May-Thurner diagnosis and management
Cardiothoracic and Vascular Surgery of
Central Mexico
Abstract
May-Thurner syndrome, known for decades as a unique pathology, has been included recently with other pelvic compression maladies in the S-V-P CEAP (symptoms-varicespathophysiology, clinical-etiology-anatomy-pathophysiology) classification sponsored by the American Venous and Lymphatic Society as part of several anatomic lesions in the abdominopelvic region that have variable clinical presentations. This classification will fully characterize and accurately describe a particular lesion; also, it will facilitate clinical interaction and precise treatment and in the long term the development of patient-reported outcome measures and clinical trials. In the interim, epidemiologic data reported so far have been questioned recently because of the lack of adequate clinical trials. The pathophysiology originally described is still currently accepted, and the clinical presentation is better known. The noninvasive vascular armamentarium (ultrasound, computed tomography, and magnetic resonance imaging) is very reliable, and the invasive methods (venography, intravascular ultrasound) allow us to assure the diagnosis and evaluate treatment. Endovascular treatment is the preferred approach to dissolve a thrombus should one be present, with then treatment of the underlying compression via stent placement. The stent most used globally is probably the Wallstent because of its results, and the dedicated nitinol venous stents are tending to show good results in long-term follow-up. There is no consensus on optimal anticoagulants given post stenting; however, the newer oral anticoagulants are used in patients with a history of thrombosis. So far, May-Thurner syndrome is underdiagnosed, but probably overtreated. That is why reviews like this are useful to avoid it. Finally, I believe that the term May-Thurner syndrome will continue to be used, alongside the new classification.
Introduction
May-Thurner syndrome (MTS) is an anatomopathologic entity described in 1957, also known as iliac vein compression syndrome or Cockett syndrome. May and Thurner named the syndrome after their findings, based on the work of Rudolph Virchow (1851)1 and his proposal of an increased incidence of left leg venous thrombosis due to compression of the left common iliac vein (LCIV) by the right common iliac artery (RCIA), and on later work of others. In the first half of the 1900s, Mc Murrich studied 107 cadavers with the same pattern of deep venous thrombosis (DVT), finding higher prevalence in the left leg (29.9%) than in the right (2.8%); this study included both neonates and adults, suggesting a possible congenital origin.2 In 1943, Ehrich reported 412 autopsies with special attention to iliac vein dissection and suggested an acquired etiology for left iliac vein obstruction (IVO).3
May and Thurner knew about the previous studies and in an effort to disclose a cause for DVT they dissected 430 cadavers; their findings indicated an important focal intimal venous thickening and septa formation in 22% of the subjects; these they named “spurs.” They hypothesized that “the repetitive trauma caused by the RCIA pulsation over the LCIV against the lumbar spine produces endothelial injury, collagen and elastin accumulating in the vein intimal layer originating webs and spurs”4 (Figure 1).5
Cockett et al in 1965 correlated the DVT incidence, postthrombotic syndrome (PTS), and iliac vein compression clinically and pathologically and they called it “iliac vein compression syndrome.” After those reports, some authors used the terms “May-Thurner-Cockett syndrome” and “iliac vein compression syndrome.”6,7
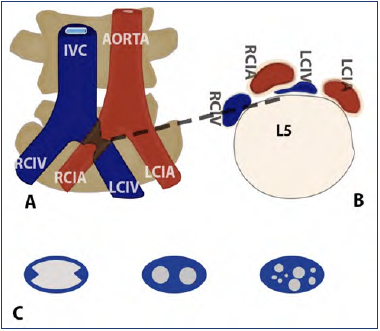
Figure 1. Anatomy of May-Thurner syndrome and types of spurs. A) Compression of LCIV between RCTA and vertebrae. B) Detail of compression. C) Types of Spurs – described in text.
Abbreviations: IVC, inferior vena cava; LCIA, left common iliac artery; LCIV, left common iliac vein; RCIA, right common iliac artery; RCIV, right common iliac vein.
After reference 5: Armenta-Flores et al. Phlebology. 2022;37(1):5-13. Digital illustration by Architect Gustavo Perez. © The Author(s) 2021. Sagepub.com/Journals.
Pathophysiology
Since the original description of the “spur theory” made by May and Thurner, little progress has been made to elucidate the pathogenesis of the LCIV obstruction, no animal model of MTS exists, and the molecular basis of venous spur development remains unknown.8
Recently, some authors have readdressed this issue with a diversity of hypotheses. Harbin et al wrote that the female pelvis has an accentuation of the lumbar lordosis that pushes lower lumbar vertebrae anteriorly, thereby compressing the LCIV against the RCIA.8-10 Urbas and Brenner dissected 100 cadavers looking at the iliocaval junction and the left iliac vein. They described 5 forms of venous spurs—central, adhesion, bridges, valves, and bands—and their conclusion was that central venous spur occur only in the venous confluence and could be remnants of ostial valves, and that the adhesions may originate from embryological development (different wall thickness). They found considerable differences in caliber and circumference in the left iliac vein due to different embryological origin of the different quarters of the cardinal vein or venous anastomotic network; all these spurs mean an obstruction to flow and may be a predisposing factor for DVT. They concluded that these 5 structures do not have a causal relationship for obstruction with compression by the RCIA.11 Despite the aforementioned theories, the most accepted is the one enunciated by May and Thurner in their original paper.4
Epidemiology
To date, no population-based studies have been made to document the prevalence or incidence of MTS.8 So far, data published shows that the exact incidence and prevalence of MTS are unknown but are likely underestimated given that most individuals with MTS anatomy are asymptomatic and require no treatment.12,13 Brazeau and others stated the difference between May-Thurner anatomy and MTS: May-Thurner anatomy is the compression of the LCIV by the overlying RCIA with no hemodynamic significance, and MTS refers to the extrinsic compression of the LCIV by the RCIA against the lumbar vertebrae alongside the presence of venous spurs, compromised venous flow, and venous collaterals with or without DVT.12-14 These differences suggested that LCIV is necessary but not sufficient to cause MTS. Studies targeting the LCIV investigating asymptomatic subjects have found significant compression and diameter reduction in up to 80% of their cohort.
