Micronized purified flavonoid fraction in adjunction to rivaroxaban improves outcomes of popliteal-femoral deep-vein thrombosis at 12-month follow-up
Ilya SCHASTLIVTSEV,1,2 MD, PhD;
Victor BARINOV,2 MD, PhD
1Pirogov Russian National Research
Medical University, Moscow, Russian
Federation;
2Clinical Hospital no.1 of the President’s
Administration of the Russian Federation
Abstract
Aim: To assess the efficacy of the long-term use of micronized purified flavonoid fraction (MPFF) in the treatment of popliteal-femoral deep-vein thrombosis (DVT). Methods: In this pilot, comparative, open-label clinical study, patients with the first episode of popliteal-femoral DVT confirmed by duplex ultrasound scan (DUS) were allocated to two groups: the control group received standard treatment with rivaroxaban for 6 months and compression stockings for 12 months, and the MPFF group received adjunctive MPFF 1000 mg/day for 12 months. During the 12-month follow-up, the degree of recanalization was assessed bi-monthly by the DUS and Marder score. Finally, patients were evaluated for post-thrombotic syndrome (PTS) via the Villalta score (score of ≥5 defined PTS). Results: Sixty patients (40 males and 20 females; mean age 56.3±13.4 years) were allocated to the MPFF or control group (n=30 in each group) and followed-up for 12 months. The median Villalta score was significantly lower in the MPFF group than in the control group (1.9±2.0 vs 5.2±2.6; P<0.001) with a smaller number of verified PTS (10% vs 53%; P=0.001). In the MPFF group, a greater reduction in the Marder score and a faster recanalization of the popliteal and the femoral veins were observed. Conclusion: The results of this pilot study suggest that long-term use of MPFF is associated with a lower incidence of PTS at 12 months and a faster recanalization of the deep veins in patients with popliteal-femoral DVT treated with rivaroxaban. These findings should be confirmed in more powerful randomized clinical trials.
Introduction
Deep-vein thrombosis (DVT) along with superficial vein thrombosis and pulmonary embolism (PE) constitute the group termed venous thromboembolism (VTE), which remains a significant medical and social problem.1,2 Standard treatment of DVT consists of using parenteral and/or oral anticoagulants for at least 3 months, with a possible prolongation of therapy for an indefinitely long period.3,4 The treatment is aimed at preventing the progression of thrombotic disease, reducing the risk of PE and fatal outcome. The introduction of direct oral anticoagulants (DOAC) has changed the paradigm of VTE treatment, making this process safer and more convenient for both the doctor and the patient. Treatment with DOACs compared with conventional therapy by low molecular- weight heparin (LMWH) switched to vitamin K antagonists (VKA) was found to be not less effective, but safer.5
After eliminating the threat to life, the risk of developing long-term complications, in particular post-thrombotic syndrome (PTS), which significantly affects the quality of life and work capacity, comes to the fore. The prevalence of PTS in 10 to 15 years after the first thrombotic episode accounts for 19% to 42% with skin ulceration in 3% to 4% of patients.6-9 The essential risk factors for PTS are as follows: proximal localization of the thrombus; ipsilateral DVT recurrence; preexisting chronic venous disease (CVD); insufficient vein recanalization and preservation of residual venous obstruction (RVO); elderly age; inadequate anticoagulant therapy; and intensive and prolonged inflammation in the thrombus and the adjacent venous wall.10
Inflammation is a crucial component of the initiation and propagation of the thrombotic process, along with other components including the venous wall and valve damage factor.11-14 In parallel, the inflammatory response is vital for the release of the vessel lumen from thrombotic masses. Therefore, a significant suppression of its intensity may affect the recanalization.15-17 Poor recanalization with the preservation of RVO increases the risk for PTS 1.6- to 2.1-fold.18-22 We hypothesized that the pharmacological modulation of the inflammatory response could protect the venous wall from the excessive injury while retaining the fundamental role of immune cells in the recanalization process. Several drugs have potential properties for such modulation, and flavonoids are at the top of the list.
Anti-inflammatory and venoprotective actions of flavonoids are well studied, and these agents are widely used to relieve symptoms and signs of CVD.23,24 Among all flavonoids, the micronized purified flavonoid fraction (MPFF) is the most established agent.25 Previous experimental studies have shown endothelial protective properties in the settings of reperfusion injury and venous hypertension,26-29 as well as suppression of the inflammatory response in patients with CVD,30 including those after sclerotherapy.31 Long-term intake of MPFF up to 12 months is associated with a low incidence of AEs, most of which are mild and do not affect health status.23,24,32 Thus, the MPFF shows a favorable risk benefit profile for long-term use as an adjunctive treatment for DVT.
