Management of special cases in chronic venous disease: what do I do?
Rebeca Reachi, MD
Vascular Surgeon Staff; Hospital Beneficencia Española A.C. San Luis Potosí, Mexico
ABSTRACT
Chronic venous disease is one of the most frequent consultations in our daily practice, so we must consider the management of complications and special cases that are more frequent than expected. If as physicians we seek to improve patients’ quality of life, we need multidisciplinary management to achieve this goal.
Introduction
If we understand the etiology, natural history, and evolution of venous disease, we know that genetics, age, female sex, obesity, pregnancy, prolonged periods of standing, and greater height are factors that contribute to the development of varicose veins.1
Age and obesity have also been described as factors that contribute to the development of varicose veins in both the Edinburgh Venous Study and the Framingham Study.2-4
Venous disease in patients with obesity
Obesity is one of the known triggering factors of chronic venous insufficiency (CVI). If we add to this the fact that most obese patients are sedentary, the percentage of chronic venous disease (CVD) is higher. Often, patients with chronic obesity also present with flat feet, which is a lowering of the plantar arch. As the arch is lower than usual, the foot falls inward, causing pain in the muscles on the inner side of the leg, resulting in tendonitis. Another cause is posterior tibial dysfunction. This means that the muscle that supports the arch stops working, causing the foot to fall further inward. Another cause of pain also occurs on the back of the foot when the arch collapses, causing the bones in the dorsal area to produce osteoarthritic spikes. Any of these causes of flatfoot, over time, creates a vicious cycle of pain, failure of the pump of the ankle joint and distal calf muscle, immobility, sedentary lifestyle, and obesity as observed in studies by Belczak et al5 and Cavalheri et al.6 In the 2013 study by Belczak et al, increasing body mass index (BMI) correlates with reduced joint mobility (Kruskal-Wallis test: P<0.0001); an increase in clinical, etiological, anatomical, and pathophysiological (CEAP) classification also correlates with decreased joint mobility; and an increase in age is associated with increased CEAP classification (Kruskal-Wallis test: P<0.0001); thus, it was concluded that obesity is associated with impaired joint mobility and worsening of CVD.5 In the 2008 study by Cavalheri et al, the clinical course of ankles affected by venous disease correlates with reduced joint mobility and hemodynamic changes, identified by plethysmography.6
In the 2002 study by Danielsson et al, it was observed that weight appears to be an independent factor for CVD. The correlation of BMI with clinical severity independent of reflux measurements indicates that the effect of being overweight may involve a mechanism independent of local effects on venous flow. Being overweight appears to be an independent risk factor for increased severity of cutaneous changes in patients with CVD.7
In the 2013 study by Vines et al,8 there was a close relationship between body weight and clinical severity of primary venous disease. Both overweight and obesity were analyzed, and both appear to be separate risk factors for increased severity in patients with chronic primary venous disease, with no correlation with disease duration. The CEAP classification and venous clinical severity score (VCSS) were used to accurately assess disease severity, with excellent correlation between the 2 scores. Concomitant primary deep venous reflux was observed more frequently in obese patients, with less abolition after superficial reflux eradication than observed in normal-weight and overweight patients. Regarding sex differences, a 2011 study by Musil et al9 on BMI, age, and severity of CVD showed that BMI, in terms of venous reflux frequency, is a risk factor in the entire group of female patients, but not in men. Multiple linear regression showed BMI, along with age, to be significant predictors of clinical CVD grade (P<0.05) according to the CEAP classification. Regarding the influence of BMI on clinical severity/grade of CVD (CEAP), the results of this study support BMI as an important risk factor.8,9
Oxidative stress is one of the possible causes of obesity increasing CVD. Increased local production of reactive oxygen species (ROS) is considered a mediator of vessel wall changes that lead to endothelial damage and may be the mechanism leading to decreased blood flow and venous stasis. Obesity is a known clinical factor influencing venous blood flow in the lower extremities. In the 2009 study by Kózka et al,10 which reviewed 31 patients with CEAP C2-C3 local ROS production was assessed based on the production of malonyldialdehyde (MDA), a lipid peroxidation product, in blood samples taken from varicose veins of the lower extremities, as well as blood collected from the forearms of patients undergoing surgery for varicose veins in the lower extremities. The correlation between MDA levels and BMI was also examined. CVD is associated with increased oxidative stress, as measured by MDA levels in blood plasma. MDA measurement may be a useful marker in the assessment of vascular changes in patients with CVD. Obesity increases the risk of lipid peroxidation and influences increased oxidative stress in the CVD patient group.10
So, how can we treat these patients? In most cases, we can talk to the patient, send them for a baropodometric study of their gait and the need for insoles, and then begin progressive exercise to improve range of motion, as well as diet and weight loss prior to surgery (Figure 1).
