Neovalve reconstruction in postthrombotic syndrome. Technique, indications, and results
Marzia LUGLI
Vascular Surgery
Hesperia Hospital
Modena, Italy
ABSTRACT
Patients with leg venous ulcer (C6 patients according to the CEAP classification) affected by deep venous reflux often fail to respond to conservative therapies, and the presence of non-healing or recurrent ulcers may lead one to consider surgery. However, even if surgery is properly indicated, traditional techniques such as femoral transposition and valve transplantation are not always suitable, and in these cases a de novo valve reconstruction represents a surgical opportunity. Our neovalve reconstruction technique consists in creating an intimal flap by performing a wall dissection. The purpose is to create an antireflux mechanism that reduces venous hypertension. This technique was applied from December 2000 in 39 selected patients (43 operations) affected by postthrombotic syndrome or valve agenesis with grade IV according to Kistner’s classification.
The results are extremely encouraging and would seem to suggest that deep venous surgery may be appropriate in cases where conservative therapies fail, and where treatment of superficial and perforator insufficiency disappoints.
INTRODUCTION
A significant section of the population suffers from chronic venous disorders, which are often the cause of severe and permanent invalidity. When associated with superficial venous insufficiency without compromise of the deep venous system, chronic venous insufficiency can be more easily controlled and healing achieved in almost all cases.1,2 By contrast, when the deep venous system is itself directly affected, healing can only be achieved in a limited number of cases. In most cases, we have to settle for a kind of “peace with honor”, a delicate balancing act. By this is meant a scenario in which discomfort, edema, and trophic lesions are controlled with conservative therapies. The most common treatment is the application of an elastic stocking,3 however, the more severe deep venous system disorders demand compression levels that are not well tolerated. It should also be mentioned that even where an elastic stocking is worn, the treated limb can deteriorate due to episodes of lymphangitis or dermatitis, particularly in warm climates. Wearing an elastic stocking can also create psychological problems for young patients, who tend to regard themselves as invalids. The result can be a drastic reduction in their quality of life. Requests for corrective surgery or definitive treatment are now common, and they come from patients who have heard media reports that describe varicose surgery as the rapid and lasting solution to their problems. When they seek medical advice, they often meet with caution, and their disappointment is all the more intense. To understand why so many colleagues are skeptical, it needs to be borne in mind that surgical tradition has always defined postthrombotic patients as inoperable.
The reasons for this are threefold.4 The first is doubtless the fear of significant complications following surgery, for instance pulmonary embolism. A complication of such gravity, possibly fatal, might not be tolerated in the context of a disease that is certainly invalidating, but with a negligible risk of death. The second reason is that venous pathophysiology presents far more complicated hemodynamic mechanisms than arterial pathophysiology. Furthermore, our experience in the field is comparatively scant and still in its initial stages. The third and not least significant reason is that the surgery is by no means simple. As such, it requires prolonged training in centers of excellence, but since these centers are few and far between, there is still widespread ignorance of the specific surgical techniques involved.
Besides these three factors, a controversial note needs to be sounded about the efficacy of such surgery. Many critics remain skeptical because at 5 years of follow-up the results show a deterioration. Our reply to such reservations, which we throw down as a provocative gauntlet, is simply this: if the exact, same criteria were used to point the finger at other branches of surgery, many operations would not be performed.
It was Kistner5 who first provided definitive evidence that the effective relief of symptoms could be achieved after neutralizing deep venous reflux. Kistner’s pioneering operation was the first surgical approach to restoring the femoral valve to its original, functional condition.
Controversial at the time, the case involved reflux in a pathophysiological configuration that today we know as primary valve insufficiency (PVI). Unlike postthrombotic syndrome, where the valves are completely destroyed, in primary valvular incompetence the valves are present but malfunctioning. Despite this difference, the pathophysiological scenario is similar. Kistner was able to restore hemodynamic equilibrium to the leg by reconstructing only one femoral valve.
A few years later, Kistner6 pioneered another operation, valve transposition, whose objective was to create a mechanism which achieved valvular continence in a leg affected by postthrombotic reflux; in other words where the valves were destroyed. Taheri7 and Raju8 followed a similar route, performing an arm-to-leg venous valve transplant.
It should be mentioned that deep venous insufficiency, which generates hypertension at deep venous system level, almost invariably entails varicose veins and perforator insufficiency. Treating superficial reflux is required, since such cases often present severe clinical symptoms and ulcers, even if in isolation. Correcting the superficial venous system without correction of the deep venous system achieves a significant improvement, but results are less lasting.