Therefore, compression of the LCIV is present in one-third of the population and both genders are equally affected.12,13,15 MTS could cause 2% to 5% of all DVT. Many cadaveric and radiographic studies estimated the prevalence to be much higher. Several autopsy studies on unselected patients showed MTS prevalence to be between 14% and 32%8,13,14 and in radiological studies with DVT patients, could be 22% to 76%.8,12-16
For all the aforementioned, the American Vein and Lymphatic Society International Group on Pelvic Venous Disorders recently published the “symptoms-varices-pathophysiology classification of pelvic venous disorders” to encompass most of the venous maladies of the pelvic-inferior extremities axis and to standardize classification and develop disease-specific outcome instruments and clinical trials. In the meantime, notation for a patient with MTS with left lower-extremity edema is written like this: SoVoPlciv.o.nt:Left C3sEseAdPo(civ).17
Clinical presentation
MTS is more common in young healthy women between ages of 20 and 50 years old, though it is not confined to this group. It is the most significant factor for left-sided DVT, being 3 to 8 times more common than right-sided DVT.12,13,18 The most frequent presentation is chronic venous insufficiency and it is present in 20% to 50% of cases of left lower-limb DVT.18,19 Patients complain of acute intermittent and progressive (activity-related) heaviness and swelling of left lower limb or venous claudication that is relieved with rest and leg elevation; they may also report tighter shoes in the affected leg at the end of the day with fatigue and swelling. The progression of the chronic venous insufficiency—manifested as pain, venous claudication, varices, skin hyperpigmentation, and ulceration—reduces quality of life (QOL). Rare symptoms include phlebitis and phlegmasia cerulea dolens. Other less common presentation is acute, spontaneous, and painful leftleg swelling (DVT) with no precipitating cause, or the disease may first present during or after pregnancy and a history of recent use of oral contraceptive pills. Many times, patients are studied for iliac vein compression after standard ablative treatment for varicose veins and poor medium- or long-term results. Actually, many patients live with progressive left-sided venous hypertension and do not recognize it. They have increased tightness or discomfort with activity but are better in the morning, so they delay medical consultation.12,13,18,20-22 Besides the ancillary presentation in May-Thurner description, other variants exist, eg, right-sided MTS and compression of the inferior vena cava (IVC) by the RCIA. Moreover, rare MTS are described, including rupture of the iliac vein, secondary to an iliac artery stent, prostate hypertrophy in patients with foramen ovale and cryptogenic stroke, and in pelvic congestion syndrome.5,23-35
A thorough history and physical exam are important to identify the clinical presence of LCIV obstruction in symptomatic patients with lower-extremity discomfort, edema, and/or discoloration. Kim et al described the 3 stages of iliac vein compression: stage I, asymptomatic iliac vein compression without any narrowing; stage II, development of venous spurs without thrombosis; and stage III, presence of left iliac vein DVT.36 Diagnosis of MTS requires demonstration of the venous stenotic lesion in an appropriate anatomic location.37 In patients with proximal DVT, history of DVT, or venous insufficiency with lower-extremity swelling, the initial investigation is via duplex ultrasound (DUS); in the absence of thrombus, computed tomography (CT) venography and/or magnetic resonance (MR) venography are indicated.5
Diagnosis
Color venous duplex ultrasound
After clinical suspicion of DVT, the initial noninvasive diagnostic test is color venous DUS (CVDU), its sensitivity is 91% and specificity is 99% using compression in proximal DVT. Whereas the first aim of CVDU is to rule out DVT, it also evaluates venous reflux time. Venous DUS findings of iliocaval DVT are as follows: absence of flow variation, narrowed iliac veins, and poststenotic turbulence (noisy signal).38-40 To evaluate the common femoral vein, a linear 4 to 7 MHz array transducer with a <60° angle of insonation is used, whereas a 2 to 3 MHz transducer should be used for iliac and caval vessels. B-mode compares vein diameter reduction at the smallest lumen area against normal vein diameter. Peak vein velocity (PVV) is measured in the pre- and poststenotic segment; a PVV gradient >2.0 is significant.12,41 However, the deep location of the proximal iliac vein plus other factors (obesity, overlying gas) interferes with ultrasound for an accurate diagnosis of MTS.41,42