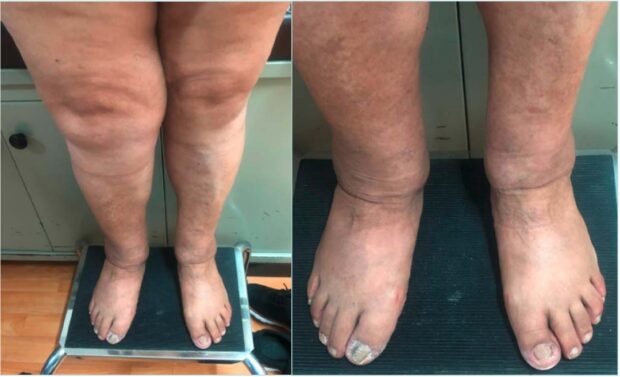
Figure 1. Patient with obesity, flat feet, lowering of the plantar arch. The foot falls inward, causing pain in the muscles on the inner side of the leg, resulting in tendonitis. In chronic cases, it can affect the knee joint, causing gonarthrosis.
Chronic venous disease in the elderly
The term “elderly” refers to patients over 65 years of age, which implies that the older the patient, the higher the prevalence of CVD. Therefore, there is an association between advanced age and more advanced clinical stages in the CEAP classification. Some of these patients ask for treatment, and after the preoperative protocol they are allowed surgery, but sometimes age is a limitation—should it be?
Life expectancy among the global population has increased, so a great proportion of elderly patients are older than 80 years old. The population over 60 years of age represented 12% of the population in 2015. By 2020, it was reported that there were more people over 60 years of age than children under 5 years of age, with an estimate that this age group will increase to 22% of the population by 2050. It is currently estimated that there are 125 million people over 80 years of age worldwide, a figure that may increase to 434 million by 2050, of whom 80% will live in low- to middle-income countries.11
Over the past decade, varicose vein treatment has shifted from the operating room to the office. Although recent studies demonstrated the safety of office-based venous ablation in the elderly, there is a paucity of published data on contemporary outcomes of varicose vein surgery in the operating room. This study, conducted by Kim et al,12 analyzed trends and outcomes of varicose vein surgery in the elderly using a large database from the American College of Surgeons’ National Surgical Quality Improvement Program from 2005 to 2017, with a total of 48 615 venous operations. Patients who underwent vein ablation or open surgery (high ligation, stripping, and phlebectomy) were identified by Current Procedural Terminology codes and principal diagnosis. Patients were stratified into 3 age groups—under 65 years, 65 to 79 years, and over 80 years— and preoperative and operative characteristics and outcomes were compared. Logistic regression was used to identify risk factors associated with any adverse event, defined as any morbidity or mortality. Varicose vein surgery was concluded to be safe in all age groups and is increasingly being offered to the elderly. High-risk patients may benefit from ablation staging and open procedures and avoiding general anesthesia can minimize adverse events. Conservative measures should be exhausted before surgery in the dialysis population.12
A 2020 study by Kibrik et al13 to assess the safety and efficacy of endovenous ablations in octogenarians, nonagenarians, and centenarians showed that whereas there is a relatively higher likelihood of endovenous heat-induced thrombosis (EHIT) and recanalization in the age group <80 years, the majority of EHITs were class 1 and class 2. According to this study, venous ablation is safe and effective in all age groups, and age alone should not be used to deny patients venous ablations.13
Another review conducted in 2020 by Garza-Herrera11 regarding the surgical treatment of CVD in octogenarians aimed at understanding the behavior of venous pathology in these age groups, as well as the safety and efficacy of its treatment, in order to clarify and determine treatment guidelines for the coming years. The investigator concluded there was superiority for endovenous ablation techniques compared with traditional surgical techniques or conservative management for treating advanced stages of CVD in patients over 80 years of age. On the basis of these results, age should not be a limitation for offering an endovenous procedure. The results of the Vascular Quality Initiative Varicose Vein Registry (VQI VVR) allow us to better understand this group of patients, who are not usually considered for clinical trials. Although the before-mentioned studies are retrospective reviews, they may provide sufficient information to consider this therapy in elderly patients.11
Chronic venous disease in patients withcoagulopathies
