Management of superficial vein thrombosis of the lower limbs: update and current recommendations
thrombosis of the lower limbs:
update and current
recommendations
Bourgoin-Jallieu, France
Abstract
Initially, superficial vein thrombosis (SVT) was considered a benign disease or a common complication of varicose veins. Recent studies have shown the potential severity of SVT and defined its place within the venous thromboembolic (VTE) diseases, along with deep vein thrombosis (DVT) and pulmonary embolism (PE). A concomitant DVT was identified in 25% to 30% of patients at presentation and a PE in 4% to 7% of patients. Subsequent VTE were reported in 3 to 20% of patients, depending on the follow-up duration. Until recently, numerous anticoagulant strategies have been tested, with no clearly demonstrated clinical benefit. However, the recent CALISTO study (Comparison of Arixtra in lower LImb Superficial vein ThrombOsis with placebo) validated an anticoagulant therapy protocol based on fondaparinux, 2.5 mg daily for 45 days, resulting in updated recommendations for the management of SVT. This article will present an update on the management of lower-leg SVT and the current recommendations and guidelines. Briefly, all patients with SVT should have a bilateral duplex scan to confirm the diagnosis of SVT, determine the precise location and extent of the SVT, and diagnose or rule out the presence of a DVT. For patients with symptomatic SVT at least 5 cm in length, it is recommended to prescribe a prophylactic dose of fondaparinux or low-molecular-weight heparin for 45 days over no anticoagulation (Grade 2B), and when the cost of treatment with fondaparinux is acceptable, it is recommended to use fondaparinux 2.5 mg daily vs lowmolecular- weight heparin (Grade 2C). However, the recommendations and guidelines have assigned these treatments with a low grade, and questions remain about SVT management. Some risk factors for subsequently developing a VTE have been identified, but further research is needed to define subgroups of patients with a higher incidence of a VTE after an SVT.
Introduction
Superficial vein thrombosis (SVT) has been considered a benign disease or common complication of varicose veins; however, recent studies have shown their potential severity and defined their place within the venous thromboembolic (VTE) diseases, along with deep vein thrombosis (DVT) and pulmonary embolism (PE).
Anticoagulant therapy is widely used today instead of nonsteroidal anti-inflammatory drugs (NSAID), which were commonly used until the last decade. A recent study has, for the first time, validated a therapeutic protocol.1 However, questions remain concerning SVT management: (i) is anticoagulant therapy required to treat all patients with SVT of the lower limbs?; (ii) should prophylactic or therapeutic doses be used?; (iii) what is the recommended treatment duration?; (iv) should the management be the same for SVT occurring in varicose veins and non-varicose veins?; (v) can the risk factors of VTE complications after SVT be predicted?; and (vi) is surgery still indicated for the management of an acute SVT?
This article will present the rationale behind the update for the management of SVTs of the legs and the current recommendations and guidelines.
Incidence of superficial vein thrombosis of the lower limbs
SVT is considered a common disease, but the actual incidence in the adult population remains unknown. A recent study, conducted in France,2 showed that the annual diagnosis rate was 0.6%. It was higher in women and increased with advancing age regardless of gender. Surprisingly, the annual diagnosis rate of SVT was lower than expected and lower than the annual diagnosis rate of DVT (about half that of DVT). According to another French study, which was conducted with comparable methods, the annual incidence of a lower limb DVT and PE was 1.24% and 0.6%, respectively.3
Superficial vein thrombosis with concomitant deep vein thrombosis at presentation
The POST (Prospective Observational Superficial Thrombophlebitis) and OPTIMEV studies (OPTimisation de l’Interrogatoire dans l’évaluation du risque throMbo- Embolique Veineux), two large observational and epidemiological studies, recently published essential data on SVT.4,5 A total of 844 patients with SVT of the legs were analyzed in the POST study,4 and a DVT or PE was identified in 25% of patients with SVT at presentation and a proximal DVT was diagnosed in 9.7% of the patients. We must emphasize that DVT was not contiguous with SVT in 41.9% of patients with DVT. A total of 788 patients with SVT were enrolled in the OPTMEV study,5 where an SVT was associated with a DVT at inclusion in 29% of patients, with distal DVT occurring in 59.5% of these patients (128/215; the exact location of DVT was missing in 12 patients).
