Micronized Purified Flavonoid Fraction in the treatment of pelvic pain associated with pelvic varicose veins
Fraction in the treatment
of pelvic pain associated
with pelvic varicose veins
Anatoly V. KARALKIN,
Ekaterina P. MOSKALENKO
Moscow, Russia
Abstract
Aim: To evaluate the benefit of micronized purified flavonoid fraction (MPFF) in women suffering from chronic pelvic pain associated with pelvic varicose veins (PVV) and possible gonadal varicose veins (GVV).
Methods: Consecutive women consulting for pelvic pain lasting more than 6 months, where a differential diagnosis of PVV had been made and possible concomitant diseases ruled out, were included in the study. Selected patients received MPFF treatment: 1000 mg a day for 8 weeks. Pelvic pain was selfassessed weekly using a visual analogue scale (VAS) during the 8 weeks of MPFF treatment and for 14 weeks after treatment was stopped. Imaging investigations using transvaginal ultrasound angioscan (USAS) and emission computer tomography (ECT) were repeated at 8 weeks, then at 6, 12, 36, and 60 months.
Results: A total of 85 women aged 28±4.6 years, of which 65 were in the PVV group and 20 in the PVV+GVV group, were enrolled in the study between 2000 and 2010. From weeks 2 to 4 of MPFF treatment, a reduction in pelvic pain was seen in either group. While a continuous pain decrease was reported by the PVV patients up to week 8 of treatment, there was no additional pain reduction in the PVV+GVV group. Over the 14 weeks following MPFF treatment, pelvic pain intensity was increased back to the pretreatment level in the PVV+GVV group, but was eliminated in the PVV group. In the latter group, the diameter of the PVVs did not significantly change over the long term (up to 60 months) as illustrated by USAS, and pelvic venous congestion declined as shown by ECT, reflecting a stabilization of the disease course.
Conclusion: In the present study, an 8-week MPFF treatment, 1000 mg up to 2000 mg per day, in women with isolated PVV, relieved them from their chronic pelvic pain in the short and long term. In patients with combined PVV and GVV, MPFF did not eliminate pain. However, MPFF may be used in women who wish a future pregnancy or in those reluctant to undergo surgery. ECT of the pelvic veinsis a reliable method for monitoring the efficacy of treatment or progression of the disease thanks to quantitative assessment of the degree of pelvic vein congestion.
Introduction
Millions of women may suffer from chronic pelvic pain at some time in their life and the frequency may be as high as 39%.1 The association with pelvic varicose veins (PVV) was first documented in 1949.2 Severe and chronic pelvic pain often results from the presence of ovarian varicose veins and PVV, reflecting chronic venous disorders in the pelvic veins. The compression of the left ovarian vein, the left renal vein, or the common iliac vein may also cause pelvic varices and pain.3
Clinical practice has shown that only one-third of patients with PVV need surgical treatment. The rest require conservative therapy, and the use of venoactive drugs has the most justification with regard to the pathogenesis and underpinnings of this pathology.4
Research objective
The aim of our research was to evaluate the benefit of a pharmacological treatment with micronized purified flavonoid fraction (MPFF)* in women suffering from chronic pelvic pain associated with PVV and possible gonadal varicose veins (GVV).
Materials and methods
Consecutive women consulting the S.I. Spasokukotskii Faculty Surgery Clinic for pelvic pain lasting greater than 6 months, where differential diagnosis of PVV had been made and other possible causes had been ruled out, were included in the study. The diagnosis of PVV was made on clinical presentation (pelvic pain, coital and postcoital pain, menstrual cycle disturbances, and dysuria), and was confirmed by imaging investigation on transvaginal ultrasound angioscan (USAS) and emission computer tomography (ECT) of the pelvic veins. Among the inclusion criteria was the absence of concomitant diseases. A bimanual pelvic examination by a urologist must reveal tenderness without induration or masses that could suspect another condition. An ultrasound investigation was systematically performed by a gynecologist to uncover pathological causes, such as endometriosis, adhesions, interstitial cystitis, and irritable bowel syndrome.
Once selected, the participants were further divided into 2 groups depending on the findings at investigation: (i) those with isolated dilation of pelvic venous plexus without any deterioration of the vulvar or ovarian veins (PVV group); and (ii) the others with more extended pelvic vein damage, combining both PVV and GVV (PVV+GVV group). Both groups underwent a conservative treatment with MPFF, at least 1000 mg per day for 8 weeks, with the goal of assessing the extent of pelvic vein damage, such that a conservative treatment could be efficient.