This pilot study aimed to assess the efficacy of the long-term use of MPFF in addition to rivaroxaban for the treatment of popliteal-femoral DVT.
Methods
This study was a pilot, single-center, open-label, comparative clinical trial with a blinded assessment of efficacy outcomes. The detailed design and findings at the 6-month follow-up have been published previously.33 Here, we present findings from the 12-month extended observation. The study protocol was approved by an Institutional Review Board of Clinical Hospital no.1 of the President’s Administration of the Russian Federation, and all patients provided signed informed consent for participation. The protocol was not registered in any open registry of clinical trials because of its pilot nature, absence of funding, and local Institutional policy. The pilot nature of the study was dictated by the lack of any experimental or clinical data on the influence of MPFF on the course of DVT, as well as the inability to perform a sample size calculation.
The study enrolled patients at the age of >18 years with the first episode of provoked or unprovoked popliteal-femoral DVT, as confirmed by DUS, who signed informed consent. The exclusion criteria have been reported previously.
The study was conducted at Clinical Hospital no.1 of the President’s Administration of the Russian Federation in 2017-2018. The pretest clinical probability by two-level Wells score34 and D-dimer was utilized according to the Institutional protocol in all patients admitted to the emergency department with suspected DVT. Treatment with LMWH, followed by DUS, was initiated in subjects with a high clinical probability of DVT or low clinical probability in combination with positive D-dimer. As soon as DVT was confirmed by DUS, patients were assessed for eligibility and enrolled in the study after signing informed consent. The allocation to the experimental (MPFF) or control group was based on the number on the patient’s medical record form. The general sequence of the diagnosis and treatment for DVT and enrollment in the study are represented in Figure 1.
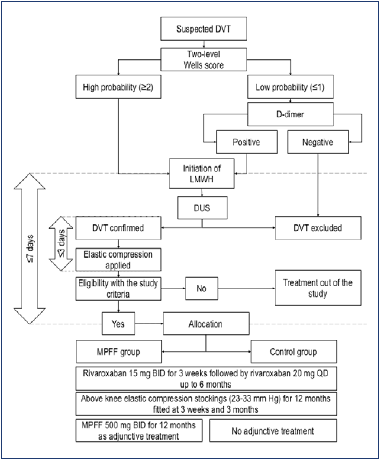
Figure 1. The general sequence of the diagnosis and treatment for deep-vein thrombosis and enrollment in the study. Abbreviations: BID, twice a day; DVT, deep-vein thrombosis; DUS, duplex ultrasound; LMWH, low-molecular-weight heparin; MPFF, micronized purified flavonoid fraction; QD, once a day.
Patients were allowed to receive therapeutic doses of LMWH (enoxaparin 1 mg/kg twice daily) during the period from hospital admission to the allocation, but not more than 7 days. After the assignment, they switched to rivaroxaban 15 mg twice daily for up to 3 weeks, followed by 20 mg once daily for up to 6 months. Within the first 3 days after DVT confirmation, patients of both groups applied above-knee elastic compression stockings with a pressure of 23 to 32 mm Hg and were recommended to use it for 12 months. Fitting for size was obligatory at 3 weeks and 3 months after the index DVT. In the MPFF group, patients received MPFF 500 mg twice daily for 12 months in adjunction to standard treatment. The first dose of MPFF was administered immediately after treatment allocation and in parallel with rivaroxaban.
Patients were followed-up for 12 months with bi-monthly clinical and ultrasound examinations. At baseline, the standard clinical data were evaluated, and the affected limb was assessed with CEAP classification (clinical, etiological, anatomical, pathophysiological classification; 2004 version)35 for preexisting CVD by the methodology described previously.36 At 6 and 12 months, the CEAP clinical class was reassessed in parallel with the evaluation of the Villalta score, venous clinical severity score (VCSS), and CIVIQ-20 score (20-item ChronIc Venous dIsease quality-of-life Questionnaire). The final decision on the presence of PTS and the severity of CVD was made by an independent expert blinded to the patient’s allocation to MPFF or control group.
A DUS was performed by use of the MyLab30 (Esaote, Italy) machine with a linear ultrasound transducer LA532 in the frequency range of 5 to 13 MHz. The common femoral vein (CFV) and femoral vein (FV) were assessed in the supine position, popliteal vein (PV) in the prone position, and calf veins in a sitting position. The whole-leg scan was performed at any time.