These data confirm previous studies showing that DVT was associated with SVT in 23% to 36% of patients and show coherence among the different studies (Table I).2,4-10
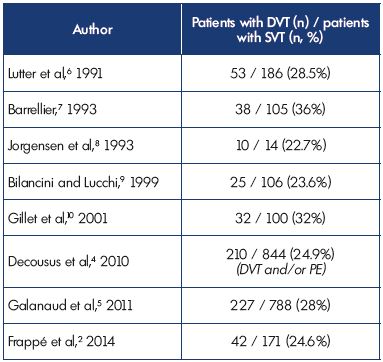
Table I. Superficial vein thrombosis with concomitant deep vein
thrombosis at presentation.
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; SVT,
superficial vein thrombosis.
Superficial vein thrombosis associated with pulmonary embolism at presentation
At inclusion, symptomatic PE was diagnosed in 3.9% and 6.9% of patients in the POST and OPTIMEV studies, respectively. However, SVT with PE, but without DVT, accounted for only 2.2% of all SVTs with DVT or PE. These data corroborate findings from previous studies (Table II).2,4-7,9,10
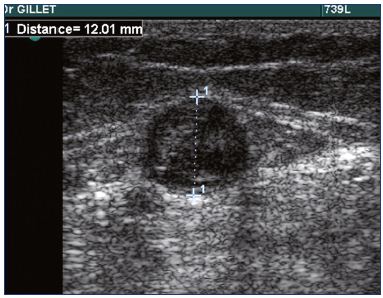
In practice, a duplex scanning examination is mandatory in patients with SVT to confirm the diagnosis (Figure 1), determine the precise location and extent of the SVT, and diagnose or rule out the presence of a DVT.
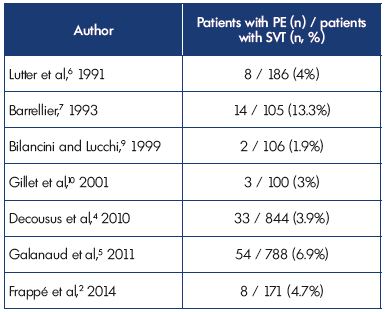
Table II. Superficial vein thrombosis with concomitant pulmonary
embolism at presentation.
Abbreviations: PE, pulmonary embolism; SVT, superficial vein thrombosis.

Outcome and venous thromboembolic recurrence
In the literature, the rate of thromboembolic recurrence ranges from 3% to 20% depending on the duration of the follow-up. In a personal study,13 we reported the occurrence of symptomatic VTEs in 16.4% of patients with isolated SVT, with a mean follow-up of 14.5 months. The VTE events included DVT (31%), PE (6%), another SVT in a different saphenous system (37.5%), and a recurrent SVT in the same saphenous system (25%).
In the POST study,4 8.3% of patients with an isolated SVT at inclusion developed at least 1 symptomatic VTE event at 3 months (symptomatic DVT, 2.8%; symptomatic PE, 0.5%; symptomatic extension of SVT, 3.3%; and symptomatic recurrence of SVT, 1.9%). In the OPTIMEV study,5 3% of patients with an isolated SVT and 5.4% of patients with an SVT associated with DVT at presentation, developed a VTE at 3 months; the rate of VTEs was 12.5% at the 3-year follow-up. In the study by Dewar and Panpher,14 a symptomatic DVT occurred in 4% of the patients with an isolated SVT at a 6-month follow-up.
These epidemiological findings show the potential severity of SVT. They should no longer be considered a benign condition. Consequently, their place has now been clearly defined within the VTE diseases.