The primary end points were:
• Reduction or even elimination of the chronic pelvic pain on a 10-cm visual analogue scale (VAS) during MPFF treatment.
• Reduction in or absence of blood deposits in the pelvic venous plexus on ECT, reflecting a diminution of the pelvic venous congestion that could be assessed by a coefficient of pelvic venous congestion (CPVC), during MPFF treatment.
The long-term results (or secondary end points) were:
• Reduction in pelvic pain on VAS.
• Reduction in pelvic vein diameter on transvaginal USAS.
• Diminution of the CPVC.
• Amelioration of the associated symptoms (coital pain, dyspareunia, menstrual pain, and dysuria).
Every week during the 8 weeks of MPFF treatment, and then during the 14-week follow-up after treatment (until week 22), the patients self-assessed their pelvic pain on VAS. Imaging investigations (ie, USAS, ECT) were repeated at different times during the study: 8 weeks, then at 6, 12, 36, 48, and 60 months.
Results
A total of 85 women were enrolled in the study between 2000 and 2010. The age ranged from 18 to 43 years, with an average of 28±4.6 years. The clinical picture of the disease was characterized by the presence of chronic pelvic pain in 100%, coital and postcoital pain in 72%, menstrual cycle disturbances in 42%, and dysuria in 34% of the enrolled women. A total of 65 women had PVV in isolation, and 20 presented with both GVV and PVV.
Pelvic pain measurement on VAS
We noticed that the starting MPFF dose of 1000 mg/day relieved most of the women, right from the first month, in both groups (58 women; 50 in the PVV group and 8 in the PVV+GVV group), but was insufficient to relieve the 27 remaining patients from their pelvic pain. In “nonrespondent” women (15 in the PVV group and 12 in the PVV+GVV group), we had to increase the MPFF dosage up to 2000 mg/day after 1 month.
From weeks 2 to 4 of treatment, a reduction in pelvic pain was seen in either group (PVV and PVV+GVV) at the dosage of MPFF 1000 mg/day. From weeks 5 to 8 of MPFF treatment, the dose increase archieved a continuous pelvic pain decrease in the PVV group. There was no additional pain reduction in the PVV+GVV group despite the dose increase (Figure 1).
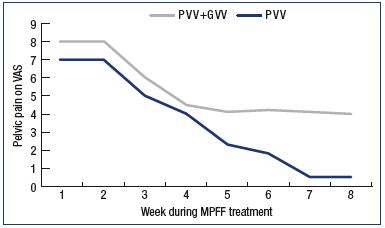
Figure 1. Assessment of pelvic venous pain.
Assessment of pelvic venous pain on a 10-cm VAS during an
8-week MPFF treatment in patients with pelvic vein dilation in
isolated PVV and with associated PVV and GVV.
Abbreviations: GVV, gonadal varicose veins; MPFF, micronized purified
flavonoid fraction; PVV, pelvic varicose veins; VAS, visual analog scale.
Pelvic venous congestion on ECT
In the 65 PVV patients who were all relieved from pelvic pain with MPFF treatment (with VAS ≤1) as evidenced in Figure 1, the comparison of ECG imaging at baseline and at week 8 of treatment showed a decline in the level of labeled erythrocytes in the venous plexus of the pelvis (Figure 2), together with a drop in the CPVC from 1.6±0.4 at baseline to 1.0±0.02 at week 8 (P<0.05).
In contrast, patients presenting with PVV+GVV did not report a complete elimination of pelvic pain after 8 weeks of MPFF treatment, even though they felt an improvement. This was confirmed by an absence of any significant changes on ECG imaging. A surgery of ovarian veins was proposed to these women and was accepted by 12 of the 20 women.
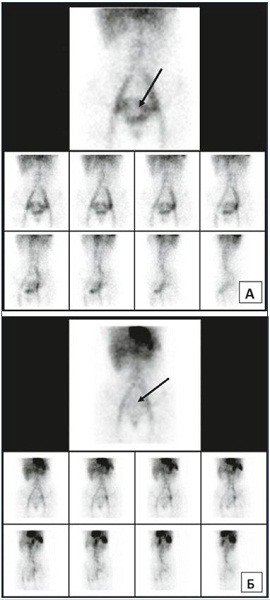
Figure 2. ECT assessment of pelvic veins before and after MPFF
treatment.
