Table III).12 A systematic review identified 32 studies from 6 continents including >300 000 adults and found a pooled C4 prevalence of 4%.13 Approximately 10% of CVD patients will develop LDS.14-16
Based on case studies, patient series, and randomized controlled trials (RCTs), middle aged and elderly women are more frequently affected by LDS. In addition, LDS also was associated with obesity in some of these studies, and although it may appear as bilateral, it is more often a unilateral lesion.1-3,11,17-19,20-28 In a retrospective analysis of 97 patients with acute and chronic LDS, 87% were women, predominantly middle aged, 66% were obese, and 85% were either overweight or obese. Bilateral involvement was found in 45% of cases.2 Patients with morbid obesity have more advanced CVD, caused by an increased abdominal pressure, a decreased calf muscle function, and inactivity. In addition, popliteal vein compression during hyperextension of the leg during standing has been observed.29-31 Together with these mechanical factors, obesity is associated with a systemic proinflammatory state that impairs venous and lymphatic return, contributing to the onset and worsening of CVD and lymphedema.32
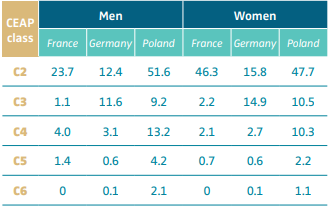
Table III. Prevalence of chronic venous disease (percentage
of population) stratified by CEAP clinical class (C2-C6).
See in particular the prevalence of C4 CEAP clinical class.
Abbreviation: CEAP, clinical, etiological, anatomical, pathophysiological
classification system. After reference 12: Nicolaides et al. Int Angiol.
2018;37(3):191-192. © 2018, Edizioni Minerva Medica.
Elderly patients more often suffer advanced stages of CVD due to associated comorbidities such as hypertension, diabetes mellitus, chronic obstructive pulmonary disease, right heart failure, and obesity; and often, a decreased calf muscle function caused by immobility, stroke, and locomotor system diseases.33
Physiopathology
The macrovascular complications associated with CVD progression are well recognized; however, the cellular and molecular mechanisms responsible for the development of LDS and subsequent venous ulceration remain unclear.
On a microcirculatory level, chronic venous hypertension produces an endothelial injury with recruitment of inflammatory cells, increased permeability, and exit of proinflammatory molecules, cells, and proteins to the interstice. The resulting perivascular inflammatory process affecting the hypodermis leads to fat necrosis and fibrosis of the skin that is defined as LDS or hypodermitis (Figure 1).34-37
Inappropriate endothelial activation in CVD is a mechanism common to other cardiovascular diseases and diabetes. A recent epidemiological study in 12 423 CVD patients found a clear association between CVD and an increased risk of cardiovascular disease and all-cause mortality. This association was reported at each stage of CVD, and the risk increased proportionally with higher CEAP classes.25,38 Epigenetic factors that cause microcirculation deterioration and inflammation can contribute to venous and lymphatic disease such as obesity, diabetes, poor lifestyle and nutritional habits, postural changes, calf pump dysfunction, drug intake, trauma, stress, and a dysfunctional autonomic nervous system.39
The venous and lymphatic systems work as a unit, the overloading of one being compensated by the other. Moreover, the overloading and failure of one produces the failure of the other.40 Lymphoscintigraphy is normal in early stages of CVD, but later it shows pathologic findings and lymph flow impairment, leading to inflammation.39 This inflammation arises when lymphatics cannot cope with the interstice overload of fluid and proinflammatory molecules and cells.39
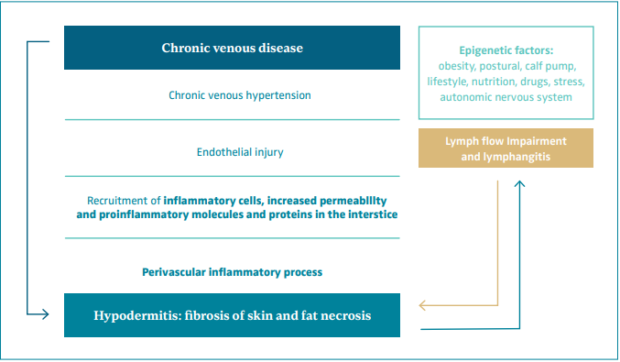
Figure 1. Physiopathology of hypodermitis.
Histopathology
Hypodermitis is characterized by a progressive septal fibrosis and necrosis of the fat lobules (Figure 2).3,22,23,41,42 An infiltrate with dilated vascular spaces is seen at first in the dermis. Thickening of the septa with discrete inflammation and necrosis of adipocytes in the center of the lobule can be observed. As the disease progresses, the septa become fibrosed, and this fibrosis progressively extends into the lobules, obliterating the adipocytes. Within the dermis, lobulated and tortuous thick-walled blood vessels, erythrocyte extravasation and siderophages, perivascular lymphocytic infiltrate with foamy macrophages and plasma cells, and dermal atrophy can be seen. Hemosiderin has been found to always be present in LDS skin.43
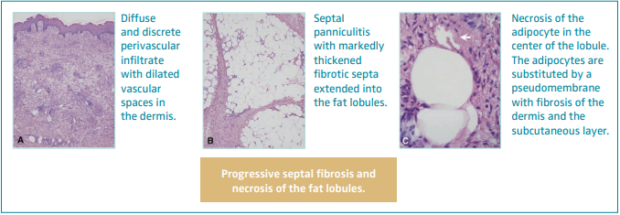
Figure 2. Histopathology of hypodermitis characterized by a progressive septal fibrosis and necrosis of the fat lobules. Images from reference 41: Chan et al. J Am Acad Dermatol. 2008;58(3):525-527. © 2018, American Academy of Dermatology, Inc. Published by Mosby, Inc. All rights reserved.
Clinical manifestation
LDS presents 2 clinical stages or phases according to the clinical manifestations: the acute-subacute phase and a chronic condition.1,2,23,24,44 The acute phase is characterized by the appearance of an erythematous and slightly indurated and warm plaque with intense local pain that is poorly demarcated in the inner surface of the lower leg (Figure 3). This acute stage is usually misdiagnosed as cellulitis, thrombophlebitis, morphea or erythema nodosum. The chronic phase is characterized by a demarcated, thickened, and indurated skin involving the gaiter region caused by extensive sclerosis of the dermis and subcutaneous tissue of the lower leg. Usually, progressive fibrosis leads to a constriction of the distal leg and imparts the characteristic shape of an inverted champagne bottle (Figures 4 and 5). The skin can be darkly hyperpigmented although it is not invariably so. Areas of atrophie blanche within LDS skin can also be present (Figure 6). Ulcers may develop in this phase. With advanced sclerosis and regression of the acute inflammatory reaction, pain tends to be duller and aching, being more frequent when the patient walks or exercises.1,2,4,22 In a retrospective study of 97 patients, Bruce et al found pain in the lower limb to be the most common symptom of LDS, seen in 43% of patients.2
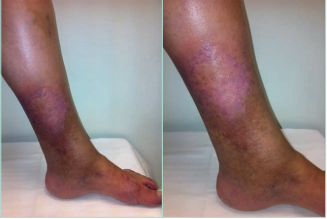
Figure 3. Acute hypodermitis or lipodermatosclerosis. Chronic venous disease of CEAP class C4b. An inflammatory plaque poorly demarcated in the inner surface of the lower third of the lower leg, which is often extremely painful. Abbreviation: CEAP, clinical, etiological, anatomical,
pathophysiological classification system. Images from: University Hospital Central Cruz Roja Collection.