Risk factors for developing a thromboembolic event
A multivariate analysis of the POST study4 identified male sex, history of DVT or PE, previous cancer, and no varicose veins as risk factors for a symptomatic VTE at 3 months, including recurrence or extension of the SVT. In the STENOX study (Superficial Thrombophlebitis treated by ENOXaparin),15 history of a VTE (DVT or PE), male sex, and severe chronic venous insufficiency were identified as independent predictive factors for a VTE at 3 months. Only severe chronic venous insufficiency was an independent predictive factor for DVT or PE. In a pooled analysis of the POST and OPTIMEV studies,16 Galanaud et al showed that male sex, cancer, personal history of VTE, and saphenofemoral or saphenopopliteal involvement significantly increased the risk of a subsequent VTE or DVT/ PE in a univariate analysis. In multivariate analyses, only male sex significantly increased the risk of a subsequent VTE or a DVT/PE recurrence. For cancer and a personal history of VTE, the adjusted hazard ratios were only slightly below the level of statistical significance (P=0.06 for both), suggesting that, for these factors, the study merely lacked sufficient statistical power.
In the STEFLUX study (Superficial Thromboembolism FLUXum),17 having a body mass index (BMI) between 25 and 30 kg/m2 and a composite of a previous SVT and/or VTE and/or family history of VTE were identified as significant independent risk factors for a VTE event (composite of symptomatic and asymptomatic DVT, PE, and SVT recurrence or extension).
Vein status
Varicose vein status has been reported to influence the risk of exhibiting DVT at presentation. In the POST4 and OPTIMEV studies,5 SVTs occurring in a non–varicose vein (NVV-SVT) were more often associated with a concomitant DVT or PE than SVTs occurring in a varicose vein (VV-SVT). Similar findings were reported by Gorty et al.18
At the 3-month follow-up in the OPTIMEV study, isolated NVV-SVT was not associated with a higher risk of adverse outcomes (ie, death, VTE recurrence, and bleeding). Isolated NVV-SVT had a higher association with symptomatic DVT or PE recurrence (2.7% vs 0.6%), but this result did not reach statistical significance (P=0.07).
In the POST study, the absence of varicose veins was identified as a risk factor for the subsequent development of a symptomatic VTE in patients with an isolated symptomatic SVT at presentation (P=0.049). In the STEFLUX study,17 the absence of varicose veins was a risk factor for VTE (P=0.004) after the treatment with low-molecular-weight heparin was stopped.
In the placebo group of the CALISTO study (Comparison of Arixtra in lower LImb Superficial vein ThrombOsis with placebo),1 thromboembolic complications occurred more often when the SVT involved the great saphenous vein (GSV), was extended to within 10 cm of the saphenofemoral junction (SFJ), involved veins above the knee, and in patients with a history of VTE.
Venous stasis is the primary mechanism of SVT in patients with varicose veins. Inflammation may play an essential role in thrombus formation in patients without varicose veins; thereby, conferring a higher risk for a more clinically serious thromboembolism. Screening for thrombophilia is not recommended for the routine management of patients with NVV-SVT, although data from the literature showed that thrombophilia was frequent in this situation. In a personal prospective study,10 we identified thrombophilia in 50% of patients with NVV-SVT, while only 15% of the patients with VV-SVT had thrombophilia. In another prospective study involving 42 patients with NVV-SVT,19 we identified thrombophilia in 20 (47.6%) patients. The most common thrombophilia was due to the heterozygous factor V Leiden mutation. In a study involving 63 patients with isolated NVV-SVT,20 Martinelli et al identified thrombophilia in 30% of patients. Screening for thrombophilia is advisable, after exclusion of an occult cancer, especially for patients with thrombus progression despite appropriate anticoagulant therapy.11,21
Treatment of superficial vein thrombosis
Treatment of SVT has always been a controversial topic. Great variations in the treatment are reported, especially regarding anticoagulant therapy. The POST study,4 which was carried out in France between March 2005 and October 2006, provided interesting information regarding SVT treatment. A total of 634 patients had an isolated SVT at inclusion. Information about the treatment they received during the 3-month observation period was available for 597 patients, with 90.5% of patients having received one or more anticoagulant drugs. Of the patients receiving anticoagulant therapy, 63% received therapeutic doses, 36.7% prophylactic doses, and 16.8% vitamin K antagonists. Treatment duration was highly variable. A total of 47.2% of patients received a topical NSAID, 8.2% an oral NSAID, and 10% had venous surgery (stripping or high-ligation).