ECT of the pelvic veins at baseline (Panel A) and after an 8-week
MPFF treatment (Panel B) in a patient with pelvic vein dilation
in isolated PVV. Deposit of labeled erythrocytes in the uterine
venous plexus is indicated by the arrows.
Abbreviations: ECT, emission computer tomography; MPFF, micronized
purified flavonoid fraction; PVV, pelvic varicose veins.
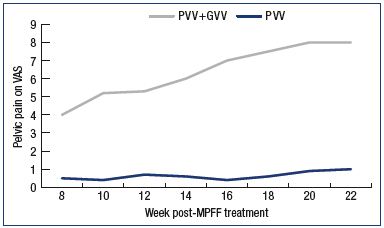
Figure 3. Assessment of pelvic venous pain.
Pelvic venous pain was measured using a 10-cm VAS in the
14 weeks following MPFF treatment in patients with pelvic vein
dilation in isolated PVV and with associated PVV and GVV.
Abbreviations: GVV, gonadal varicose veins; MPFF, micronized purified
flavonoid fraction; PVV, pelvic varicose veins; VAS, visual analog scale.
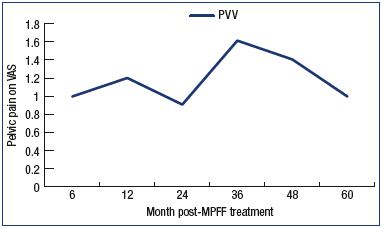
Figure 4. Assessment of pelvic venous pain.
Pelvic venous pain was measured using a 10-cm VAS in the
long term (with a mean of three 3-month MPFF treatments/year)
in 57 women with pelvic vein dilation in isolated PVV only.
Abbreviations: MPFF, micronized purified flavonoid fraction; PVV, pelvic
varicose veins; VAS, visual analog scale.
After MPFF treatment (week 9 to week 22)
Patients in the PVV group who self-assessed their pain during the 14 weeks following MPFF treatment (week 9 to week 22) were almost relieved of their pelvic pain in the long term. Contrarily, those who belonged to the group with more extended PVV (PVV+GVV group) reported an increase in pelvic pain that reached the pre-MPFF treatment level within the 10 weeks that followed the treatment (Figure 3).
Over the 60 months of observation of patients with PVV in isolation (PVV group), additional courses of MPFF therapy were undertaken using the previously recommended dose and duration. A total of 8 patients were excluded for the long-term observation due to concomitant pathology that occurred in the meantime: 4 women developed endometriosis; 2, vulvar varicose veins; 1, an adhesive process; and 1, acute cystitis. Of the 57 PVV women who followed several, long-term treatment courses, 7 took MPFF for preventive purposes and the others only when the symptoms reoccurred (pelvic pain, dyspareunia, coital and postcoital pain, etc).
Table I summarizes the number of MPFF-treatment courses undertaken by 57 PVV women at the different times of the study. Of note, the number of courses per year usually did not exceed 3, and the duration of administration ranged from 2 to 3.2 months.
Long-term pelvic pain measurement
Over a 60-month period, pelvic pain scores in PVV patients were maintained around 1 cm according to the VAS assessment, with an increase at 36 and 48 months, corresponding to noncompliance with MPFF treatment in some patients. The P V V+GVV group of patients remained with a high level of pelvic pain similar to that before MPFF treatment despite a drop in pain from VAS 8 cm to VAS 4 cm after a 2-month MPFF treatment (1000 mg/day) was seen. The patients’ flow diagram is summarized in Figure 5.
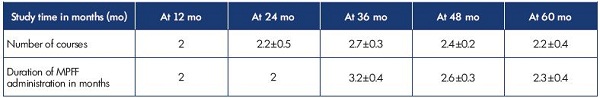
Table I. Number of courses and duration of MPFF treatment in 57 patients with pelvic vein dilation in isolated PVV over a 60-month
observation period.
Abbreviations: MPFF, micronized purified flavonoid fraction; PVV, pelvic varicose veins.