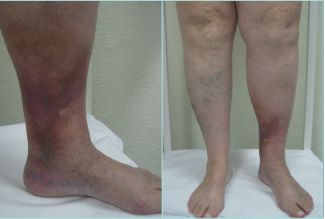
Figure 4. Chronic hypodermitis or lipodermatosclerosis. Chronic Venous Disease of CEAP class C4b. A well-demarcated area of hyperpigmented, thickened, and indurated skin involving the gaiter region of the lower leg caused by extensive fibrosis and sclerosis of the dermis and subcutaneous tissue of the lower leg. Usually, fibrosis leads to a constriction of the distal leg giving to the leg the appearance of an inverted champagne bottle. An ulcer may develop. Abbreviation: CEAP, clinical, etiological, anatomical, pathophysiological classification system. Images from: University Hospital Central Cruz Roja Collection
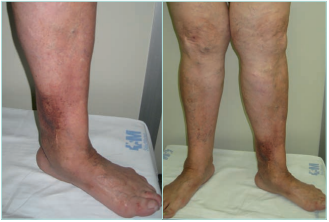
Figure 5. Chronic hypodermitis or lipodermatosclerosis. Fibrosis and sclerosis of the dermis and the subcutaneous layer that manifests as an induration of the skin and the sign of the inverted champagne bottle. It can be the start of an ulcer. Images from: University Hospital Central Cruz Roja Collection.
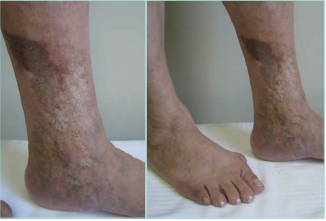
Figure 6. Chronic hypodermitis or lipodermatosclerosis. It can be associated with stasis purpuric dermatitis and atrophie blanche. Images from: University Hospital Central Cruz Roja Collection.
The chronic phase most often develops following the acute phase (in months or even more than a year) or may occur independently. It is unclear whether each patient with chronic LDS undergoes an acute phase. Based on patient history, it appears that an acute phase is not necessary or that its severity may be variable, thus a patient may not recall such a phase or have sought medical attention.1,4 Once installed, acute inflammatory phases can occur again during the chronic phase, named the “acute on chronic” form of LDS.4,44 In the retrospective case-control study of Suehiro et al, in 6 legs out of 30, acute LDS recurred 9 (3-38) months after the initial attack, exclusively in legs with persistent induration, hyperpigmentation, and edema.28 In CVD, LDS is usually a chronic condition in which acute phases may occur.44
Clinical diagnosis
The diagnosis of LDS is clinical. However, although chronic LDS is readily recognized clinically, acute lesions are frequently misdiagnosed since they lack the sharp demarcation, hard consistency, and sometimes obvious clinical signs of venous disease associated with chronicity.
The acute phase is frequently mistaken as a cellulitis and treated ineffectively with antibiotics. The key points to differentiate acute LDS from cellulitis/erysipelas are the absence of fever, systemic symptoms, inguinal lymphadenopathy, normal laboratory inflammatory parameters such as leukocytes, and lack of antibiotic treatment effectiveness.22 The diagnosis of lower-limb cellulitis is incorrect in as many as one-third of patients. The most common disorders mistaken for lower-limb cellulitis are venous eczema, LDS, and lymphedema.45
Erythema nodosum is observed especially on the anterior aspects of the lower legs, and erythema induratum is found mainly on the calves. Panniculitis during lupus erythematosus course usually appears on the trunks or arms.22,23
Biopsy
Biopsy can provoke a nonhealing ulcer; therefore, a histologic study is only recommended in selected cases to do a differential diagnosis with other panniculitis or diseases.
Skin ultrasound examination
Skin ultrasound examination, a “noninvasive biopsy,” is an excellent morphological evaluation of the cutaneous (epidermis and dermis) and subcutaneous layers, using the same probes dedicated to routine vascular investigation.46 The author of this review refers the readers to the publication of Caggiatti et al46 to further investigate this topic.
Findings in normal skin are illustrated in Figure 7.46 The epidermis appears as a thin hyperechoic band because of the echoes created between the gel and the skin surface. The papillary dermis (PD) appears as a thin and low-echogenic band parallel to the skin surface, immediately below the hyperechoic epidermis. It is called the “subendothelial-low-echogenic-band” (SLEB). The hypoechogenicity of the PD is related to its high water content. The reticular dermis (RD) appears as a regular band, with homogeneous thickness and echogenicity. The echoes from the reticular layer originate from the boundaries between the collagen fibers and the surrounding ground substance and cells.
In normal conditions, the dermo-hypodermic junction (D-HJ) appears as an uninterrupted line, easily recognizable thanks to the marked difference in echogenicity of the RD and the subcutaneous layer (Figure 7). The subcutaneous tissue consists of hypoechoic fat lobules separated by echolucent connective trabeculae. The prevalence of adipose tissue makes the subcutaneous layer markedly less echogenic than the overlying dermis. The thickness of the trabeculae varies greatly between individuals and within the same subject, depending on the evaluated area (Figure 7).46
Hypodermitis is characterized by inflammatory edema in initial phases and liposclerosis in advanced cases. The ultrasound pattern is greatly variable, with different combinations of cutaneous and subcutaneous changes. To simplify the matter, it is possible to designate 2 main ultrasound patterns: scleroedematous and fibrosclerotic.46
Acute hypodermitis: scleroedematous pattern
There is an inflammatory edema manifested by thickening of the dermis, disappearance of PD and the D-HJ, and thickening and hyperechogenicity of the hypodermis (Figure 8).46
Chronic hypodermitis: fibrosclerotic pattern
The fibrosclerotic pattern of chronic hypodermitis is characterized by progressive dermal sclerosis with disappearance of the PD and D-HJ, and subcutaneous layer thinning and hyperechogenicity by progressive fibrous proliferation and disappearance of fat lobules (liposclerosis).46 Figure 9.
A prospective study of 14 limbs with CEAP class C4-C6 CVD found on high-frequency ultrasound examination showed that dermis thickness and the dermis and subcutaneous layer echogenicity were higher in the areas of LDS than in normal thigh skin. Subcutaneous layer and venous wall calcification and fibrosis of the affected skin were also detected. Compression static elastography showed lower compliance of the subcutaneous layer than muscle.47
The retrospective case-control study published recently by Suehiro et al has shown differences in the dermal and subcutaneous ultrasound findings in patients diagnosed with acute LDS between patients that progressed to chronic LDS and those who did not.28 These results may have opened a line of investigation on the prognosis of CVD.
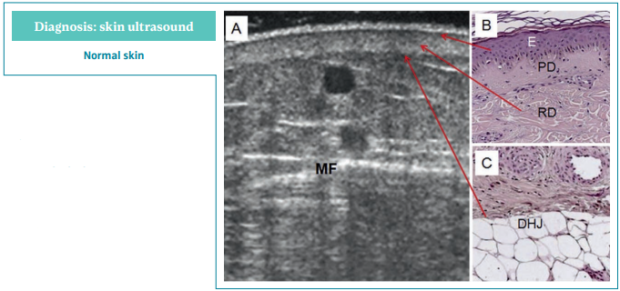
Figure 7. Normal skin ultrasound.
Ultrasonography of skin changes in
legs with chronic venous disease.
A) Normal skin, 12 MHz sonography.
B,C) Light microscopy.
Abbreviations: DHJ, dermo-hypodermic
junction; E, epidermis; MF, muscular fascia;
PD, papillary dermis; RD, reticular dermis.
After reference 46: Caggiati. Eur J Vasc
Endovasc Surg. 2016;52(4):534-542.
© 2016, European Society for Vascular
Surgery. Published by Elsevier, Ltd.