These data showed the necessity of clarifying the role of anticoagulant therapy in SVT management. The use of anticoagulant therapy in patients presenting with an SVT was first reported in 1962 by Zollinger et al,22 after observing the occurrence of a PE, which was fatal in 34 (10.1%) of a series of 335 patients with an SVT. Until recently, although numerous anticoagulant strategies had been tested, including unfractionated heparin or low molecular- weight heparin, at prophylactic or therapeutic doses for various durations, none had clearly demonstrated any clinical benefit.
The STENOX study23 was a randomized double-blind trial involving 427 patients, comparing low-molecular-weight heparin (enoxaparin at therapeutic and prophylactic doses) with NSAID and placebo. Patients were treated for 10 days, with a 3-month follow-up. At 10 days, there were more VTEs in the placebo group (P<0.01) than the other groups, but at 3 months there was no difference, suggesting that the 10-day treatment period was too short. The VESALIO study24 compared therapeutic vs prophylactic doses of nodraparin in 163 patients with an isolated SVT in the GSV, and patients were treated for 1 month. At the 3-month follow-up, the results were similar in both groups (7.2% and 8.6% occurrence of a VTE, respectively; P=0.7), showing no benefit of the therapeutic dosage. A “catch-up” or rebound phenomenon was observed during the follow-up, as many VTEs were reported, especially in the group of patients treated with the therapeutic dosage. A “catch-up” phenomenon was also observed after discontinuing low-molecular-weight heparin treatment after 1 month in the STEFLUX study.25 These findings, like those of the STENOX trial, plead for the choice of prophylactic doses of low-molecular-weight heparin in SVT. The occurrence of the majority of VTEs during the 2 and 3 months after discontinuing treatment with low-molecular-weight heparin in the group receiving therapeutic doses, once again highlights the issue of the optimal duration of anticoagulant therapy.
The randomized, double-blind CALISTO study1 compared fondaparinux 2.5 mg daily for 45 days with placebo in 3002 patients with an isolated symptomatic lower limb SVT that was at least 5 cm in length. The main exclusion criteria were treatment for cancer within the previous 6 months, DVT or PE within the previous 6 months, SVT located within 3 cm of the SFJ, and severe renal insufficiency (creatinine clearance <30 mL/min). According to Decousus et al,26 the 2.5 mg dose of fondaparinux was selected on the idea that a prophylactic dose would be sufficient to treat patients with SVT. In addition, this dose was shown to be more effective in preventing VTEs after major orthopedic surgery than a prophylactic dose of low-molecular-weight heparin, and as effective as the therapeutic dose of low-molecular-weight heparin in patients with acute coronary syndromes, suggesting that 2.5 mg fondaparinux would match the 2008 American College of Chest Physicians (ACCP) recommendations27 that advocate the use of either prophylactic or intermediate doses of low-molecular-weight heparin to treat patients with SVT. The duration of 45 days was chosen because a treatment period of 30 days or less might be too short, as most symptomatic VTEs occur after treatment discontinuation. The primary efficacy outcome was a composite of death from any cause, a symptomatic PE, a symptomatic DVT, a symptomatic extension to the SFJ, or a symptomatic recurrence of SVT at day 47. There was a 77-day follow-up period for the patients.
The primary efficacy outcome occurred in 0.9% of patients in the fondaparinux group and 5.9% in the placebo group (P<0.001). The incidence of each component of the primary efficacy outcome was significantly reduced in the fondaparinux group as compared with the placebo group, except for death (0.1% in both groups). The rate of PE or DVT was 85% lower in the fondaparinux group.
Unlike previous studies with low-molecular-weight heparin, and using shorter courses of treatment, similar risk reductions were seen at day 77, 1 month after discontinuing fondaparinux. Based on the results from the CALISTO study, fondaparinux was granted European market authorization for treatment of isolated SVT and the ACCP guidelines were updated.28

It is interesting to notice the change of recommendations from the 2008 ACCP guidelines.27 However, we must take into account that they are low-grade recommendations (Grade 2B or 2C).
In the update of the Cochrane Database Systematic Review on “Treatment for superficial thrombophlebitis of the leg,”29,30 Di Nisio et al reached the same conclusions. This review was based on the analysis of 30 randomized controlled trials involving 6507 participants with SVT of the legs. The authors’ conclude that a prophylactic dose of fondaparinux, given for 6 weeks, appears to be a valid therapeutic option for SVT of the legs. The evidence on oral treatments, topical treatment, or surgery is too limited and does not provide information for use in clinical practice about the effects of these treatments in terms of VTE and SVT progression.