A recent maneuver in asymptomatic patients showed the presence of illusory MTS: even a well-hydrated patient in supine position can show compression of the LCIV; moving the patient to a semi-sitting 45° position (Semi-Fowler) releases the gravitational overload and flow recovery occurs in the LCIV (Zamboni maneuver); this has been corroborated by plethysmography, either in the semi-settle or in supine position, with and without leg elevation. The real MTS is nonreversible and/or associated with intraluminal defects. This maneuver could become an initial screening to avoid more invasive orexpensive diagnostic steps.43,44 Current ultrasound technology allows for a greater penetration depth with improved image resolution, and DUS planimetry has recently been proposed to diagnose an obstruction better than hemodynamic criteria. DUS permits a full examination of the abdominopelvic and lower-limb venous system in a single session. It shows obstruction and reflux, detects intraluminal vs extraluminal causes of stenosis, and allows ongoing assessments after intervention and treatment. Transabdominal ultrasound is comparable to IVUS for the detection of IVO, and DUS planimetry measures are more reliable than hemodynamic measures. Nowadays, the results for the utility of DUS as a noninvasive workup diagnostic tool for IVO and tracking tool for IVO treatment, show that it can be used to guide clinicians and help determine which patients should be offered IVUS and IVUS-guided treatment.45-47
Cross-sectional imaging: CT/MR venography
Both imaging methods have more than 95% sensitivity and specificity in MTS but require particular protocols in order to obtain better imaging. CT venography (Figure 2) using 3-mm to 5-mm cuts allows visualization of structural details (spurs, webs), rules out extrinsic compression, identifies location and stenosis degree in nonthrombosed veins, and shows DVT and collateral pathways.48-55 If the contrast opacification is suboptimal with the standard (indirect) method, a direct technique could be used with good results.49,50
As with ultrasound, the patients can be put in different positions (supine or prone) or the Valsalva maneuver can be used to identify an illusory MTS.43,56 CT venography advantages over CDUS or venography include lack of operator dependence, clearer pelvic vein images, and shorter exam time. However, because of the radiation dose, it should be avoided in pregnancy, and the use of contrast medium contraindicates its use in patients with renal failure.49,50
With recent advances in software and technology, eg, 3-dimensional CT venography, some researchers have compared it with IVUS and described noninferiority for the evaluation of the degree of stenosis, its length and luminal caliber of the left iliac vein, and for the prediction of stent sizing, rendering it a good tool for diagnosis and treatment of chronic iliofemoral venous obstruction.57,58
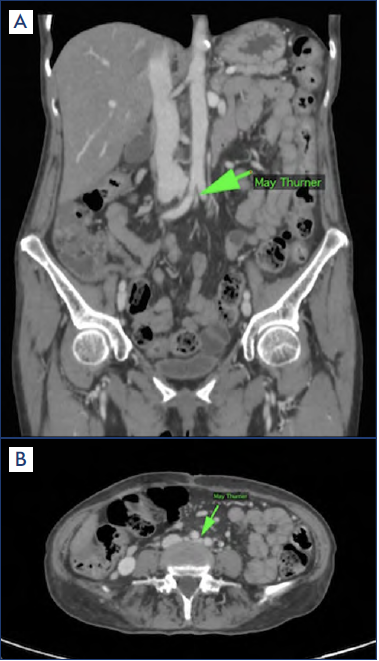
Figure 2. Contrast computed tomography in May-Thurner syndrome. A) Coronal view. B) Axial view.
Green arrow, left common iliac vein compressed.
Images used by permission: Dr Rafael Paz. Departamento de radiología. Hospital Medica Campestre, Leon, Gto. Mexico.
MR venography provides information similar to CT venography with better characterization of the pathology in pelvic and spinal structures, including lumbar vertebral degeneration, bulging or protruding intervertebral disks, osteophytes, or spondylolisthesis59; and further assessment of hemodynamic significance by demonstrating pelvic collaterals and flow reversal (on time-of-flight pulse sequence) within the ascending lumbar veins. With advances of techniques, high-resolution variable-flip-angle turbo-spin-echo (CUBE) MR imaging allows separation of the vessel wall and lumen and can demonstrate DVT. A few studies showed the use of noncontrast MR venography to diagnose MTS without using a control cohort, and they concluded that MR venography and CUBE have similar results in assessing the degree of luminal stenosis, the site, and number of compressions in MTS; however, they underestimate the narrowest diameter in comparison with CTV; hence, high clinical suspicion of an occlusive left iliac vein stenosis with a suggestive color DUS could lead to performance of invasive venous imaging; but, if there is any anatomical concern, cross-sectional imaging— preferentially CT venography—is in order.12,13,60,61
Invasive venous imaging
Catheter venography
Catheter venography was the gold standard in diagnosing MTS until recently. It is nowadays reserved for cases in which cardiovascular intervention is planned or diagnosis by noninvasive modalities is equivocal.21 It measures pressure gradients across the stenotic area; a gradient >2 mm Hg at rest and >3 mm Hg during exercise has hemodynamic significance.12,18,21,55 It determines location and severity of the stenosis; to improve its accuracy, multiplanar views—anterior posterior and lateral projections—are obtained during injection to avoid “the pancaked vein effect” (externally compressed in the AP plane).18,20,21,50. So far, no study has validated a specific diameter threshold for a stenotic lesion in the venous system leading to symptoms; that is due to various factors, including compliance of veins, volume status, and position of the patient. However, a stenosis of more than 50% has been accepted empirically to stent for relieving symptoms.37,39,40,50,62
Confirmation of a stenotic lesion in MTS is made by pressure measurements. There are various methods, but the more accurate is the pullback method, which measures the pressure in the lower IVC, comparing it with the distal iliac vein, and a gradient pressure is obtained.52,55 Venography helps to define collaterals or the presence of congenital venous anomalies; it shows blood flow patterns and the presence of thrombi (Figure 3A).