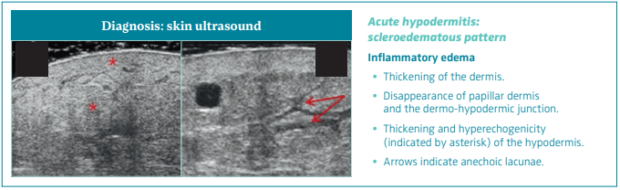
Figure 8. Skin ultrasound of acute hypodermitis: scleroedematous pattern. Ultrasonography of skin changes in legs
with chronic venous disease.
After reference 46: Caggiati. Eur J Vasc Endovasc Surg. 2016;52(4):534-542. © 2016, European Society for Vascular Surgery.
Published by Elsevier, Ltd.
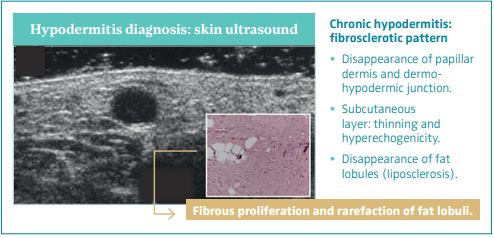
Figure 9. Skin ultrasound
of chronic hypodermitis:
fibrosclerotic pattern.
Ultrasonography of skin
changes in legs with chronic
venous disease.
After reference 46: Caggiati.
Eur J Vasc Endovasc Surg.
2016;52(4):534-542. © 2016,
European Society for Vascular
Surgery. Published by Elsevier, Ltd.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is another potentially useful alternative to skin biopsy that reveals thickening of the skin with typical distinct fibrous septa in the hypodermis with a “honey-comb” appearance, which is in concordance with the histopathologic features of LDS.41
Indication of investigations in CEAP C4b
The European Venous Forum (EVF), International Union of Phlebology (UIP), and International Union of Angiology (UIA) clinical guidelines recommend doing a lower-limb and abdominal echo-doppler examination. If venous obstruction is suspected, it is recommended to do a plethysmography, computed tomography venography (CTV), magnetic resonance venography (MRV), venography, pressure measures, and/or intravascular ultrasound (IVUS). If phlebolymphedema is suspected, it is recommended to do lower-limb isotopic lymphoscintigraphy (Figure 10).48
According to the European Society for Vascular Surgery (ESVS) clinical guidelines, if suprainguinal pathology is suspected, it is recommended to do an abdominal echo-doppler, abdominal CTV, abdominal MRV, or abdominal venography.49
Lower-limb echo-doppler
Lower-limb echo-doppler is the primary test of choice. One must evaluate the deep venous system, the superficial venous system, and the perforant veins in the vicinity of severe skin changes (Figures 11 and 12).
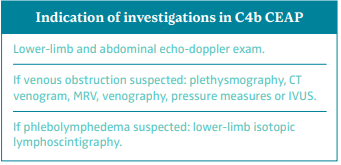
Figure 10. Indication of investigations in class C4b of CEAP
classification according to the guidelines of UIP, EVF, and UIA.
Abbreviations: CEAP, clinical, etiological, anatomical,
pathophysiological classification system; CT, computed tomography;
EVF, European Venous Forum; IVUS, intravascular ultrasound;
MRV, magnetic resonance venography; UIA, International Union of
Angiology; UIP, International Union of Phlebology.
Based on reference 48: Nicolaides et al. Int Angiol.
2018;37(3):181-232.
The definition of a perforant vein as being pathological when surpassing an outward flow >0.5 seconds and a diameter >3.5 mm in area of skin changes is controversial.49-51
Of patients with CVD, 40% to 70% have superficial venous reflux (SVR), associated or not with reflux of perforant veins or in the deep venous system.52,53 This reflux can be eliminated through invasive procedures.49-51,54
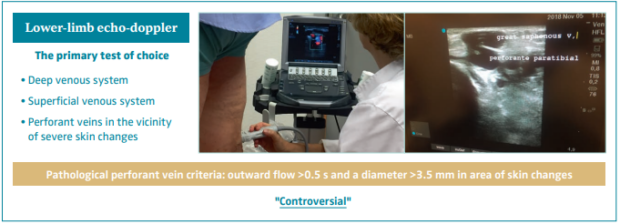
Figure 11. Lower-limb echo-doppler is the primary test of choice. Definition of pathological perforant veins remains
controversial.
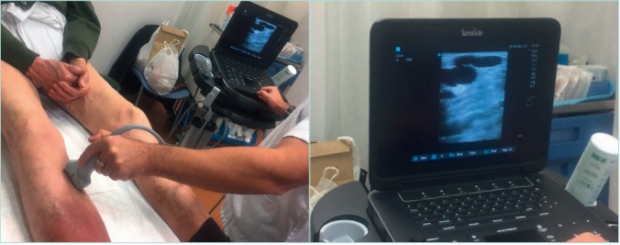
Figure 12. Lower limb echo-doppler examination of a patient with hypodermitis and incompetence of perforant tibial vein.
Images from: University Hospital Central Cruz Roja Collection.
Treatment
The objective of treatment should be to eliminate the chronic ambulatory venous hypertension and its secondary inflammatory process in the microcirculation. To achieve the first goal, one can eliminate the SVR and/or the deep venous reflux or obstruction by invasive procedures and conservative measures such as compression therapy, calf muscle activation, and elevation of the leg. Venoactive drugs (VADs) and compression therapy are indicated to counter the inflammatory process at the microcirculatory level.48-51,55
Treatment of lymphatic insufficiency associated with advanced stages of CVD
The treatment of the lymphatic insufficiency associated with advanced stages of CVD (phlebolymphedema) should not be forgotten. This objective will be gained by compression therapy through complex decongestive therapy with manual lymphatic drainage in acute and intensive care and in the maintenance phase, skin care, exercise, drugs, and nutraceuticals.56,57
Treatment of nonvascular factors that impact venous and lymphatic disease
An interdisciplinary medical pathway that approaches both diabetes and obesity is encouraged. This combined syndrome is a true pandemic worldwide, sharing its endothelial lesion with the venous and lymphatic disease.32 The treatment strategy should approach the commonalities of these interconnected diseases.58 Other therapeutic alternatives such as new drugs and nutraceuticals, measures to improve parasympathetic systems, gene treatment, stem cell treatment, and improvement of poor health habits are being investigated.32,39
Treatment for the acute phase
The acute phase requires rapid treatment. Oral analgesics are essential to reduce the pain. Compression therapy is the first-line treatment, preferably by multilayer bandaging (MLB) and adjustable compression garments (ACG). Using elastic compression stockings (ECS) may be difficult because of pain.1,27,28,59
Reich-Schupke et al observed in a case series a lower recurrence of acute episodes in those patients in which SVR was eliminated.22
Among other general measures are the nonsteroidal anti-inflammatory drugs, the topical/oral corticosteroids, and skin care with zinc oxide and emollients, although there is poor evidence to support them. Various treatments including nonsteroidal anti-inflammatory drugs, antibiotics, and topical/ oral steroids were provided in 28 patients with acute-subacute LDS for 8 weeks (2-52 weeks) without improvement.28
Venoactive drugs (VADs)
The 2018 UIP, UIA, and EVF clinical guidelines scrutinized both old and new meta-analyses addressing the effect of individual VADs on individual symptoms and signs of CVD. The only drug that had been evaluated in placebo-controlled, double-blind, RCTs on its effect on leg redness and skin changes was the micronized purified flavonoid fraction (MPFF). The analyses showed improvement in these signs with a level of evidence B and A, respectively, the clinical guidelines giving a strong recommendation for this drug in this indication (Tables IV and V).60,61 In a pooled analysis of 4 RCTs evaluating the effects of MPFF treatment on skin trophic disorders, the risk ratio (RR) of 0.87 (95% CI, 0.81- 0.94) for persistence of the skin trophic disorder with MPFF treatment vs placebo indicated a statistically significant benefit with MPFF.62,63
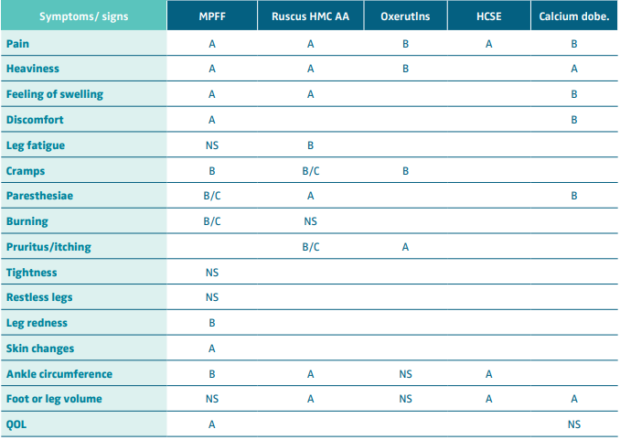
Table IV. Effect of venoactive drugs on symptoms and signs of chronic venous disease. Level of evidence.