Surgery versus anticoagulant therapy
A review of studies comparing surgery with anticoagulant therapy does not show any benefit for surgical treatment. The rates of SVT progression were similar while the incidence of VTE and complications were higher with surgery.31 Lozano et al showed no difference between surgery and enoxaparin for 4 weeks.32

Figure 2. Ultrasound of an extension of a thrombosis of the
great saphenous vein into the common femoral vein.
Abbreviations: CFV, common femoral vein; GSV, great saphenous vein

In practice, most experts recommend treating patients with SVT extended at the SFJ or SPJ with anticoagulant therapy at therapeutic doses for 3 months.
Conclusion
SVT should no longer be considered a benign disease. Recent epidemiological studies, which have included a large number of patients, have shown the potential severity of SVTs and have clearly defined their place within the VTE diseases. A concomitant DVT was identified in 25% to 30% of patients at presentation and a PE in 4% to 7% of patients. Consequently, all patients with SVT should have bilateral duplex scanning to confirm the diagnosis of SVT, determine the precise location and extent of the SVT, and diagnose or rule out the presence of a DVT. Today, SVT management has changed, with anticoagulant therapy being widely used instead of NSAIDs. Until the recent CALISTO study, no anticoagulant protocol had demonstrated a clear clinical benefit. The recommendations were updated after the CALISTO study validated the anticoagulant therapy protocol based on fondaparinux 2.5 mg daily for45 days. For patients with a symptomatic SVT of the legs at least 5 cm in length, a prophylactic dose of fondaparinux or low-molecular-weight heparin for 45 days is recommended over no anticoagulation (Grade 2B). When the cost of treatment with fondaparinux is acceptable, using fondaparinux 2.5 mg daily over a prophylactic dose of low-molecular-weight heparin is recommended (Grade 2C). However, the recommendations and guidelines are of a low grade, and questions remain concerning SVT management. Some risk factors for subsequently developing a VTE have been identified, but further research is needed to clearly define subgroups of patients with a higher incidence of VTE after SVT.

1. D ecousus H, Prandoni P, Mismetti P, et al; CALISTO Study Group. Fondaparinux for the treatment of superficial vein thrombosis in the legs. N Engl J Med. 2010;363:1222-1232.
2. Frappé P, Buchmuller-Cordier A, Bertoletti L, et al; STEPH Study Group. Annual diagnosis rate of superficial vein thrombosis of the lower limbs: the STEPH community-based study. J Thromb Haemost. 2014;12:831-838.
3. Oger E; EPI-GETBO Study Group. Incidence of venous thromboembolism: a community-based study in Western France. Thromb Haemost. 2000;83:657- 660.
4. D ecousus H, Quéré I, Presles E, et al; POST Study Group. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152:218-224.
5. G alanaud JP, Genty C, Sevestre MA, et al; OPTIMEV SFMV Investigators. Predictive factors for concurrent deepvein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. Thromb Haemost. 2011;105:31-39.
6. Lutter KS, Kerr TM, Roedersheimer R, Lohr JM, Sampson MG, Cranley JJ. Superficial thrombophlebitis diagnosed by duplex scanning. Surgery. 1991;100:42-46.
7. Barrellier MT. Thromboses veineuses superficielles des membres inférieurs [in French]. Actual Vasc Int. 1993;17:7-9.
8. Jorgensen JO, Hanel KC, Mogan AM, Hunt JM. The incidence of deep venous thrombosis in patients with superficial thrombophlebitis of the lower limbs. J Vasc Surg. 1993;18:70-73.
9. Bilancini S, Lucchi M. Les thromboses veineuses superficielles sont-elles polymorphes [in French]? Phlébologie. 1999;52:41-43.
10. G illet JL, Perrin M, Cayman R. Superficial venous thrombosis of the lower limbs: prospective analysis in 100 patients [in French]. J Mal Vasc. 2001;26:16-22.