However, venography is invasive, time consuming with an increased bleeding risk, and does not contribute to extravascular information; also, patients are exposed to radiation and contrast dye.12,13,18,21
Intravascular ultrasound
Nowadays, the gold standard for MTS is venography plus intravascular ultrasound. IVUS is more sensitive than venography (>98%). It provides high-resolution images through high-frequency sound waves from the ultrasound transducer on the catheter. IVUS shows precisely the morphology of the spur and estimates the severity and distribution of pathology.18,37,40,41,55,62,63 There are 2 types of IVUS available: mechanical and solid state (digital and rotational catheters). IVUS catheters use a 0.035-inch wire and are chosen on the basis of their maximal imaging diameter and transducer frequency, eg, Volcano 60 mm, 12 Mhz.12,18,52,55
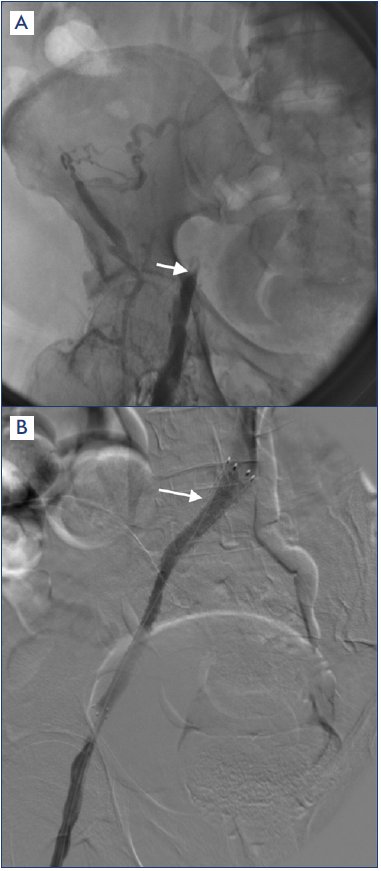
Figure 3. Catheter venography in May-Thurner syndrome. A) Deep venous thrombosis with collateral circulation. B) Left common iliac vein, stented.
After reference 5: Armenta-Flores et al. Phlebology. 2022;37(1):5-13. © The Author(s) 2021. Sagepub.com/
Journals.
IVUS provides data on minimal luminal area at the compression site, reference lumen area, and signs of fibrosis within the vein. Since the inception of IVUS in the turn of this century, it has been considered an integral part of stent deployment. It has advantages in subtle iliac vein pathology and is useful before intervention—for proper vessel sizing— and after therapeutic interventional procedures. It measures cross-sectional area gain, stent placement, its expansion, and in-stent restenosis (Figure 4). Specific measurements of luminal areas and diameters can be obtained without the requirement of contrast and lateral projection imaging.
IVUS visualizes wall thickening caused by compression and adjacent structures, eg, iliac artery. It identifies subtle stenosis when the vein wall and lumen appear otherwise normal. IVUS does not utilize contrast or ionizing radiation.
IVUS is useful for measuring iliac luminal areas and diameters, which are important in selecting stent size and length for placement of the optimal stent for the patient, and thereby minimize the chance of stent migration. Finally, IVUS allows for more precise stent deployment by facilitating accurate identification of the iliac vein confluence. This assists in a right deployment by reducing the chance that a portion of the stent will unduly obstruct the RCIV. The limitations of IVUS are invasiveness of the procedure, limited extravascular information, and in some places, lack of availability.12,18,20,37,41,52,55,62,64
Management
Current treatment of MTS
Management depends upon the presence of symptoms, severity, and whether or not DVT is present.
1) In patients with nonthrombotic iliac vein lesions (NIVL) who are symptomless or with mild symptoms (CEAP 1–3), conservative treatment with compression stockings is enough.12,13,18
2) In patients with nonthrombotic MTS with moderate to severe symptoms (CEAP 4–6), angioplasty and stenting is indicated.12,20,62
3) For thrombotic MTS without contraindication to lytic therapy, initially, anticoagulation is indicated, then catheter-directed thrombolysis and/or pharmacomechanical thrombolysis, and finally, angioplasty and stenting; after this, the rate of PTS is less than 10%; without treatment it is 80% to 90%.12,20,62
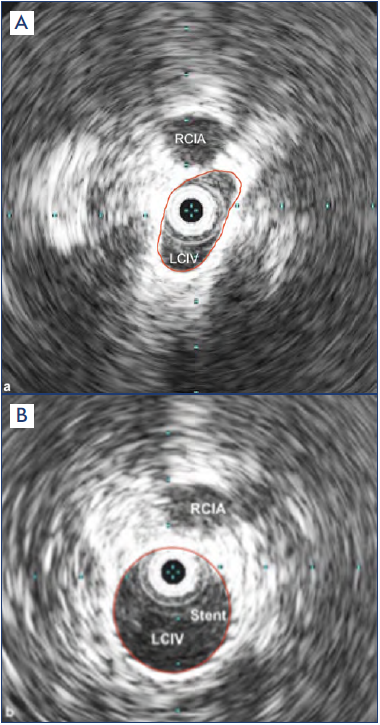
Figure 4. Intravascular ultrasound (IVUS). A) Stenotic left common iliac vein, compressed by right common iliac artery. B) Left common iliac vein, stented.
Abbreviations: LCIV, left common iliac vein; RCIA, right common iliac artery.
After reference 5: Armenta-Flores et al. Phlebology. 2022;37(1):5-13. © The Author(s) 2021. Sagepub.com/ Journals.