Abbreviations: MPFF, micronized purified flavonoid fraction; Ruscus HMC AA, Ruscus aculeatus extract, hesperidin methyl chalcone
and ascorbic acid; HCSE, horse chestnut extract; Calcium dobe, Calcium dobesilate; QOL, quality of life.
After reference 60: Nicholaides et al. Int Angiol. 2018;37(3):232-254. © 2018, Edizioni Minerva Medica.
However, studies focusing on the use of MPFF-based conservative treatment in patients with CEAP class C4 CVD are scarce. Bogachev et al recently published a prospective, observational study with 365 patients with CEAP class C4 CVD treated with MPFF, associated with other conservative measures, such as compression for 6 months, and showed a significant improvement in subcutaneous adipose thickness assessed by ultrasound and a significant reduction in lesion area and skin density measured by curvimetry and durometry, respectively. The VCSS and symptoms typical of C4 class (itching, skin tightening, burning, pain) evaluated by a 10- cm visual analog scale (VAS) and the health-related quality of life (HRQoL) evaluated by the CIVIQ-14 scale (14-item ChronIc Venous Insufficiency Quality of Life Questionnaire) were also improved. No adverse reactions were reported.25
A 3-month treatment with sulodexide significantly improved objective signs of erythema, skin temperature and induration, and all subjective symptoms of CVD in an open, uncontrolled observational study in 450 CVD patients.63,64
The ESVS clinical guidelines have provided a single generic recommendation on VADs, recommending “For patients with symptomatic chronic venous disease, who are not undergoing interventional treatment, are awaiting intervention, or have persisting symptoms and/or oedema after intervention, medical treatment with venoactive drugs should be considered to reduce venous symptoms and oedema, based on the available evidence for each individual drug. Class IIa, Level A.”49,61,65,66
Compression therapy
Compression therapy by ECS has been shown to reduce skin induration in patients with hypodermitis in 2 RCTs.11,67
Firstly, in a RCT with 77 patients treated with ECS and 81 patients that were not, Vandongen et al showed that below knee 35-45-mm-Hg ECS reduced the area of LDS and ulcer recurrence in C5 CEAP.11
The second one is a pilot study of 17 patients with bilateral LDS,67 where each leg was treated with a different stocking. One of the patient’s legs was treated using a stocking permanently impregnated with copper oxide ions. Copper oxide has been shown to have biocidal and antimicrobial effects and is an essential element for normal skin function and is involved in several processes crucial for wound healing.
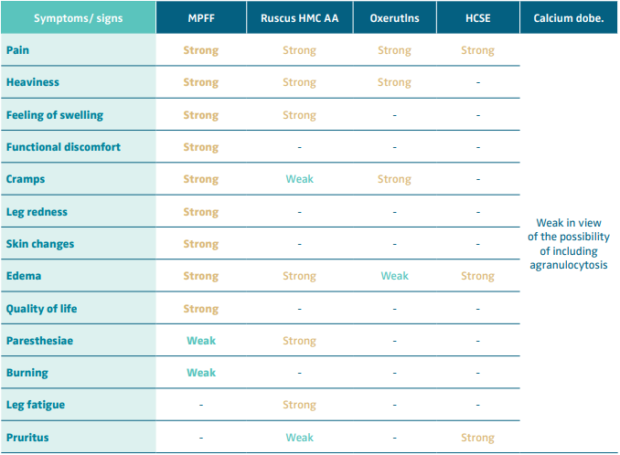
Table V. Recommendation level for venoactive drug (VAD) treatment of symptoms and signs of chronic venous disease.
Abbreviations: MPFF, micronized purified flavonoid fraction; Ruscus HMC AA, Ruscus aculeatus extract, hesperidin methyl chalcone
and ascorbic acid; HCSE, horse chestnut extract; Calcium dobe, Calcium dobesilate.
Based on reference 60: Nicolaides et al. Int Angiol. 2018;37(3):232-254.
The leg treated with nonimpregnated ECS was the control group. Below-knee 14-18-mm-Hg graduated ECS impregnated with copper reduced the area of LDS in C4b CEAP.67
The ESVS clinical guidelines recommend: “For patients with chronic venous disease and lipodermatofibrosis and/or atrophie blanche (CEAP clinical class C4b), using below-knee ECS, exerting a pressure of 20-40 mm Hg at the ankle, is recommended to reduce skin induration. Class I, Level B.”49
Multilayer bandaging
MLB achieving an interface pressure of >40 mm Hg is a more suitable treatment for the acute-subacute phase of LDS than ECS or superimposed stockings, providing higher pressure and more effectiveness in relieving pain. Using ECS is difficult for patients with acute LDS because of pain.1,4 In a case-report study, MLB eased the pain in 8 of 9 patients within 2 to 7 weeks of treatment with only occasional use of painkillers and no other treatment.27 In another case-report study of 30 patients with acute LDS, in all cases, the symptoms subsided within 5 weeks (2-11 weeks) after treatment with MLB exerting an interface pressure >40 mm Hg. Patients did not require particular medications, except for the occasional use of analgesics. Six legs out of 30 patients had a recurrent acute LDS. The treatment with MLB controlled symptoms and prevented re-recurrence.28 Nevertheless, MLB is sometimes neither possible nor tolerated; 1 of 9 patients in the study of Suehiro did not tolerate it.27 It must be taken into account that LDS modifies the limb shape, therefore ECS and MLB may have limitations.59 Moreover, compression therapy in patients with acute-subacute LDS is unlikely to prevent the progression to chronic LDS.28
Adjustable compression garments
ACGs are made of stiff material with self-adhesive straps, usually applied from the ankle to the knee. The more the straps are stretched around the leg, the higher the compression pressure. Inelastic compression devices are more effective than inelastic bandages because they can be readjusted. They are easy to apply after a short training, allowing for self-management (Figures 13-15).68,69 ACGs allow for compression therapy in morbid obesity, for nonstandard legs, for skin that needs daily care, and allow the use of footwear (Figure 16).70 ACG have been used to treat the very painful phase of acute LDS.59 Similarly to the MLB, ACG can achieve higher pressure than ECS, helping to control pain more easily. In contrast to MLB, ACG can be adapted to limbs with chronic LDS and an inverted champagne-bottle shape.

Figure 13. Adjustable compression garments for the treatment of chronic lipodermatosclerosis.
Images from: University Hospital Central Cruz Roja Collection.