Subjects with truncal varicose veins and those with CVI had higher levels of each hemostatic factor than those without truncal varicose veins and without CVI. Although unit increases in tissue-type plasminogen activator (t-PA) and von Willebrand factor (vWF) were initially associated with a significantly higher risk of CVI in men, and both factors with an elevated risk of truncal varicose veins in women, multiple adjustment for age, smoking, and BMI reduced the odds ratios to nonsignificance. However, this does not completely rule out the possibility of a pathogenetic role for hemostatic factors in venous disease; rather, it indicates the need for further experimental and epidemiological studies.14
Chronic venous disease in patients with coagulopathies In an observational study of a cohort of 132 adult patients with CVD, symptoms reported by patients with CVD of the leg were recorded and correlated with systemic inflammatory markers, including vWf. No correlation was found between patient-reported symptoms and the internationally agreed clinical stages of venous disease from C2 to C5. There was also no correlation between levels of inflammatory mediators and patient symptoms. The symptoms reported by patients with CVD cannot be explained by the anatomical distribution of venous disease in the lower extremity veins or by the systemic inflammatory response in venous disease.15
Conclusions
Each patient should have a complete medical history, as well as a physical examination complemented by color duplex Doppler ultrasound. This evaluation should be carried out individually. Depending on their BMI, comorbidities such as age, medical history, anticoagulant medication use, or other concomitant diseases, the type of treatment can be chosen. This may include just taking venoactive drugs, use of compression stockings, and in the absence of contraindications, performance of one of the current endovenous procedures, whether thermal or nonthermal, which have been shown to be safe, effective, and long-lasting in most cases.
CORRESPONDING AUTHOR
Rebeca Reachi
Mariano Arista #943 int 27. Col. Tequisquiapan, San Luis Potosi, S.L.P México CP 78250
e-mail: rbkreachi@yahoo.com
References
1. Bergan JJ, Schmid-Schönbein GW, Coleridge Smit, PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-488.
2. Evans CJ, Fowkes FGR, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53:149-153.
3. Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4:96-101.
4. Laurikka J, Sisto T, Auvinen O, Tarkka M, Läärä E, Hakama M. Varicose veins in a Finnish population aged 40- 60. J Epidemiol Community Health. 1993;48:212-213.
5. Belczak CEQ, de Godoy JMP, Belzack SQ, Ramos RN, Caffaro RA. Obesity and worsening of chronic venous disease and joint mobility. Phlebology. 2013;29(8):500-504.
6. Cavalheri G, de Godoy JMP, Belczak CEQ. Correlation of haemodynamics and ankle mobility with clinical classes of clinical, aetiological, anatomical and pathological classification in venous disease. Phlebology. 2008;23(3):120-124.
7. Danielsson G, Eklof B, Grandinetti A, Kistner RL. The influence of obesity on chronic venous disease. J Vasc Endovasc Surg. 2002;36(4):271-276.
8. Vines L, Gemayel G, Christenson JT. The relationship between increased body mass index and primary venous disease severity and concomitant deep primary venous reflux. J Vasc Surg Venous Lymphat Disord. 2013;1(3):239-244.
9. Musil D, Kaletova M, Herman J. Age, body mass index and severity of primary chronic venous disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(4):367-371.
10. Kózka M, Krzyściak W, Pietrzycka A, Stepniewski M. Obesity and its’ influence on reactive oxygen species (ROS) in the blood of patients with varicose veins of the lower limbs [article in Polish]. Przegl Lek. 2009;66(5):213-217.
11. Garza-Herrera R. Tratamiento quirúrgico de la enfermedad venosa crónica en octogenarios. Rev Mex Angiol. 2020;48(3):96-99.
12. Kim TI, Zhang Y, Guzman RJ, Ochoa Chaar CI. Trends of hospital-based surgery for varicose veins in the elderly. J Vasc Surg Venous Lymphat Disord. 2021;9(1):146- 153.e2
13. Kibrik P, Chait J, Arustamyan M, et al. Safety and efficacy of endovenous ablations in octogenarians, nonagenarians, and centenarians. J Vasc Surg Venous Lymphat Disord. 2020;8(1):95-99.
14. Lee AJ, Lowe GD, Rumley A, Ruckley CV, Fowkes FG. Haemostatic factors and risk of varicose veins and chronic venous insufficiency: Edinburgh Vein Study. Blood Coagul Fibrinolysis. 2000;11(8):775-781.
15. Howlader MH, Smith PDC. Symptoms of chronic venous disease and association with systemic inflammatory markers. J Vasc Surg. 2003;38(5):950-954.