Attempts to create a neovalve in a devalvulated axis have also involved experiments with homograft and allograft implants in animals. However, such operations have not yielded satisfactory results in humans.9,10 Other surgical attempts at creating an antireflux mechanism have been reported,11,12 and the operation we are proposing can be situated in this research field. In a nutshell, neovalve reconstruction aims at creating an antireflux mechanism by fashioning one or two flaps constructed by dissecting thickened vein wall tissue. These neovalves perform a similar function to a physiological venous valve as we reported in previous papers.13,14
TECHNIQUE
The anatomical structure of the human valve is so complex that we are not at present able to attempt a precise reproduction. It is for this reason that our objective is to create a mechanism that has the same function as nature’s valve, though not necessarily the same morphology. The neovalve is based on the creation of a flap that allows the blood to flow in one direction only, preventing its return in the opposite direction. The idea came to us when we observed that in cases of postthrombotic syndrome the vein wall is thickened by a process of fibrosis. This thickened tissue, it occurred to us, might be used to obtain a flap that could be fashioned so as to imitate the action of the valve (Figure 1). To create a neovalve it is first necessary to perform a phlebotomy, a perpendicular parietal incision and dissection so as to obtain a leaflet or flap. If the vein wall were always of an even thickness, the procedure would be based on a few simple guidelines: the length of the parietal incision and the depth of the dissection (Figure 2). The bicuspid valve, in which two pockets are fashioned, would require less depth of incision than in the monocuspid, where just one deeper pocket is fashioned. However, in postthrombotic disorders the fibrosis is so uneven and anarchic that in the patients we have operated on we have never found two cases similar in terms of anatomy and lesions. This means that each neovalve construction varies from case to case. In the absence of standard scenarios, the only common denominator in our series of patients was that of actual flap creation. Preoperative assessments and intraoperative manual palpation can partly help in determining the construction site, but in the majority of cases it is only possible to decide on how to proceed after the phlebotomy has been performed and the precise nature of the lesions ascertained. The fibrosis is often so thick that it creates a double channel inside the vein and this particular anatomical feature can be exploited to create the neovalve. Once the pocket has been created and its efficiency tested, experience shows that there is a risk of recurrent adhesion. To prevent this from happening it is enough to fix the flap in the semi-open position.
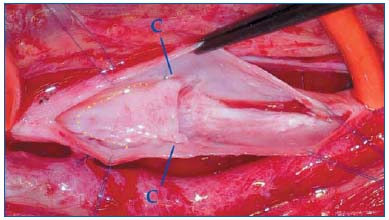
Figure 1: Postthrombotic wall damage
a) line of parietal dissection
b) fibrotic native valve
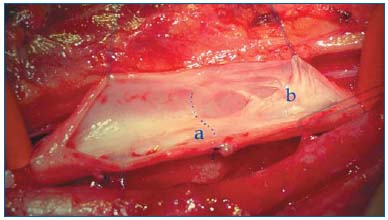
Figure 2: Neovalve and endophlebectomy
c) stitch fixing the flap in semi-open position dotted line:
depth of dissection
The ideal site for the neovalve would be the popliteal vein, since an antireflux mechanism here would obviate a reflux in several axes, femoral and profunda veins flowing together in this area. However, this rarely proves practicable for technical reasons, hence the preferred site is the femoral vein. The valve’s efficiency is tested during the operation by applying a suture on the proximal side of the phlebotomy and inspecting the pocket’s continence (bulge in valve) on the distal side while the phlebotomy is still open. This is most important and allows us to make minor adjustments before concluding the operation. It is not always possible to create a flap, but given that our series has an intention-to-treat rate of 3/43, failure is infrequent.
MATERIAL AND METHODS
From December 2000 to December 2007 we performed 43 neovalve construction operations in 39 patients (20 males, 19 females, median age 57, range 29 – 82) affected by severe chronic venous insufficiency. All patients presented an ulcer resistant to conservative therapy, and treatments on the superficial venous system and perforators did not prove feasible. The ulcer had been present or recurrent for over 5 years and caused the patient major discomfort. Thirty-five patients were affected by postthrombotic syndrome and presented deep venous thrombosis almost 10 years previously; 4 patients were affected by valve agenesis. All patients underwent descending venography that showed a reflux grade IV according to Kistner. Two patients presented thrombophilia: one patient presented a Leiden-factor alteration and the other a congenital defect of antithrombin III. No patient had a contraindication to anticoagulant therapy. This group of patients had been excluded from conventional antireflux surgery, such as femoral transposition or valve transplant. All patients underwent duplex scanning and air plethysmography detecting venous filling index (VFI) and ejection fraction (EF). Associated comorbidities were not sufficient to dissuade us from operating. All patients were adequately informed about the innovative nature of this technique and received a detailed informed consent form.
Apart from 4 operations under general anesthesia, the others were performed under epidural anesthesia. The position of the patients on the operating table was supine with the leg rotated externally and the knee slightly flexed. The operation is performed through an incision of 10 cm in length in most cases at the intermediate third of the thigh, but could also be performed at the proximal or distal third. We proceed with careful dissection of the femoral vein, taking care not to damage the lymphatic vessels so as to avoid loss of lymph in the postoperative period. Particular refluxes in the deep collateral circle, the result of postthrombotic syndrome, should be tied, as should reflux in the doubled femoral vein.