Supervised physical exercises and walking associated with compression therapy
Patients with advanced stages of CVD present significant limitations of ankle and foot-joint mobility. LDS often covers the structures of foot joints, particularly the ankle joints and the Achilles tendon. The stiffness of the ankle joint limits foot mobility and disturbs the proper biomechanics of walking.71 As 2 RCTs have shown, patients should be encouraged to regularly exercise (standing on tiptoes, foot bending, using a training bike) and walk to increase foot and ankle joint mobility and to improve calf muscle pump function.72,73 Compression increases the function of the calf muscle pump.74
Elimination of superficial venous reflux
Traditional surgery by stripping is unpopular or impossible in elderly patients with skin lesions or comorbidity.54 Endovenous echo-guided techniques are less invasive, can be performed without anesthesia or local anesthesia, are less painful with a short return to daily activities, have less major complications, and prevent incision of the skin (Figure 17).75 Therefore, they are preferred in patients with skin lesions (C4b), in the elderly with comorbidities, obesity, or lymphedema.
The UIP, UIA, and EVF clinical guidelines55 give a strong recommendation based on high-quality evidence (A) for treatment of the great saphenous vein (GSV) with modern open echo-guided surgery (1A), thermal ablation with endolaser or radiofrequency (1A), and echo-guided sclerotherapy with foam (1A). Ablation with steam, cyanoacrylate, and mechanochemical ablation (MOCA) have a recommendation of 1B, awaiting long-term results. These clinical guidelines recommend the treatment of tributaries with sclerotherapy or phlebectomy at the same time or at a second stage.55
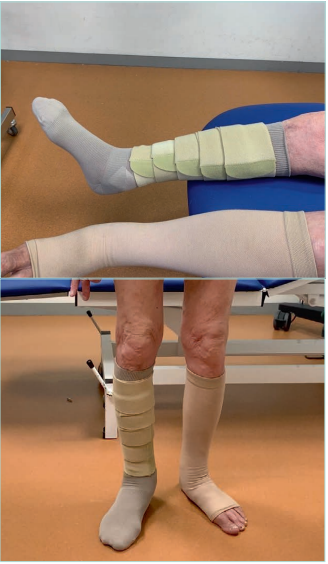
Figure 14. Elastic compression stockings (ECS) and
adjustable compression garments (ACG) in bilateral
lipodermatosclerosis.
Images from: University Hospital Central Cruz Roja Collection.
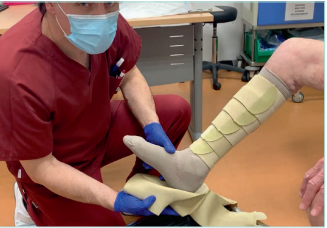
Figure 15. Adjustable compression garments (ACG) with the
garment for the foot.
Images from: University Hospital Central Cruz Roja Collection.
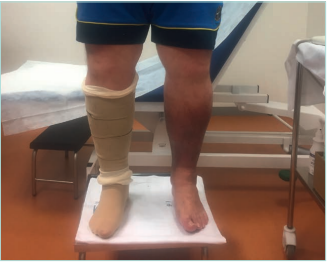
Figure 16. Adjustable compression garments (ACG)
in a patient with morbid obesity and bilateral
lipodermatosclerosis.
Images from: University Hospital Central Cruz Roja Collection.
Ultrasound-guided foam sclerotherapy (UGFS) is the most frequent procedure in the treatment of SVR. The advantages of UGFS compared with thermal ablation and phlebectomy is that LDS and lymphedema limit open surgery, thermal ablation, and tumescent anesthesia. Although the long-term anatomical effectiveness of UGFS is lower, it can be repeated. Sclerotherapy is the simplest and fastest and imposes no limitation on normal activity.Figure 18.
The ESVS clinical guidelines49 recommend:
• “For patients with saphenous trunk incompetence undergoing treatment, ultrasound guided foam sclerotherapy may be considered for treating saphenous trunk with a diameter less than 6 mm. Class IIb, Level B.” “In patients with clinical class C4b, ultrasound guided foam sclerotherapy is a better alternative to phlebectomy for the treatment of tributaries. Phlebectomy may be complicated by delayed wound healing.”
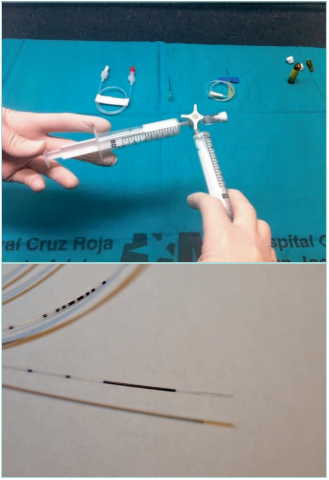
Figure 17. Echo-guided procedures in the ablation of
the superficial venous reflux. Up: hand-made foam with
Tessari technique. Down: endolaser fiber.
Images from: University Hospital Central Cruz Roja Collection.
• “For patients with great saphenous vein incompetence requiring treatment, endovenous thermal ablation is recommended as first choice treatment, in preference to high ligation/stripping and ultrasound guided foam sclerotherapy. Class I Level A.” Figure 19.
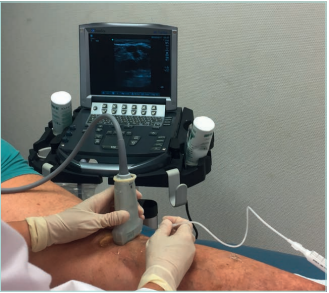
Figure 18. Echo-guided sclerotherapy of small saphenous
vein in a patient with clinical class C4b of CEAP classification.
Images from: University Hospital Central Cruz Roja Collection.
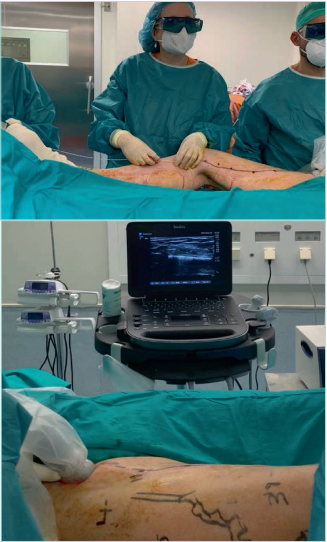
Figure 19. Thermal ablation of the great saphenous vein
with endolaser. Down: ultrasound image of the thermal
ablation of the vein.
Images from: University Hospital Central Cruz Roja Collection.
• “For patients with great saphenous vein incompetence requiring treatment, cyanoacrylate adhesive closure should be considered when a non-thermal non-tumescent technique is preferred. Class IIa, Level A.” Figure 20.
• “For patients with great saphenous vein incompetence requiring treatment, mechanochemical ablation may be considered when a non-tumescent technique is preferred. Class IIb, Level A.” Figure 21.
• “For patients with small saphenous vein incompetence requiring treatment, endovenous thermal ablation is recommended in preference to surgery or foam sclerotherapy. Class I, Level A.”
• “For patients with small saphenous vein incompetence requiring treatment, endovenous non-thermal non tumescent ablation may be considered. Class IIb, Level B.”
• “For patients with incompetence of the anterior accessory saphenous vein requiring treatment, endovenous thermal ablation should be considered. Class IIa, Level C.”
• “For patients with incompetence of the anterior accessory saphenous vein requiring treatment, ultrasound guided foam sclerotherapy may be considered. Class IIb, Level C.”
Treatment of incompetent perforant veins
UGFS is the most commonly used technique (Figure 22).