The criteria for DVT were incompressibility of the vein during compression by the ultrasound probe and the absence of blood flow on the color mapping mode with provocation maneuvers. A modified Marder score was used to assess the extension of the thrombotic lesion as described previously.33,37 Briefly, occlusion of different venous segments was scored from 1 (calf veins) to 8 (iliac veins) with the maximal score of 34 for each limb. A higher score corresponds to a higher extension of the thrombotic lesion. The recanalization degree (RD; the inverse criteria of RVO) at the narrowest point of PV, FV, and CFV was calculated as previously described (Figure 2).33,37 All ultrasound studies were performed by a specialist blinded to the patient’s allocation to the MPFF or control group. The DUS could be performed in an unscheduled and urgent manner if any clinical suspicion for VTE recurrence arose.
The primary outcome of the extended study was the detection of PTS at 12 months. The PTS was diagnosed with the Villalta score by the blinded expert. The score of 0-4 defined the absence of disease; 5-9, mild disease; 10-14, moderate disease; and score of ≥15 or the presence of venous ulcer, severe disease.
The secondary efficacy outcomes were as follows: diagnosis of PTS at 6 months (the primary outcome of the previous report); diagnosis of severe PTS at 6 and 12 months; severity of PTS by the Villalta score at 6 and 12 months; symptomatic or asymptomatic DVT recurrence and symptomatic PE within the follow-up period; progression of CVD at 6 and 12 months by CEAP clinical class; severity of CVD by VCSS score at 6 and 12 months; quality of life by CIVIQ-20 questionnaire at 6 and 12 months; complete recanalization of the PV, FV, and CFV at 12 months; change in recanalization degree at the PV, FV, and CFV within the follow-up period; and change in thrombus burden by the Marder score within the follow-up period.
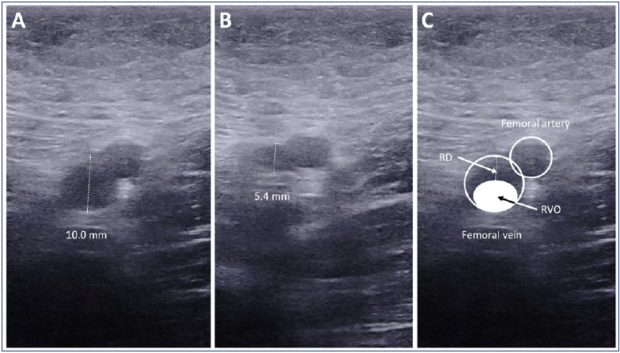
Figure 2. An example of the assessment for recanalization degree on the femoral vein.A) The diameter of the vein without compression (10.0 mm). B) The diameter of the vein under maximal compression at the narrowest point (5.4 mm). C) The relationship between RVO and RD. Residual venous obstruction (RVO) = d(under compression) / d(without compression) x 100% = 5.4/10.0 x 100% = 54%. Recanalization degree (RD) = [d(without compression) – d(under compression)] / d(without compression) x 100% = (10.0-5.4)/10.0 x 100% = 46%. RD = 100% – RVO = 100% – 54% = 46%.
The symptomatic DVT recurrence was defined as an increase in edema, pain, or skin hyperemia of the affected limb, or the occurrence of the same signs in the intact leg. It had to be confirmed by the scheduled or unscheduled DUS. The asymptomatic DVT recurrence was defined as the occurrence of total occlusion in a previously recanalized venous segment or appearance of the new occlusion in the primary intact vein as detected by scheduled DUS. PE could be suspected in the presence of typical clinical signs (shortness of breath, chest pain, cough, increased heart rate, and decreased arterial blood pressure) and confirmed by appropriate imaging tests.4 The progression of CVD was defined as a transition from a lower CEAP clinical class to a higher class, eg, C0 to C1 to C2 to C4. Complete vein recanalization was suggested as clearance of thrombotic masses by 80% or more with RVO <20% or RD ≥80%.
The safety outcomes were represented by major or clinically relevant nonmajor (CRNM) bleeding, as defined by the International Society of Thrombosis and Hemostasis (ISTH),36,38 or minor bleeding (any other hemorrhage not fulfilling the criteria of major or CRNM bleeding), and any other adverse event (AE), including serious adverse event (SAE). All safety outcomes were assessed by the three authors and two independent experts in vascular surgery and cardiology for the casual relationship with studied drugs. Bleeding events were considered as expected AEs related to rivaroxaban if they occurred within the period of anticoagulation treatment and 3 days after cessation and were analyzed as prespecified safety outcomes.
Specific measuring for compliance with MPFF, rivaroxaban, or compression stockings was not prespecified. Patients were asked to report any preliminary cessation of studied drugs or elastic compression. The subjects with expected low adherence were not included according to the study design.