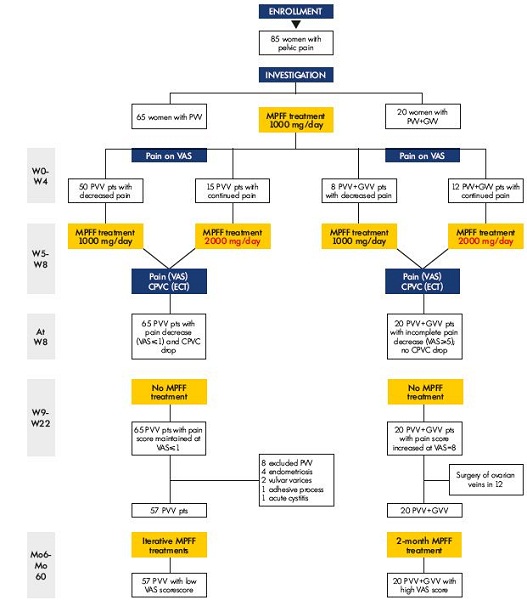
Figure 5. Patients flow-diagram.
Abbreviations: CPVC, coefficient of pelvic venous congestion; ECT, emission computer tomography; GVV, gonadal varicose vein; Mo,
month; MPFF, micronized purified flavonoid fraction; pts, patients; PVV, pelvic varicose vein; VAS, visual analogue scale; W, week.
Pelvic venous congestion on ECT
In PVV patients who were all relieved from pelvic pain and congestion of the pelvis with MPFF treatment (Figure 1 and Figure 2), the comparison of ECT imaging at baseline, and at 6, 12, 36, and 60 months showed results similar to 8 weeks post-MPFF treatment: the level of labeled erythrocytes in the venous plexus of the pelvis declined (Table II) and the CPVC remained at a low level (1.3±0.1) even at the 60-month follow-up.
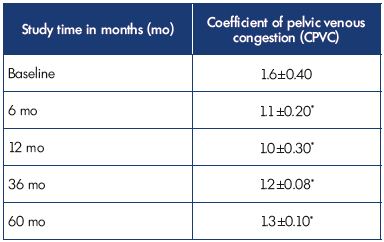
Table II. Results of ECT in 57 patients with pelvic dilation in
isolated P V V assessed using the CPVC.
*P0.05 compared with baseline.
Abbreviations: CPVC, coefficient of pelvic vein congestion; ECT, emission
computer tomography; P V V, pelvic varicose veins.
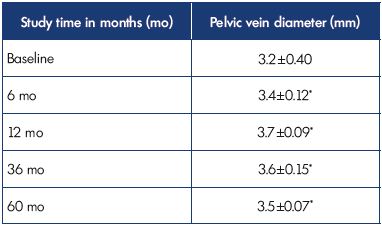
Table III. Results of tranvaginal USAS in 57 patients with pelvic
dilation in isolated P V V using the diameter of the pelvic vein.
*P=NS compared with baseline.
Abbreviations: P V V, pelvic varicose veins; USAS, ultrasound angioscan.
Pelvic vein diameter on transvaginal USAS
The diameter of P V V did not significantly change over the long term, reflecting a stabilization of the disease course with MPFF treatment (Table III).
Side effects
Over a 60-month follow-up period with iterative MPFF treatments, no side effects were noted, except a few manifestations of gastric dyspepsia, which disappeared after a short time (2 to 3 days) when the drug was withdrawn and did not recur.
Conclusion
In the present study, an 8-week MPFF treatment, 1000 or 2000 mg per day, in women with isolated P V V, relieved them from their chronic pelvic pain. In the long term (up to 60 months), iterative MPFF treatments of an average of three 3-month courses helped to eliminate pelvic pain in these P V V patients. In patients with combined P V V and GVV, MPFF treatment failed to eliminate pelvic pain. However, MPFF may be used in women who wish a future pregnancy or in those reluctant to undergo surgery. ECT of the pelvic veins is a reliable method of monitoring the efficacy of treatment or progression of the disease thanks to quantitative assessment of the degree of pelvic vein congestion.

1. I gnacio EA, Dua R, Sarin S, et al. Pelvic congestion syndrome: diagnosis and treatment. Semin Intervent Radiol. 2008;25:361-368.
2. T aylor HC Jr. Vascular congestion and hyperemia; their effect on structure and function in the female reproductive system. Am J Obstet Gynecol. 1949;57:211-230.
3. T u FF, Hahn D, Steege JF. Pelvic congestion syndrome-associated pelvic pain: a systematic review of diagnosis and management. Obstet Gynecol Surg. 2010;65:332-340.
4. Simsek M, Burak F, Taskin O. Effects of micronized purified flavonoid fraction on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clin Exp Obstet Gynecol. 2007;34:96-98.