No treatment is defined as superior because there are no randomized studies comparing the different techniques. In general, smaller incompetent perforant veins are treated with UGFS and larger ones with cyanoacrylate adhesive closure (CAC) or thermal ablation.49-51
The ESVS clinical guidelines recommend: “For patients with CVD requiring treatment of incompetent perforating veins, endovenous ablation, division, or ligation should be considered. Class IIa, Level C.”49
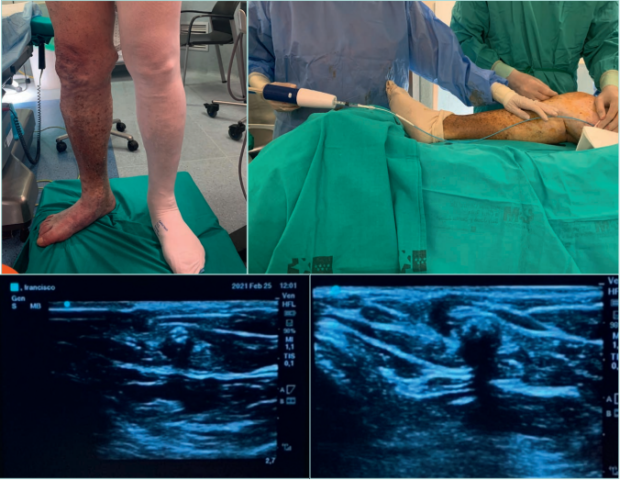
Figure 20. Endovenous closure of the great saphenous vein with cyanoacrylate adhesive with the VenaSeal technique.
Up: VenaSeal Procedure. Down left: ultrasound images of the VenoSeal catheter inside the great saphenous vein.
Down right: cyanoacrylate adhesive inside the great saphenous vein with acoustic shadow.
Images from: University Hospital Central Cruz Roja Collection.
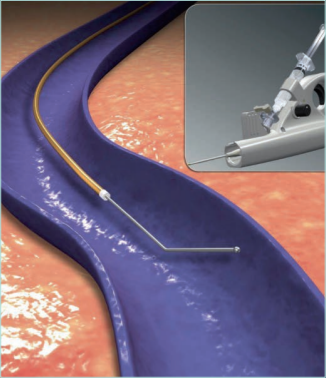
Figure 21. Mechanochemical ablation of the saphenous
trunks with catheter ClariVein.
Based on reference 82: Reina and Solares. Angiología.
2018;70(1):25-32. https://doi.org/10.1016/j.angio.2017.10
© 2017, SEACU. Published by Elsevier España, S.L.U.
Images from: University Hospital Central Cruz Roja Collection.
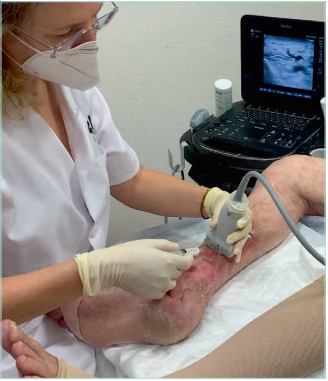
Figure 22. Echo-guided foam sclerotherapy of pathologic
perforant vein in a patient with lipodermatosclerosis.
Images from: University Hospital Central Cruz Roja Collection.
Which technique do we choose?
We should take into account the following considerations: clinical guidelines recommendations and RCT results, local conditions determined by hospital or outpatient setting, refund and device availability, experience and preferences of the medical staff, and clinical condition and preferences of the patient.
What is the current trend?
The trend is to apply thermal ablation of the GSV in young patients with acceptable surgery risk, using UGFS of the tributaries.
In patients with lymphedema, obesity, advanced age, high risk for surgery or tumescent anesthesia, incompetence of SSV, and tributaries in the area of LDS, and taking into account the preference of the patient, the nonthermal, nontumescent techniques represented by UGFS, CAC, and MOCA are frequently performed.
Deep venous system and pelvic venous disorders
Interventional techniques to eliminate reflux or obstruction of the deep venous system in the lower limbs or abdominal veins or to treat pelvic venous disorders should be done in specialized centers in selected patients (Figure 23-26).49-51
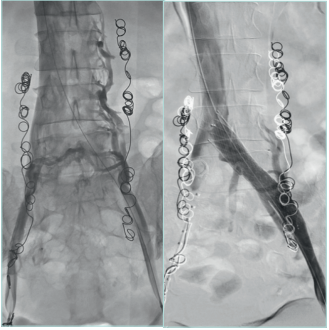
Figure 23. Stent in left iliac vein obstructed after a deep
venous thrombosis.
Courtesy of: Dr Angel Sanchez and Dr Roberto Villar. Interventional
Radiology Department. University Hospital 12 de Octubre, Madrid.
University Hospital Central Cruz Roja Collection.
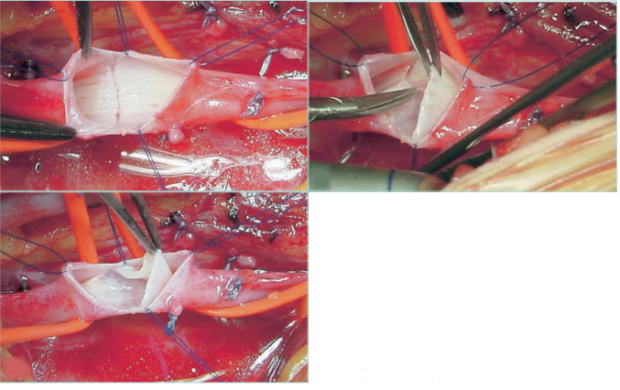
Figure 24. Deep venous disease of the lower limbs.
Construction of a neovalve in deep veins of lower limbs
with congenital absence of valves.
Images courtesy of: Dr Oscar Maleti and Dr Maria Lugli, published
in reference 8: Perrin et al. The Vein Glossary. Institut la Conference
Hippocrate; 2018. © 2018, Institut la Conference Hippocrate.
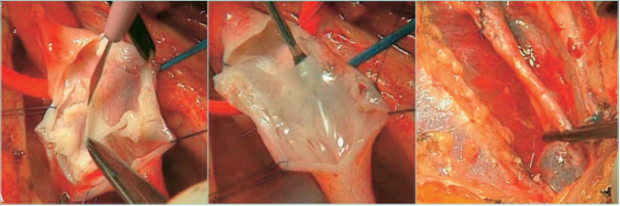
Figure 25. Deep venous disease of the lower limbs. Construction of a neovalve in deep veins of lower limbs after a deep
venous thrombosis.
Images courtesy of: Dr Oscar Maleti and Dr Maria Lugli, published in reference 8: Perrin et al. The Vein Glossary. Institut la Conference
Hippocrate; 2018. © 2018, Institut la Conference Hippocrate.
Other surgical approaches
The above-described invasive venous procedures can eliminate chronic venous hypertension, but the long-term accumulated liquefied fatty and necrotic subcutaneous tissue cannot be directly removed. Percutaneous drainage with multiple tiny incisions of the skin showing LDS and blunt dissection associated with saphenous vein stripping has been shown to relieve the tenderness, induration, redness, and swelling.76,77 In a RCT of 60 patients with CEAP class C4-C6 CVD, this surgical drainage decreased the calf and ankle circumference, improved the subcutaneous thickness and Venous Clinical Severity Score (VCSS), inhibited the expression of adhesion molecules as well as the inflammatory response, improved the microcirculation of the lower extremities, reduced the mRNA expression of collagen type I alpha 1 chain 1, and improved QOL at 6 months after surgery.78
The excision of LDS tissue by radical surgery associated with saphenous vein stripping in the management of C5/ C6 patients with LDS might be considered for very select patients who fail to respond to endovenous techniques, but evidence is lacking.79-81
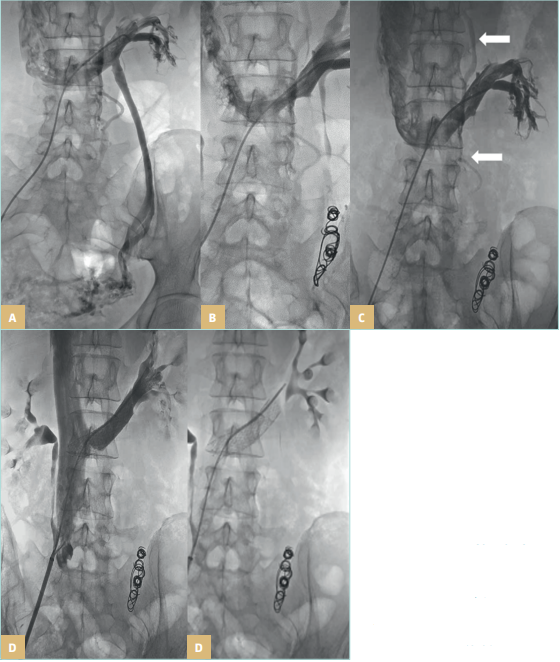
Figure 26. Pelvic venous disorders.