As this was a pilot study, the minimal sample size was not calculated. All absolute values are presented as the mean with the standard deviation (SD) and relative values, presented as percent with a 95% confidence interval (CI) as calculated by Wilson. The comparisons were performed using the t-test for continuous variables or the two-sided Fisher’s exact test and a chi-square test for categorical variables. A comparison of mean values with time was performed by assessment of within- and between-subject effects, as well as their within-subject interaction via the general linear model for repeated measurements (GLMRM), a kind of dispersion analysis (ANOVA). Time to event was represented by survival curves and compared via the Kaplan-Meier test. Statistical analysis was carried out using the IBM SPSS Statistics v.26 software package. The relative risk and its 95% CI were calculated with a free online calculator by MedCalc (https://www.medcalc.org). Differences were considered statistically significant if the P-value was less than 0.05.
Results
During the enrollment period, 132 patients with suspected DVT were admitted to the hospital, and the diagnosis was confirmed in 104 cases. Of these, 68 patients fulfilled the criteria of eligibility, and eight patients refused to participate. The remaining 60 patients were allocated to one of the two treatment groups (n=30 in each group), and all of them completed the 12-months follow-up (Figure 3).
The clinical characteristics of the enrolled patients are presented in Table I. Participants in the control group receiving a standard treatment had a higher prevalence of CVD prior to the DVT, in particular, the CEAP clinical classes of C2 to C4 (73% vs 50%; P=0.110). The prevalence of thrombotic occlusion in popliteal, femoral, and CFVs was comparable in both groups. However, the total thrombus extension by the Marder score was higher in the MPFF group due to the higher involvement of the calf veins. The other characteristics were comparable among participants in both groups.
The results for the primary and secondary outcomes are summarized in Table II. At 12 months, PTS was reported in 3 of 30 (10%; 95% CI, 3.5%-25.6%) patients who received adjunctive MPFF in comparison to 16 of 30 (53%; 95% CI, 35.8%-69.5%) who were treated only with rivaroxaban and compression stockings. Interestingly, when compared with the results obtained at 6 months, the number of established PTS at 12 months decreased from 20% to 10% (minus three patients) in the MPFF group and from 57% to 53% (minus one patient) in the control group. At the final assessment, most of the cases were classified as mild PTS. Only two patients in the control group had moderate disease, and none developed severe disease. The Villalta score was significantly lower in the MPFF group than in the control group (1.9±2.0 vs 5.2±2.6; P<0.001). These figures decreased during extended observation from 2.9±2.7 to 1.9±2.0 in the MPFF group and from 5.8±3.0 to 5.2±2.6 in the control group (P<0.001). Thereby, the addition of MPFF to the standard treatment of popliteal-femoral DVT significantly reduced the risk of PTS by 81% at 12 months.
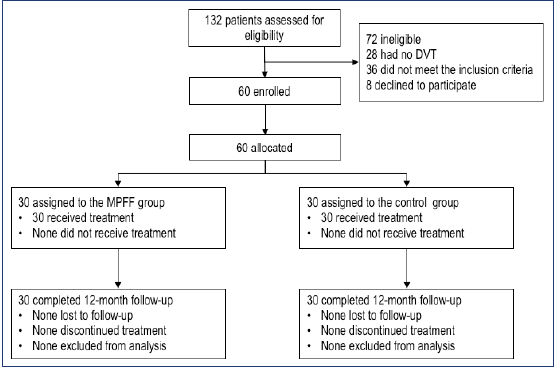
Figure 3. Trial profile (CONSORT flow diagram). Abbreviation: DVT, deep-vein thrombosis; MPFF, purified flavonoid fraction.
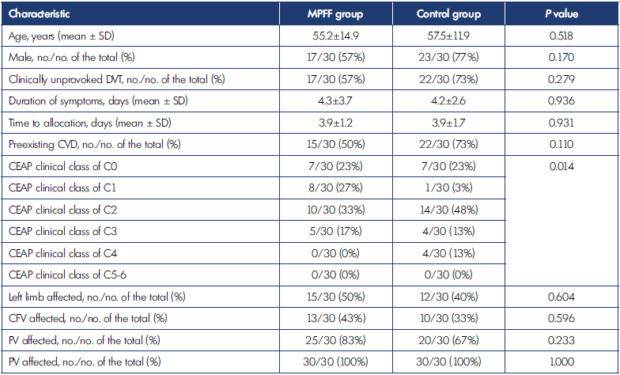
CEAP, clinical, etiological, anatomical, pathophysiological classification; CFV, common femoral vein; CVD, chronic venous disease; DVT, deep-vein thrombosis; MPFF, micronized purified flavonoid fraction; FV, femoral vein; PV, popliteal vein; SD, standard deviation. Table I. The clinical characteristics of patients enrolled in the study.