4) In patients with thrombotic MTS with contraindications to lytic therapy, mechanical (suction) thrombectomy or open surgical thrombectomy are indicated, then angioplasty and stenting.12,18,20,62,64 Application of IVC filter is not recommended unless an IVC thrombus is present.5,12,13,18
The endovascular approach begins as follows: 1) a presumptive MTS based on clinical suspicion; 2) CDVU with the Zamboni maneuver to avoid an illusory image56; then, either CT venography or MR venography; 3) venography and IVUS to demonstrate and confirm the degree of LCIV stenosis, the affected vein stenosis segment; and finally, 5) stenting. (Figure 3B).12,13,18,43,64
DVT and PTS are 2 of the clinical maladies more visible in angiology practice; it is estimated that PTS develops in 20% to 50% of DVT cases.65 However, the risk is greatest after thrombosis in the iliofemoral region. Alternatively, obstructive chronic deep venous disease can occur owing to NIVL, of which MTS is an example.
Imaging studies showed that NIVL can be present in 24% of asymptomatic patients and up to 60% of patients with chronic deep venous disease. The development of improved endovascular skill sets in various specialties treating venous diseases has led to the offering of endovascular treatment in outpatient clinics; also, the availability of dedicated venous stents is a factor in this enthusiasm for the treatment of venous outflow obstruction.
The use of self-expanding stents for the treatment of venous outflow obstruction was reported by Drs Neglen and Raju more than 20 years ago. Their described technique included the use of the venous Wallstent endoprosthesis stent (Boston Scientific Corporation), a braided, self-expanding stent composed of Elgiloy (a cobalt-chromium-nickel alloy).66
Although the venous Wallstent was not initially designed as a venous stent, its large diameters, compression (crush) resistance, radial force, and fracture resistance lent itself well to venous stenting.67
The Wallstent is the stent most used in the United States. It improves patency and symptoms compared with angioplasty alone. However, it has a high rate of recoil and significant foreshortening (83.75%) when deployed, making it difficult to position accurately at the compression site; for this, the proximal landing zone must be 3 to 5 cm in the IVC.22,68-70 Clinical experience with the Wallstent in the iliofemoral venous region in the last 2 decades showed its efficacy as reported in one of the largest retrospective studies, including 982 lesions; the 5-year primary patency, assisted-primary patency, and secondary cumulative patency rates were 79%, 100%, and 100% in nonthrombotic disease and 57%, 80%, and 86% in thrombotic disease, respectively.22,68,69 The last several years have witnessed the shift from use of the Wallstent alone to a combination of Wallstent and Z stent (Cook Medical)—a composite stent. This approach enables better handling of the iliocaval confluence, and the overall patency seems to be comparable with the use of Wallstents alone. Additionally, use of the composite stent configuration not only decreases the need for contralateral stenting from chronic obstruction, but also decreases the incidence of contralateral iliofemoral deep venous thrombosis; this result argues for the use of a composite stent configuration in patients undergoing iliofemoral venous stenting as opposed to Wallstents alone; it will allow for comparison of outcomes with the use of dedicated venous stents.71
Dedicated venous stents were developed to overcome the Wallstent problems. Nitinol stents do not foreshorten as much as the Wallstent; the implanted length should be near nominal of the intended when sized properly, so the foreshortening is not significant, providing a more accurate positioning of the stent.22,70,72 Hence, nitinol stents are not put in the IVC. Several of these stents have good outward and compression radial force and crush resistance. There is no comparative data between the Wallstent and the new nitinol stents with relation to patency and target-lesion revascularization; the stent must be large enough to bypass the stenotic area, and the distal landing zone has to be wide enough to avoid blood flow perturbations.68
There is no comparative data between venous stents following angioplasty and stenting for MTS.22
The ideal venous stent would be adaptable to a variety of venous anatomic features, available in a wide range of diameters and lengths, strong and able to resist both recoil and compressive forces, flexible and able to negotiate the curves of the venous anatomy in the pelvis without kinking or distorting the vein, durable and able to withstand repetitive movement without loss of integrity, and able to offer accurate and precise deployment at both the stent ends. By the year 2021,67,70 the US Federal Drug Administration (FDA) had approved 5 dedicated venous stents; but 2 withdrew voluntarily, allegedly due to issues with stent deployment and migration (Vici and Venovo). Hence, nowadays, there are 3 brands available: Wallstent (Boston Scientific), Zilver (Cook), and Abre (Medtronic).67
The Zilver Vena stent has a distinctive venous-specific design; it has flexibility, kink resistance, and balanced radial force, giving it conformability in venous anatomy. There is no stent foreshortening beyond 5 mm, and a stent length up to 14 cm is available, suitable for stenting the diseased venous segments with 1 or 2 stents only from CIV to common femoral vein. There would be no need to extend through the IVC. Mohamed-Salem et al report a 5-year experience using the Zilver Vena stent in 58 patients.73 In the follow-up, primary, assisted primary, and secondary patency of 60.3%, 65.5%, and 81% were observed, respectively; they concluded that the Zilver Vena stent is a good choice and is noninferior to the Wallstent with respect to 1-year and 5-year patency and provides good clinical improvement.73
The most recent dedicated venous stent approved by the FDA is the Abre (Medtronic), supported by a multicenter, prospective, nonrandomized, single-arm international study in patients with symptomatic iliofemoral venous outflow obstruction (IFVOO). The Abre venous self-expanding nitinol stent has an open-cell structure tailored to perform in the iliofemoral veins. The primary objective of the study was to evaluate the safety and effectiveness in patients with symptomatic IFVOO. The study included 200 patients from 24 international study centers between the years 2018/2019 and the follow-up at 12 months.