Left renal vein compression (A)
treated with left gonadal vein
embolization (B), with lumbar
collateral circulation after
embolization marked with long,
white arrows (C), treated with a
second stage renal vein stent (D).
Images courtesy of: Dr Angel Sanchez
and Dr Roberto Villar. Interventional
Radiology Department. University
Hospital 12 de Octubre Madrid, Spain.
University Hospital Central Cruz Roja
Collection.
Conclusion
The management of advanced-stage CVD is a challenge that begins with the understanding of its physiopathology, above all at the microcirculatory and molecular level. This seems to connect CVD with other common chronic disorders such as obesity and diabetes mellitus among others. The diagnosis of hypodermitis is based on clinical findings and may not be easy during the acute phase, being frequently misdiagnosed as cellulitis and other panniculitides, which delay optimal treatment. Ultrasound examination with probes used in the routine venous examination of lower limbs can easily identify the histopathologic changes that affect the dermis and subcutaneous layer in CEAP class C4b CVD. Treatment must focus on eliminating ambulatory chronic venous hypertension through conservative and invasive measures and controlling the factors that determine the onset or progression of CVD.
References
1. Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol. 1993;28:623-627.
2. Bruce A, Bennett D, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol. 2002;46:187-192.
3. Jorizzo JL, White WL, Zanolli MD, Greer KE, Solomon AR, Jetton RL. Sclerosing panniculitis: a clinicopathologic assessment. Arch Dermatol. 1991;127:554-558.
4. Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther. 2010;23:375-388.
5. Huriez C, Legache G, Desmons F, et al. Leg ulcers and trophic disorders of venous origin; data from the study of one thousand hospitalized patients with ulcers. Article in French. Rev Prat. 1955;5(26):2703-2721.
6. Sánchez-Saldaña L, Sáenz-Anduaga E, Thomas-Gavelan E. Paniculitis. Parte I: Paniculitis lobular. Dermatol Peru. 2006;16(3):36-61.
7. Bondi E, Margolis DJ, Lazarus GS. Paniculitis. In: Fitzpatrick TB ed. Dermatología en Medicina General. 5 ed. Éd. Medica Panamericana; 2001:1341- 1356.
8. Perrin M, Eklof Bo, Maleti O. The Vein Glossary. Institut la Conference Hippocrate; 2018.
9. Lurie F, Passman M, Meisner M, et al. The 2020 classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8:342-352.
10. Nemeth A, Eaglestein WH, Falanga V. Clinical parameters and transcutaneous oxygen measurements for the prognosis of venous ulcers. J Am Acad Dermatol. 1989;20:186-190.
11. Vandongen VK, Stacey MC. Graduated compression elastic stockings reduce lipodermatosclerosis and ulcer recurrence. Phlebology. 2000;15:33-37.
12. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines According to Scientific Evidence. Part I. Int Angiol. 2018;37(3):191-192.
13. Salim S, Machin M, Patterson BO, Onida S, Davis AH. Global epidemiology of chronic venous disease: a systematic review with pooled prevalence analysis. Ann Surg. 2021;274:971-976.
14. Lee AJ, Evans CJ, Allan PL, et al. Lifestyle factors and the risk of varicose veins Edinburgh Vein Study. J Clin Epidemiol. 2003;56:171-179.
15. Pannier F, Rabe E. Progression in venous pathology. Phlebology. 2015;30(1 suppl):95-97.
16. Rabe E, Pannier F, Ko A, Berboth G, Hoffmann B, Hertel S. Incidence of varicose veins, chronic venous insufficiency, and progression of the disease in the Bonn vein study II. J Vasc Surg. 2010;51(3):791.
17. Naschitz J, Yeshurun D, Schwartz H, et al. Pathogenesis of lipodermatosclerosis of venous disease: the lesson learned from eosinophilic fasciitis. Cardiovasc Surg. 1993;1:524-529.
18. Buurnand KG, Whimsterm I, Naidoo A, Browse NL. Pericapillary fibrin in the ulcer bearing skin of the leg: the cause of lipodermatosclerosis and venous ulceration. Br Med J (Clin Res Ed). 1982;285:1071-1072.
19. Gniadecka M. Dermal oedema in lipodermatosclerosis distribution, effects on posture and compressive therapy evaluated by high frequency ultrasonography. Acta Derm Venereol. 1995;75:120-124.
20. Gross E, Wood CR, Lazarus GS, Margolis DJ. Venous leg ulcers: an analysis of the underlying venous disease. Br J Dermatol. 1993;129:270-274.
21. Snow J, Su WPD. Lipomembranous (membranocytic) fat necrosis: clinicopathologic correlation of 38 cases. Am J Dermatophatol. 1996;18:151-155.
22. Reich-Schupke S, Kreuter A, Altmeyer P, Stücker M. Wrong diagnosis erysipelas: hypodermitis-case series and review of literature. Article in German. J Dtsch Dermatol Ges. 2009;3:222-225.
23. Huang TM and Lee JYY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
24. Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
25. Bogachev V, Boldin B, Turkin P, Samenkov A, Dzhenina O. Micronized purified flavonoid fraction-based conservative treatment of chronic venous disease in a real-world setting. Future Cardiol. 2022;18(10):777-785.
26. Arendsen LP, Vig S, Thakar R, Sultan AH. Impact of copper compression stockings on venous insufficiency and lipodermatosclerosis: a randomized controlled trial. Phlebology. 2019;34(4):224-230.
27. Suehiro K, Morikage N, Harada T, et al. Compression therapy using bandages succesfully manages acute or subacute lipodermatosclerosis. Ann Vasc Dis. 2019;12(1):77-79.
28. Suehiro K, Morikage N, Harada T, et al. Post-treatment course of acute lipodermatosclerosis. Phlebology. 2023;38(2):73-79.
29. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
30. Millen RN, Thomas KN, Versteeg MPT, van Rij AM. Popliteal vein compression, obesity, and chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2022;10(1):200- 208.
31. Lane RJ, Cuzzilla ML, Harris RA, Phillips MN. Popliteal vein compression syndrome: obesity, venous disease and the popliteal connection. Phlebology. 2009;24(5):201- 207.
32. Cavezzi A. Medicine and phlebolymphology: time to change? J Clin Med. 2020;9:4091.
33. Labrapoulos N. How does chronic venous disease progress from the first symptoms to the advanced stages? A review. Adv Ther. 2019;36(suppl 1):13-19.
34. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;139:333- 346.
35. Mansilha A, Sousa J. Pathophysiological mechanism of chronic venous disease and implications for venoactive drugs therapy. Int J Mol Sci. 2018;19:1669.
36. Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The role of endothelial dysfunction and inflammation in chronic venous disease. Ann Vasc Surg. 2018;46:380-393.
37. Mannello F, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide modulates inflammatory pathways in chronic venous disease. Int Angiol. 2014;33:236-242.