The progression of CVD was observed in 1 of 30 (3%; 95% CI, 0.5%-16.2%) patients who received MPFF in comparison with 7 of 30 (23%; 95% CI, 11.6%-40.6%) patients who did not. In the MPFF group, the only subject who had preexisting untreated varicose veins (clinical class of C2) developed a disease progression to hyperpigmentation (clinical class of C4). In contrast, in the control group, two subjects with C0 progressed to C1 and C3, respectively, and five subjects with C2 progressed to C3 (n=4) or C4 (n=1). In total, CVD progression showed a significant association with the development of PTS. Only 11 of 52 (21%) patients without CVD progression were diagnosed with PTS in comparison to 8 of 8 (100%) subjects with CVD progression (P<0.001).
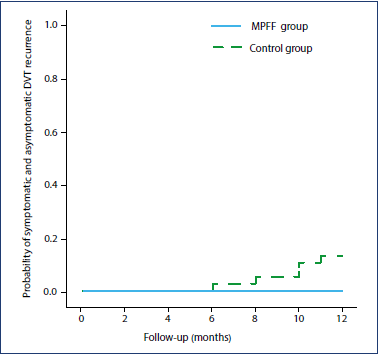
Figure 4. Probability of symptomatic and asymptomatic deepvein thrombosis recurrence. Kaplan-Meier statistics and logrank test (P=0.021). Abbreviations: DVT, deep-vein thrombosis; MPFF, micronized purified flavonoid fraction.
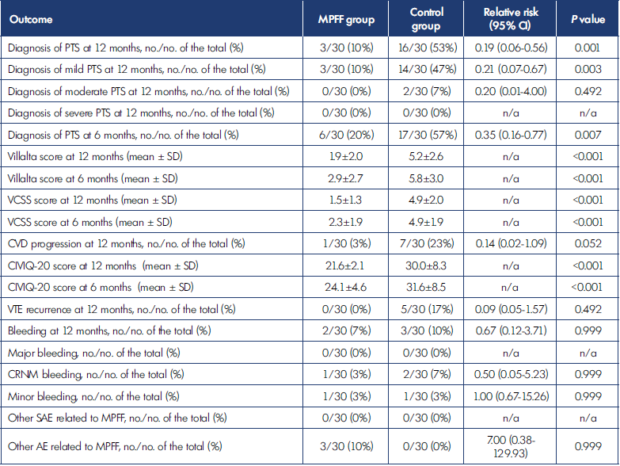
AE, adverse event; CI, confidence interval; CIVIQ-20, 20-item ChronIc Venous dIsease quality-of-life Questionnaire; CRNM, clinically relevant non-major bleeding; CVD, chronic venous disease; MPFF, micronized purified flavonoid fraction; n/a, nonavailable; PTS, post-thrombotic syndrome; SAE, serious adverse event; SD, standard deviation; VTE, venous thromboembolic event Table II. Primary and secondary outcomes in the MPFF and control groups.
The severity of CVD assessed by the VCSS score was significantly lower in patients who received MPFF: 1.5±1.3 vs 4.9±2.0 (P<0.001). A similar tendency in score reduction from 6 to 12 months was observed only in the MPFF group (P<0.001). The CIVIQ-20 score was significantly lower in the MPFF group, corresponding with better quality of life: 21.6±2.1 vs 30.0±8.3 (P<0.001). A further reduction in the score in both groups after 6 months was observed as well (P<0.001). No episode of symptomatic PE was detected. The recurrence of DVT was found in none of the patients in the MPFF group (0%; 95% CI, 0.0%-11.4%) compared with 5 (17%, 95% CI, 7.6%-33.9%) patients in the control group. The time-to event is represented in Figure 4. Four of five recurrences were observed after cessation of anticoagulation at 8 to 11 months. Only two episodes were symptomatic, and scheduled DUS revealed the other three. The contralateral DVT represented three cases; the re-occlusion of the previously recanalized vein, the only case; and the new occlusion of the previously unaffected ipsilateral vein, the last case. In four patients, anticoagulation treatment was reinitiated if it had been previously stopped. The only patient who developed recurrence within the period of oral anticoagulation was switched to LMWH. All these patients were analyzed for clinical outcomes with the exclusion of the new ipsilateral venous lesions from the analysis of ultrasound endpoints. The appearance of recurrent DVT significantly affected the risk of PTS development. Four of five (80%) subjects with recurrent thrombosis developed PTS in comparison with only 15 of 55 (27%) patients free of recurrence (P=0.031).