Venous obstruction was classified as acute DVT (16.5%, 33/200), PTS (47.5%, 95/200), or NIVL (36.0%, 72/200). Primary patency at 12 months was 88.0% (162/184). Twelvemonth primary-assisted and secondary patency were 91.8% (169/184) and 92.9% (171/184) respectively. Mean target limb Villalta score decreased from 11.2± 5.6 at baseline to 4.1&plusm;4.8 (88/200) at 12 months. The authors concluded that symptomatic IFVOO can be successfully treated with an Abre venous stent; the study showed a high patency rate with a good safety profile, and patients had a significant reduction in clinical symptoms and improvement in QOL through 12-month follow-up.74
The success or failure of a venous outflow intervention does not end with stent placement; there is no comparative data between venous stenting after angioplasty and stenting for MTS.22 In PTS patients, oral anticoagulation for at least 6 to 12 months or indefinitely in patients with a history of DVT or thrombophilia is indicated.2,22 For nonthrombotic MTS, compression, antiplatelets or anticoagulants or both are used. There is no randomized controlled trial (RCT) comparing these antithrombotic strategies.12,22,75-77. There is no comparative data between direct oral anticoagulants and warfarin in postvenous stenting.22,78
Finally, after successful iliac stenting solving leg venous hypertension, compression stockings are no longer needed.79.
So far, most studies on follow-up QOL after endovascular treatment have relied on the Villalta score—useful but partial in describing some important factors—and the adoption of the VEINES-QOL classification (VEnous INsufficiency Epidemiological and economic Study on Quality of Life), which is the most validated venous disease–specific measurement scale nowadays. Several authors have reported their results and generally agree that QOL improved significantly after the endovascular treatment either in thrombotic or in NIVL, and this improvement is sustained for years.80-84
Conclusion
MTS is underdiagnosed; hence, when evaluating patients with left leg DVT, it should be considered. Early diagnosis and adequate treatment are paramount; so, clinical presentation and imaging findings can help. Venography and IVUS are now the gold standard for precise diagnosis and treatment. The minimal invasive endovascular treatment is now first choice: in thrombotic iliac vein occlusion, pharmacomechanic thrombectomy, angioplasty, and stenting will minimize morbidity of PTS; in NIVL, stenting has showed better patency rates on long-term follow-up. The venous stents in use now are near the ideal of what a prosthesis must be; and very-longterm results are in progress. Overall, MTS and its variants will be a subject of continuous research in coming years, so, we will see whether the MTS eponym is still used as time goes by.

REFERENCES
1. Virchow R. Ueber die erweiterung kleinerer geffasse. Arch Pathol Anat. 1851;3:427- 462.
2. Mc Murrich JP. The occurrence of congenital adhesions in the common iliac veins, and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci. 1908;135:342-345.
3. Ehrich WE, Krumbhaar EB. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J. 1943;26:737-750.
4. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427.
5. Armenta-Flores R, Armenta-Villalobos D, Ramirez E. May-Turner syndrome: sixty years later. Phlebology. 2022;37(1):5-13.
6. Cockett FB and Thomas ML. The iliac compression syndrome. Br J Surg. 1965;52:816-821.
7. Cockett FB, Thomas ML, Negus D. Iliac vein compression—its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J. 1967;2:14-19.
8. Harbin MM, Lutsey PL. May-Thurner Syndrome—history of understanding and need for defining population prevalence. J Thrombosis Haemost. 2020;18(3):534- 542.
9. Berry A. Sonography’s role in the diagnosis of May-Thurner Syndrome. J Diagnostic Medical Sonography. 2018;34:65-70.
10. Fraser DG, Moody AR, Martel A, Morgan PC. Re-evaluation of iliac compression syndrome using magnetic resonance imaging in patients with acute deep vein thromboses. J Vasc Surg. 2004;40:604- 611.
11. Urbas D, Brener E. May-Thurner syndrome—the pelvic spur in modern times. Phlebologie. 2021;50:184-95.
12. Mousa AY. May-Thurner syndrome. Up to date. Accessed June 16, 2022. http:// www.uptodate.org
13. Mangla A, Hamad H. May-Thurner syndrome. StatPearls. Accessed June 17, 2022. http://www.statpearls.org
14. Brazeau NF, Harvey HB, Pintoec, et al. May-Thurner syndrome diagnosis and management. Vasa. 2013;42:96-105.
15. Toonder I. The myth of May-Thurner. Phlebologie. 2020;49:230-232.
16. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937-943.
17. Meissner MK, Khilnani NM, Labropoulus N, et al. The symptoms–varicespathophysiology classification of pelvic venous disorders: a report of the American Vein and Lymphatic Society International working Group on Pelvic Venous Disorders. J Vasc Surg Venous Lymphat Disord. 2020;9(3):568-584.
18. Birn J, Vedamtham S. May-Thurner Syndrome and other obstructive iliac vein lesions. Vasc Med. 2015;20:74-83.
19. Neglen P, Thrasher TL, Raju S. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38:879-885.
20. Knuttinen MG, Naidu S, Oklu R, et al. May-Thurner syndrome diagnosis and endovascular management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S159-S164.
21. Poyyamoli S, Mehta P, Cherian M. May- Thurner syndrome. Cardiovasc Diagn Ther. 2021;11(5):1104-1111.
22. Radaideh Q, Patel NM, Shammas NW. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag. 2019;15:115-122.
23. Burke RM, Rayan SS, Kasirajan K, et al. Unusual case of right-sided May-Thurner syndrome and review of its management. Vascular. 2006;14:47-50.