11. Kalodiki E, Stvrtinova V, Allegra C, et al. Superficial vein thrombosis: a consensus statement. Int Angiol. 2012;31:203-216.
12. Nicolaides A. Superficial vein thrombosis in prevention and treatment of venous thromboembolism. Int Angiol. 2013;32:237-242.
13. G illet JL, Perrin M, Cayman R. Thromboembolic recurrence after superficial thrombophlebitis of the lower limbs. J Phlebology. 2002;2:103-118.
14. D ewar C, Panpher S. Incidence of deep vein thrombosis in patients diagnosed with superficial thrombophlebitis after presenting to an emergency department outpatient deep vein thrombosis service. Emerg Med J. 2010;27:758-761.
15. Quenet S, Laporte S, Décousus H, Leizorovicz A, Epinat M, Mismetti P; STENOX Group. Factors predictive of venous thrombotic complications in patients with isolated superficial vein thrombosis. J Vasc Surg. 2003;38:944- 949.
16. G alanaud JP, Bosson JL, Genty C, et al. Superficial vein thrombosis and recurrent venous thromboembolism: a pooled analysis of two observational studies. J Thromb Haemost. 2012;10:1004-1011.
17. Cosmi B, Filippini M, Campana F, et al; STEFLUX Investigators. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial SteFlux (Superficial Thromboembolism Fluxum). Thromb Res. 2014;133:196-202.
18. G orty S, Patton-Adkins J, DaLanno M, Starr J, Dean S, Satiani B. Superficial venous thrombosis of the lower extremities: analysis of risk factors, and recurrence and role of anticoagulation. Vasc Med. 2004;9:1-6.
19. G illet JL, Allaert FA, Perrin M. Superficial thrombophlebitis in non-varicose veins of the lower limbs. A prospective analysis in 42 patients [in French]. J Mal Vasc. 2004;29:263-272.
20. Martinelli I, Cattaneo M, Taioli E, de Stefano V, Chiusolo P, Mannucci PM. Genetic risk factors for superficial vein thrombosis. Thromb Haemost. 1999;82:1215-1217.
21. Milio G, Siragusa S, Malato A, Grimaudo S, Pinto A. Superficial venous thrombosis: role of inherited deficiency of natural anticoagulants in extension to deep veins. Int Angiol. 2009;28:298- 302.
22. Zollinger RW, Williams RD, Briggs DO. Problems in the diagnosis and treatment of thrombophlebitis. Arch Surg. 1962;85:34-40.
23. D ecousus H; Enoxaparin Study Group. A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Int Med. 2003;163:1657-1663.
24. Prandoni P, Tormene D, Pesavento R; Vesalio Investigators Group. High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind, randomized trial. J Thromb Haemost. 2005;3:1152-1157.
25. Cosmi B, Filippini M, Tonti D, Avruscio G, Ghirarduzzi A, Bucherini E; STEFLUX Investigators. A randomized doubleblind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost. 2012;10:1026-1035.
26. D ecousus H, Frappé P, Accassat S, et al. Epidemiology, diagnosis, treatment and management of superficial-vein thrombosis of the legs. Best Pract Res Clin Haematol. 2012;25:275-284.
27. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):454S-545S.
28. Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e419S-e494S.
29. D i Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2013;30;4:CD004982.
30. D i Nisio M, Middeldorp S. Treatment of lower extremity superficial thrombophlebitis. JAMA. 2014;311:729- 730.
31. Sullivan V, Denk PM, Sonnad SS, Eagleton MJ, Wakefield TW. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg. 2001;193:556-562.
32. Lozano FS, Almazan A. Low-molecularweight heparin versus saphenofemoral disconnection for the treatment of above-knee greater saphenous thrombophlebitis: a prospective study. Vasc Endovascular Surg. 2003;37:415- 420.
33. Chengelis DL, Bendick PJ, Glover JL, Brown OW, Ranval TJ. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg. 1996;24:745-749.
34. Hingorani A, Ascher E. Superficial venous thrombophlebitis. In: Gloviczki P, ed. Handbook of Venous Disorders. Guidelines of the American Venous Forum. 3rd ed. London, UK: Hodder Arnold; 2009:314-319.