No new safety outcome besides that previously published was reported beyond 6 months of observation. One CRNM and one minor rectal bleeding incident were detected in the MPFF group. Two CRNM macrohematuria and one minor epistaxis were observed in the control group. No SAE related to MPFF was identified. Three patients in the MPFF group reported a mild dyspeptic disorder, which appeared within the first month of therapy, did not require treatment discontinuation, and was relieved by changing the time of MPFF intake with the consumption of food. All these events were classified as AE certainly related to MPFF.
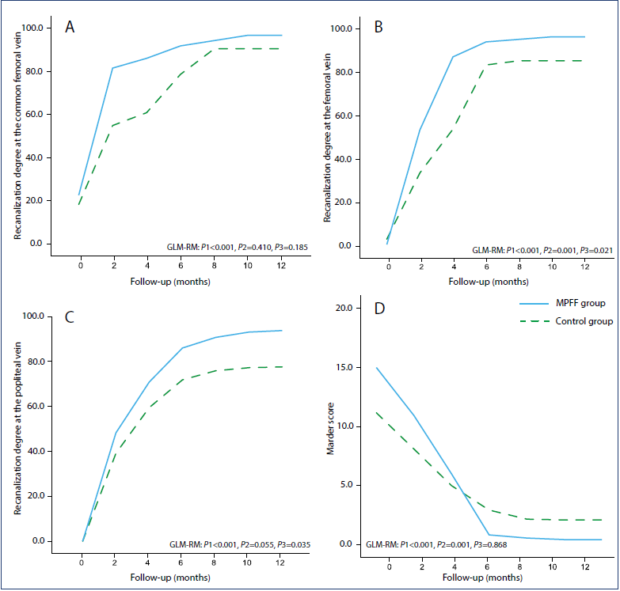
Figure 5. The recanalization of the main venous segments and thrombotic burden. Changes in recanalization degree at the (A)
common femoral vein, (B) femoral vein, and (C) popliteal vein; (D) dynamics of the Marder score. GLM-RP (generalized linear
model repeated measures): P1, within-subject effect “time” (P<0.05 interpreted as significant changes over time in both groups);
P2, within-subject interaction “time x group” (P<0.05 interpreted as a significant difference in the slope of curves related to faster
recanalization); P3, between-subject effect “group” (P<0.05 interpreted as a significant deviation of the curves related to complete
recanalization).
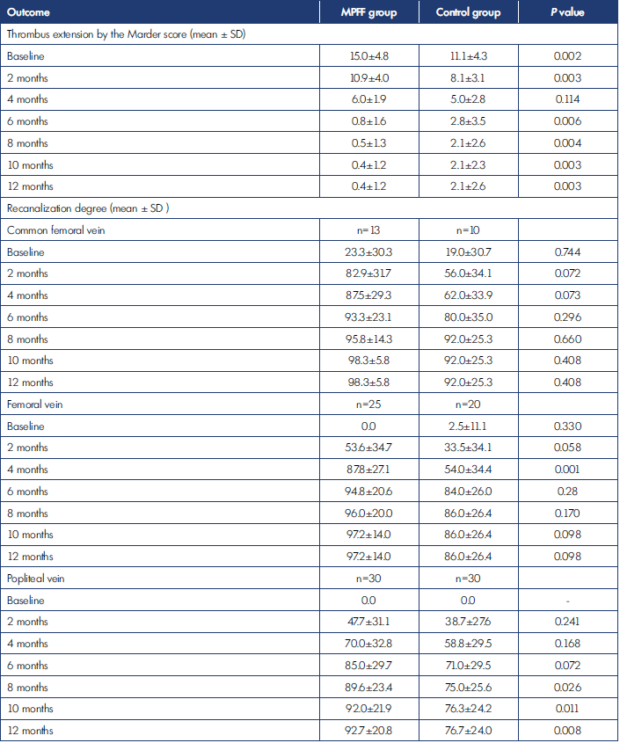
MPFF, micronized purified flavonoid fraction; SD, standard deviation.
Table III. Ultrasound outcomes in the experimental and control groups.
The ultrasound outcomes are represented in Table III and Figure 5. A significant trend toward the progressive clearance of thrombotic masses from the vessel lumen was observed on PV, FV, and CFV in both groups. No difference in the intensity of recanalization was found on the CFV. Complete recanalization was seen on all of 13 (100%) veins in the MPFF group and on 9 of 10 (90%) veins in the control group (P=0.434). In contrast, the speed of recanalization on FV and PV was higher in the MPFF group. At the end of the observation period, complete recanalization on FV was identified in 24 of 25 (95%) patients in the MPFF group compared with 15 of 20 (75%) patients in the control group (P=0.074). As for the PV, the adjunctive use of MPFF led to an increase in complete recanalization from 60% to 90% (P=0.015).