24. Abboud G, Midulla M, Lions C, et al. “Right-sided” May-Thurner syndrome. Cardiovasc Intervent Radiol. 2010;33:1056-1059.
25. Hassell DR, Reifsteck JE, Harshfield DL, et al. Unilateral left leg edema: a variation of the May-Thurner syndrome. Cardiovasc Intervent Radiol. 1987;10:89-91.
26. Steinberg JB, Jacocks MA. May-Thurner syndrome. A previously unreported variant. Ann Vasc Surg. 1993;7:577-581.
27. Fretz V, Binkert CA. Compression of the inferior vena cava by the right iliac artery: a rare variant of May-Thurner syndrome. Cardiovasc Intervent Radiol. 2010;33:1060-1063.
28. Moudgill N, Hager E, Gonsalves C, et al. May-Thurner syndrome: case report and review of the literature involving modern endovascular therapy. Vascular. 2009;17:330-335.
29. Hosn MA, Katragunta N, Kresowik T, et al. May-Thurner syndrome presenting as spontaneous left iliac vein rupture. J Vasc Surg Venous Lymphat Disord. 2016;4:479- 481.
30. Hermany PL, Badheka AO, Mena-Hurtado CI, et al. A unique case of May-Thurner syndrome: extrinsic compression of the common iliac vein after iliac artery stenting. ACC Cardiovasc Interv. 2016;9:e39-e41.
31. Pandit AS, Hayes M, Guiney-Borgelt S, et al. Iatrogenic May-Thurner syndrome after EVAR. Ann Vasc Surg. 2014;28:739.e17- 739.e20.
32. Hung JB, Hsu CW, Tsai SH. Prostatism and May-Thurner syndrome. Am J Emerg Med. 2013;31:445.e1-445.e2.
33. Kiernan TJ, Yan BP, Cubeddu RJ, et al. May-Thurner syndrome in patients with cryptogenic stroke and patent foramen ovale:an important clinical association. Stroke. 2009;40:1502-1504.
34. Greer DM, Buonanno FS. Cerebral infarction in conjunction with patent foramen ovale and May-Thurner syndrome. J Neuroimaging. 2001;11:432-434.
35. Gavrilov SG, Vasilyev AV, Krasavin GV et al. Endovascular interventions in the treatment of pelvic compression syndrome caused by May-Thurner Syndrome. J Vasc Surg Venous Lymphat Disord. 2020;8(6):1049-1057.
36. Kim JY, Choi D, Ko YG, et al. Treatment of May-Thurner syndrome with catheterguided local thrombolysis and stent insertion. Korean Circ J. 2004;34:655-659.
37. Brinegar KN, Sheth RA, Khademhosseini A, et al. Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J Radiol. 2015;7:375-381.
38. Labropoulos N, Borge M, Pierce K, et al. Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg. 2007;46:101-107.
39. Hurst DR, Forauer AR, Bloom JR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106-113.
40. Zucker EJ, Gnguli S, Ghoshhajra BB, et al. Imaging of venous compression syndromes. Therapy. 2016;6:519.
41. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118-126.
42. Mousa AY, Broce M, Yacoub M, et al. Validation of venous duplex ultrasound imaging in determining iliac vein stenosis after standard treatment of active chronic venous ulcers. J Vasc Surg Venous Lymphat Disord. 2016;4:307-312.
43. Zamboni P, Franceschi C, Del Frate R. The over-treatment of illusory May Thurner syndrome. Veins Lymphatics. 2019;8:8020.
44. Lattimer CR, Mendoza E, Kalodiki E. The current status of air-plethysmography in evaluating non-thrombotic iliac vein lesions. Phlebology. 2018;33:3-4.
45. Villalba LM, Graydler V, Sherman P, et al. Our protocol for transabdominal pelvic vein duplex ultrasound. Endovascular Today. 2018;17:54-58.
46. Souto-Barros F, Salles-Cunha SX, Herman- Roelke L, et al. Arterial compression of left iliac veins: five-year patency rates of endovascular treatment. J Vasc Ultrasound. 2018;42(1):11-17.
47. Villalba l, Larkin T. Transabdominal duplex ultrasound and intravascular ultrasound planimetry measures of common iliac veins stenosis are significantly correlated in a symptomatic population. J Vasc Surg Venous Lymphat Disord. 2021;9:1273- 1281.
48. Chung JW, Yoon CJ, Jung SI, et al. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004;15:249-256.
49. Oguzkurt L, Tercan F, Pourbagher MA, et al. Computed tomography findings in 10 cases of iliac vein compression (May-Thurner) syndrome. Eur J Radiol. 2005;55:421-425.
50. Jeon UB, Chung JW, Jae HJ, et al. May- Thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long-term stent patency and changes in the iliac vein. AJR Am J Roentgenol. 2010;195:751-757.
51. Wu WL, Tzeng WS, Wu RH, et al. Comprehensive multidetector computed tomography evaluation of patients with suspected May-Thurner syndrome. AJR Am J Roentgenol. 2012;199:W638-W645.
52. Butros JR, Liv R, Oliveira GR, et al. Venous compression syndromes: clinical features, imaging findings and management. Br J Radiol. 2013;86:20130284.
53. Rossi FH, Gama CA, Fonseca IY, et al. CT venography diagnosis of iliocaval venous obstruction in advance chronic venous insufficiency. J Vasc Bras. 2014;13:306-311.