38. Prochaska JH, Arnold N, Falcke A, et al. Chronic venous insufficiency, cardiovascular disease, and mortality: a population study. Eur Heart J. 2021;42(40):4157-4165.
39. Cavezzi A et al. Lymphology and translational medicine. Int Angiol. 2020;39:422-432.
40. Lee BB, Andrade M, Antignani PL, et al. Diagnosis and treatment of primary lymphedema consensus document of the International Union of Phlebology (IUP)- 2013. Int Angiol. 2013;32(6):541-574.
41. Chan CC, Yang CY, Chu CY. Magnetic resonance imaging as a diagnostic tool for extensive lipodermatosclerosis. J Am Acad Dermatol. 2008;58(3):525-527.
42. Ayele A, Tidman MJ, Biswas A. Pseudomembranous changes in the dermis: a novel observation and potential clue for evolving lipodermatosclerosis? J Cutan Pathol. 2017;44:1070-1074.
43. Caggiati A, Rosi C, Casini A, et al. Skin iron deposition characterizes lipodermatosclerosis and leg ulcer. Eur J Vasc Endovasc Surg. 2010;40:777-782.
44. Greenberg AS, Hasan A, Montalvo BM, et al. Acute lipodermatosclerosisis associated with venous insufficiency. J Am Acad Dermatol. 1996;35:566-568.
45. Hirschmann JV, Raugi GJ. Lower limbs cellulitis and its mimics. Part II. Conditions that simulate lower limb cellulitis. . J Am Acad Dermatol. 2012;67:177-179.
46. Caggiati A. Ultrasonography of skin changes in legs with chronic venous disease. Eur J Vasc Endovasc Surg. 2016;52(4):534-542.
47. Wozniak W, Danowska A, Mlosek RK. The use of high-frequency skin ultrasound in the diagnosis of lipodermatosclerosis. J Ultrason. 2021;20(83):e284-e290.
48. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181-232.
49. Maeseneer MG, Kakkos SK, Aherne T, et al. ESVS 2022 Clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63:184-267.
50. O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2014;60:35-59.
51. Mosti G, Maeseneer M, Cavezzi A, et al. Society for Vascular Surgery and American Venous Forum guidelines on the management of venous leg ulcers: the point of view of the International Union of Phlebology. Int Angiol. 2015;34(3):202- 218.
52. Robertson LA, Evans CJ, Lee AJ, Allan PL, Ruckley CV, Fowkes FG. Incidence and risk factors for venous reflux in the general population: Edinburgh Vein Study. Eur J Vasc Endovasc Surg. 2014;48;208-214.
53. Myers KA, Ziegenbein RW, Zeng GH, Matthews PG. Duplex ultrasonography scanning for chronic venous disease: patterns of reflux. J Vasc Surg. 1995;21:605-612.
54. Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363(9424):1854-1859.
55. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part II. Chapters 9-18. EVF and UIP. Int Angiol. 2020;39(3):175- 240.
56. Mosti G, Cavezzi A. Compression therapy in lymphedema: between past and recent scientific data. Phlebology. 2019;34:515- 522.
57. Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53:3-19.
58. Gastaldi G, Pannier F, Roztočil K, et al. Chronic venous disease and diabetic microangiopathy: pathophysiology and commonalities. Int Angiol. 2021;40(6):457-469.
59. Rastel D, Pichot O. Auto-adjustable medical compression device to treat acute lipodermatosclerosis in superficial chronic venous disease: a case report. J Med Vasc. 2022;47(3):141-144.
60. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence Part I. Chapter 8. Venoactive drugs. Int Angiol. 2018;37(3):232-254.
61. Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (Daflon) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double blind placebo-controlled trials. Int Angiol. 2018;37:143-154.
62. Martinez-Zapata MJ, Vernooij RW, Uriona Tuma SM, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2016;4(4):CD003229.
63. Mansilha A, Sousa J. Pathophysiological mechanism of chronic venous disease and implications for venoactive drugs therapy. Int J Mol Sci. 2018;19:1669.
64. Elleuch N, Zidi H, Bellamine Z, Hamdane A, Guerchi M, Jezalli N. Sulodexide in patients with chronic venous disease of the lower limbs: clinical efficacy and impact on quality of life. Adv Ther. 2016;33:1536- 1549.
65. Bignamini AA, Matuska J. Sulodexide for the symptoms and signs of chronic venous disease: a systematic review and meta analysis. Adv Ther. 2020;37:1013-1033.
66. Kakkos SK, Allaert FA. Efficacy of ruscus estract, HMC and vitamin C, constituents of Cyclo 3 fort®, on improving individual venous symptoms and edema: a systematic review and meta-analysis of randomized double-blind placebo controlled trials. Int Angiol. 2017;36:93- 106.
67. Arendsen LP, Vig S, Thakar R, Sultan AH. Impact of copper compression stockings on venous insufficiency and lipodermatosclerosis: a randomised controlled trial. Phlebology. 2019;34(4):224-230.
68. Mosti G. Self-management by firm non elastic adjustable compression wrap device. Veins Lymphatics. 2017;6:7003.
69. National Institute for Health and Care Excellence. The Juxtacures adjustable compression system for treating leg venous ulcers. Medtech innovation briefing [mib25]. Published March 25, 2015. https://www.nice.org.uk/advice/mib25
70. Blecken SR, Villavicencio JL, Kao TC. Comparison of elastic versus non elastic compression in bilateral venous ulcer: a randomised trial. J Vasc Surg. 2005;42(6):1150-1155.
71. Orsted HL, Radke L, Gorst R. The impact of musculoskeletal changes on the dynamics of the calf muscle pump. Ostomy Wound Manage. 2001;47:18-24.
72. Padberg FT Jr, Jonhnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. J Vasc Surg. 2004;39:79-87.
73. Szewczyk MT, Jawien A, Cwajda-Biatasik J, Cierzniakowsk K, Moscicka P, Hancke E. Randomized study assessing the influence of supervised exercises on ankle joint mobility in patients with venous ulcerations. Arch Med Sci. 2010;6(6):956- 963.
74. Hirai M. Changes in interface pressure under elastic and short-stretch bandages during posture changes and exercise. Phlebology. 1998;13:25-28.
75. Gohel M S, Heatley F, Liu X, Bradbury A, et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med. 2018;378:2105-2114.
76. Li Y, Zhou H, Ning Y, Wan B, Zhu Y. Efficacy of point-of-care drainage surgery in chronic venous insufficiency of the lower extremities with lipodystrophy. China Clin Med. 2015;22:218-221.
77. Sun H. Effectiveness of point-of-care drainage surgery in chronic venous insufficiency of the lower extremities with lipid scleroderma. Biped Health Care. 2018;27:90-92.
78. Zhou H, Ma Z, Lu G, Wan B. The effects of percutaneous drainage using tiny incisions on the inflammatory factors in chronic venous insufficiency combined with lipodermatosclerosis of the lower extremities. Am J Transl Res. 2021;13(4):2635-2643.
79. Martis G, Laczik R. The role of radical surgery in the management of CEAP C5/ C6 and lipodermatosclerosis. Phlebology. 2016;31(10):753-768.
80. Whiteley M. Letter regarding the role of radical surgery in the management of CEAP C5/C6 and lipodermatosclerosis. Phlebology. 2016;31(10):769.
81. Martis G. Reply letter to the editor, regarding the role of radical surgery in the management of CEAP C5/C6 and lipodermatosclerosis. Phlebology. 2016;31(10):770-771.
82. Reina-Gutierrez L, Fernández Solares JI. Clarivein mechano-chemical ablation of the great saphenous vein: technique details and literature review. Article in Spanish. Angiología. 2018;70(1):25-32. https:// doi.org/10.1016/j.angio.2017.10.002