The reduction in thrombotic burden as assessed by the Marder score was more intensive with the adjunctive use of MPFF (Figure 5). At baseline, the thrombus extension in the MPFF group was significantly higher than in the control group (15.0±4.8 vs 11.1±4.3, respectively; P=0.002), but after 12 months of therapy, it was significantly lower (0.4±1.2 vs 2.1±2.6, respectively; P=0.003).
The specific measure for compliance with MPFF, rivaroxaban, and elastic compression stockings was not prespecified. However, none of the enrolled patients reported preliminary cessation of study drugs or elastic compression.
Discussion
Here, we present the findings of extended follow-up on 60 patients who were treated for popliteal-femoral DVT. The results obtained at 6 months have already shown a significant decrease in PTS by long-term use of MPFF.33 Surprisingly, the cumulative incidence of PTS did not increase during the extended follow-up. In contrast, three patients received MPFF plus compression stockings, and one patient who used compression stockings alone dropped out of the criteria for PTS. That shows the high efficacy of conservative therapy to control the symptoms and signs of CVD.
Preexisting CVD is a well-established predisposing factor that increases the risk of PTS 1.5- to 3.2-fold.10 This fact may be related to misdiagnosis of PTS by the Villalta score, for example, when preexisting CVD is considered a new PTS even without worsening of symptoms and signs,39 or to true disease progression. In the current study, we encountered a higher prevalence of preexisting CVD in the control patients. This fact could affect the results, providing a higher incidence of PTS in the control group. However, preexisting CVD did not increase the risk of PTS in this study: 35% of patients with CVD compared with 27% of patients without CVD were diagnosed with PTS (P=0.581). In contrast, CVD progression was strongly associated with PTS, and treatment with MPFF slowed this progression. Our data support the previous experimental findings on MPFF treatment reducing venous disease development and progression.28,29
The important findings from the extended follow-up concern the rate of DVT recurrence. Four new thrombotic events were observed after cessation of anticoagulation in control patients only. The significant trend for reduction in the recurrence rate with MPFF treatment was observed. These data should be interpreted with caution and be confirmed in more powerful trials. However, they may suggest some slight protective effects of MPFF on recurrent DVT owing to its anti-inflammatory action similar to statins.40
In comparison with ultrasound outcomes obtained at 6 months, no further significant recanalization was found. Thus, the most intensive process of thrombus clearance was observed within the first half-year. Treatment with MPFF improved deep-vein recanalization, probably due to its anti-inflammatory actions. This idea should be confirmed in future trials assessing inflammatory biomarkers. The most appropriate candidates are interleukin-6, intercellular adhesion molecule 1, soluble P-selectin, matrix metalloproteinase-9, and C-reactive protein.14,41
It is an essential finding that continuous treatment with MPFF was safe. No new AEs were reported after the first 6 months. This study is one of the few20 that confirms the possibility of long-term treatment with MPFF without serious consequences.
The limitations of the study are related to its pilot and open design, small sample size, absence of placebo, and appropriate randomization. However, this study is the first to provide information on the influence of MPFF on the course of DVT and the size of this effect. That will allow making appropriate sample size calculations for further randomized controlled trials that should overcome the limitations of the current one. A new study evaluating the level of biomarkers is suggested.
Conclusion
The results of this pilot study suggest that long-term treatment with MPFF can increase the speed of deep vein recanalization and reduce the incidence of PTS at 12 months in patients with popliteal-femoral DVT treated with rivaroxaban. Continued MPFF intake may have an influence on DVT recurrence after cessation of anticoagulation treatment. These findings should be confirmed in more powerful randomized clinical trials.

REFERENCES
1. LaMori JC, Shoheiber O, Mody SH, Bookhart BK. Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther. 2015;37(1):62-70.
2. Braekkan SK, Grosse SD, Okoroh EM, et al. Venous thromboembolism and subsequent permanent workrelated disability. J Thromb Haemost. 2016;14(10):1978-1987.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352.
4. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603.
5. Roussin A. Effective management of acute deep vein thrombosis: direct oral anticoagulants. Int Angiol. 2015;34(1):16-29.
6. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7.
7. Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clinic Proceedings: Elsevier; 2000:1249-1256.
8. Franzeck UK, Schalch I, Jager KA, Schneider E, Grimm J, Bollinger A. Prospective 12-year follow-up study of clinical and hemodynamic sequelae after deep vein thrombosis in low-risk patients (Zurich study). Circulation. 1996;93(1):74-79.