54. Lamba R, Tanner DT, Sekhon S, et al. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. 2014;34:2019.
55. Shammas NW, Shammas GA, Jones-Miller S, et al. Predicting iliac vein compression with computed tomography angiography and venography: correlation with intravascular ultrasound. J Invasive Cardiol. 2018;30:452.
56. van Vuuren TMJ, Kurstjens RLM, Wittens CHA, et al. Illusory angiographic signs of significant iliac vein compression in healthy volunteers. Eur J Vasc Endovasc Surg. 2018;56(6):874-879.
57. J ayaraj A, Raju S. Three-dimensional computed tomography venogram enables accurate diagnosis and treatment of patients presenting with symptomatic chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9(1):73-80.e1.
58. Raju S, Walker W, Noel C, et al. The twosegment caliber method of diagnosing iliac vein stenosis on routine computed tomography with contrast enhancement. J Vasc Surg Venous Lymphat Disord. 2020;8:970-977.
59. Wolpert LM, Rahmani O, Stein B, et al. Magnetic resonance venography in the diagnosis and management of May- Thurner syndrome. Vasc Endovascular Surg. 2002;36:51-57.
60. Hsu YC. Using non-contrast-enhance MRV for the evaluation of May-Thurner syndrome in patients with renal insufficiency. Medicine (Baltimore). 2019,98:e18427.
61. Shen S, Shan C, Lan Y. Combined highresolution 3D-CUBE T1-weighted imaging and non-contrast-enhance magnetic resonance venography for evaluation of vein stenosis in May-Thurner syndrome. Phlebology. 2022;37(1):14-20.
62. White JM, Comerota AJ. Venous compression syndromes. Vasc Endovascular Surg. 2017;51:155-168.
63. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein trombosis by realtime B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
64. Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosis and treating iliofemoral vein obstruction. J Vasc Surg Venous lymphat Disord. 2017;5:678-687.
65. Raju S, Neglen PJ. Prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44:136- 144.
66. Neglen PJ, Raju S. Ballon dilatation an stenting of chronic venous outflow obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2007;7:79-91.
67. Gibson K. Iliac vein stenting: best practices for patient safety and successful outcome. Endovascular Today. Published July 2021. Accessed June 29, 2022. https://www. evtoday.com
68. Neglen P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979-990.e1.
69. Gloviczki P, Lawrence PF. Iliac vein stenting and contralateral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2017;5:5-6.
70. Raju S. Ten lessons learned in iliac venous stenting. Endovascular Today. Published July 2016. Accessed June 29, 2022. https://evtoday.com/articles/2016- july/ ten-lessons-learned-in-iliac-venous-stenting
71. Jayaraj A, Chandler N, Kuykendall R, et al. Long-term outcomes following use of a composite Wallstent-Z stent approach to iliofemoral venous stenting. J Vasc Surg Venous Lymph Disord. 2021;9:393-400.
72. Murphy EH. Surveying the 2019 venous stent landscape. Published 2019. Accessed June 30, 2022. https://evtoday.com
73. Salem ARM, AboElNeel HA, Fakhr ME. Long-term outcome of dedicated venous stents in management of chronic iliofemora obstruction. J Vasc Surg Venous Lymphat Disord. 2022;10:52-59.
74. Murphy E, Gibson K, Sapoval M, et al. Pivotal study evaluating the safety and effectiveness of the Abre venous self- expanding stent system in patients with symptomatic iliofemoral venous outflow obstruction. Circ Cardiovasc Interv. 2022;15:192-204.
75. Milinis K, Thapar A, Shalhoub J, et al. Antithrombotic therapy following venous stenting: international Delphi consensus. Eur J Vasc Endovasc Surg. 2018;55:537- 544.
76. Endo M, Jahangiri Y, Horikawa M, et al. Antiplatelet therapy is associated with stent patency after iliocaval venous stenting. Cardiovasc Intervent Radiol. 2018;41:1691- 1698.
77. Padrnos LJ, Garcia D. May-Thurner syndrome and thrombosis: a systematic review of antithrombotic use after endovascular stent placement. Res Pract Thromb Haemost. 2019;3:70.
78. Chopard R, albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA. 2020;324(17):1765-1776.
79. Raju S, Lurie F, O’Donnell T Jr. Compression use in the era of venous intervention and wound care centers. J Vasc Surg Venous Lymphat Disord. 2016;4:346-354.
80. Wik HS, Enden TR, Ghanima W, Engeseth M, Kahn SR, Sandset PM. Diagnostic scales for the post-thrombotic syndrome. Thromb Res. 2018;164:110-115.
81. Catarinella FS, Nieman FHM, Wittens CHA. An overview of the most commonly used venous quality of life and clinical outcome measurements. J Vasc Surg Venous Lymphat Disord. 2015;3:333-340.
82. Abenhaim L, Kurz X. The VEINES study (Venous Insufficiency Epidemiologic and Economic Study): an international cohort study on chronic venous disorders of the leg. VEINES Group. Angiology. 1997;48:59-66.
83. Kahn SR, Lamping DL, Ducruet T, et al. VEINES-QoL/Sym questionnaire was a reliable and valid diseasespecific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049-1056.
84. Morris RI, Pouncey Al, Quintana B, et al. Quality of life outcomes for patients undergoing venous stenting for chronic deep venous disease. J Vasc Sur Venous Lymphat Disord. 2021;9:1185-1192.