9. Beyth RJ, Cohen AM, Landefeld CS. Long-term outcomes of deepvein thrombosis. Arch Intern Med. 1995;155(10):1031-1037.
10. Kahn S, Comerota A, Cushman M. Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130(18):1636-1661.
11. Budnik I, Brill A. Immune Factors in Deep Vein Thrombosis Initiation. Trends Immunol. 2018;39(8):610-623.
12. Henke PK, Wakefield T. Thrombus resolution and vein wall injury: dependence on chemokines and leukocytes. Thromb Res. 2009;123(suppl 4):S72-S78.
13. DeRoo S, Deatrick KB, Henke PK. The vessel wall: a forgotten player in post thrombotic syndrome. Thromb Haemost. 2010;104(4):681-692.
14. Mosevoll KA, Johansen S, Wendelbo O, Nepstad I, Bruserud O, Reikvam H. Cytokines, adhesion molecules, and matrix metalloproteases as predisposing, diagnostic, and prognostic factors in venous thrombosis. Front Med (Lausanne). 2018;5:147.
15. Varma MR, Moaveni DM, Dewyer NA, et al. Deep vein thrombosis resolution is not accelerated with increased neovascularization. J Vasc Surg. 2004;40(3):536-542.
16. Henke PK, Pearce CG, Moaveni DM, et al. Targeted deletion of CCR2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006;177(5):3388-3397.
17. Henke PK, Varga A, De S, et al. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24(6):1130-1137.
18. Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost. 2005;3(2):401-402.
19. Vedovetto V, Dalla Valle F, Milan M, Pesavento R, Prandoni P. Residual vein thrombosis and trans-popliteal reflux in patients with and without the postthrombotic syndrome. Thromb Haemost. 2013;110(4):854.
20. Galanaud J, Holcroft C, Rodger M, et al. Predictors of post-thrombotic syndrome in a population with a first deep vein thrombosis and no primary venous insufficiency. J Thromb Haemost. 2013;11(3):474-480.
21. Tick LW, Kramer MH, Rosendaal FR, Faber WR, Doggen CJ. Risk factors for post-thrombotic syndrome in patients with a first deep venous thrombosis. J Thromb Haemost. 2008;6(12):2075- 2081.
22. Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55(3):768-773.
23. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs – guidelines according to scientific evidence. Int Angiol. 2014;33(2):87-208.
24. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181-254.
25. Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (MPFF) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and metaanalysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37(2):143-154.
26. Korthuis RJ, Gute DC. Adhesion molecule expression in postischemic microvascular dysfunction: activity of a micronized purified flavonoid fraction. J Vasc Res. 1999;36(suppl 1):15-23.
27. Takase S, Lerond L, Bergan JJ, Schmid- Schonbein GW. The inflammatory reaction during venous hypertension in the rat. Microcirculation. 2000;7(1):41- 52.
28. Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schonbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28(5):484-493.
29. Maria das Graças C, Cyrino FZ, de Carvalho JJ, Blanc-Guillemaud V, Bouskela E. Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. Eur J Vasc Endovasc Surg. 2018;55(5):694- 702.
30. Shoab SS, Porter JB, Scurr JH, Coleridge- Smith PD. Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: a pilot study. J Vasc Surg. 2000;31(3):456-461.
31. Bogachev VY, Boldin BV, Lobanov VN. Benefits of micronized purified flavonoid fraction as adjuvant therapy on inflammatory response after sclerotherapy. Int Angiol. 2018;37(1):71- 78.
32. Guillot B, Guilhou JJ, de Champvallins M, Mallet C, Moccatti D, Pointel JP. A long term treatment with a venotropic drug. Results on efficacy and safety of MPFF at a dose of 500 mg in chronic venous insufficiency. Int Angiol. 1989;8(4 suppl):67-71.
33. Lobastov K, Schastlivtsev I, Barinov V. Use of micronized purified flavonoid fraction together with rivaroxaban improves clinical and ultrasound outcomes in femoropopliteal venous thrombosis: results of a pilot clinical trial. Adv Ther. 2019;36(1):72-85.
34. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349(13):1227-1235.
35. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248-1252.
36. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in nonsurgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
37. Lobastov K, Ryzhkin V, Vorontsova A, et al. Correlation of clinical and ultrasound parameters used to assess the severity of post-thrombotic syndrome in patients after popliteal-femoral DVT. Int Angiol. 2018;37(1 [suppl 1]):62 (Abstract).
38. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694.
39. Ning J, Ma W, Fish J, Trihn F, Lurie F. Biases of Villalta scale in classifying post-thrombotic syndrome in patients with pre-existing chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2020 Mar 21. Epub ahead of print. doi:10.1016/j.jvsv.2020.01.018.
40. Kunutsor SK, Seidu S, Khunti K. Statins and secondary prevention of venous thromboembolism: pooled analysis of published observational cohort studies. Eur Heart J. 2017;38(20):1608-1612.
41. Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94(2):362-365.