A foot-thigh elastic stocking (35 mm Hg) was applied in the postoperative period. Movement of the leg was encouraged and certain patients underwent physiotherapy and distal 30° bed-elevation. On the second postoperative day movement was resumed and anticoagulant therapy was replaced by oral anticoagulation therapy to obtain an international normalized ratio of 2.5–3 for a six-month period. In other cases subcutaneous heparin was administered for one month. Patients were advised to use a Class I elastic stocking for at least one month after the healing of the ulcer. Ulcer healing was finally achieved with a shortstretch bandage.
RESULTS
During follow-up, all lower extremities were screened with duplex scanning at 1 and 6 months after the operation and thereafter at one-year intervals. Air plethysmography was performed one month after surgery to assess VFI and EF variations, and thereafter annually. The average follow-up was 33 months (range 1-84) at December 2007.
The operative and postoperative mortality rate was 0% and no cases were complicated by pulmonary embolism. The median hospital stay was 6 days.
Minor postoperative complications occurred in 7 patients (17.5%): 3 wound hematomas, 3 seromas, and 1 wound infection.
Early deep vein thrombosis located below the neovalve site was detected in two patients (5%). One patient stopped anticoagulant therapy for reasons unknown. No early thrombosis at the operative site was detected. Early deep vein thrombosis below the neovalve site occurred in 2 cases (4.7%). Late deep vein thrombosis occurred in one patient (2.3%), 8 months after the operation when she resumed oral contraception. Ulcer healing was observed in 39 cases (90.7%), while ulcer recurrence was observed in 3 cases (7%).
Neoalve competence was established in 37 cases (86%). There were 6 cases of valve failure during the follow-up period, making for 14% overall neovalve incontinence. Postoperative instrumental assessment shows statistically significant improvement in VFI and EF. Postoperative venography was performed in 27 patients before discharge.
DISCUSSION
In the majority of patients classified CEAP C6 or C5, conservative therapies are selected. Various types of bandage are applied to achieve ulcer healing when, as happens periodically, the ulcers reappear. Elastic stockings are also used as reinforcement, but the high levels of compression required usually cause discomfort that meets with reluctance on the patient’s part. Requests from patients seeking a more radical solution also meet with reluctance on the part of doctors, who manifest misgivings about saphenous vein stripping in patients with a history of deep venous thrombosis. It is easy to imagine that treatment of the deep venous system is still regarded with particular suspicion and diffidence. Aging goes hand in hand with the increase in problems related both to deterioration of muscle compliance and to the presentation of diseases affecting joints and arteries. This all aggravates problems to do with compression stockings. Young patients, by contrast, in an attempt to strike a balance between their health and a normal social life, are often inconstant in their use of containment stockings, and this can lead to a rapid deterioration in their condition, complicated further by problems of self-image and, in some patients, depression. In terms of hemodynamics the health of these patients is compromised on various levels: deep, superficial, and perforator. Correcting a superficial-system reflux can restore equilibrium to the system, and it should be observed that deep venous system reflux is not necessarily synonymous with serious symptoms or cutaneous ulcers. Not infrequently, we come across young patients with a deep reflux which is well compensated by physical exercise and an efficient muscular pump, provided, of course, that there is careful monitoring of the superficial system.15 When the problem is related to perforator insufficiency, results can be achieved by treating this alone, but the presence of deep venous reflux often highlights the long-term inadequacy of such treatment and the risk of recurrence is high. Sclerotherapy also helps in maintaining homeostasis, and the various systems work in synergy to achieve acceptable clinical conditions, provided compression is not excessive. Should such therapies fail, and in the absence of contraindications of a general and local nature, serious consideration should be given to an operation on the deep venous system. Treating postthrombotic syndrome is not easy since we still lack a means of gauging thoroughly the full extent of an occlusion or the significance of a stenosis and reflux.16 Clinical observation reveals that reflux is often less tolerated than occlusions, but this distinction is often not valid since the two processes, reflux and stenosisocclusion, are frequent bedfellows. Raju points out the key role of stenosis-occlusion of the proximal iliocaval system rather than subinguinal reflux pathology.17 The availability of endoluminal corrective surgery has revolutionized the treatment of postthrombotic syndrome in this area. However, our personal experience is that a persistent, Grade IV subinguinal reflux provokes a state of hypertension both in patients with a serious primary iliocaval disease as well as in those where iliocaval flow has been restored. This hypertension is quite capable of maintaining the clinical situation under discussion. One can quite readily understand how in hybrid diseases reopening the iliocaval axis can result in a limb’s improvement. However, if this were all that is needed, the logical conclusion would be that the subinguinal tract can manage quite happily, free of symptoms, in the absence of a valve. This flies in the face of daily clinical evidence.
The association of refluxing segments with occluded segments—anarchic and refluxing subfascial collateral pathways, the presence of postthrombotic pseudovalves, obstructing and hence antiphysiological reflux—all contribute to making postthrombotic syndrome a multifaceted scenario which is difficult to assess.
In straightforward primary venous insufficiency, we are restoring valves that don’t work to functionality. As Kistner has shown, significant physiological results can be achieved, even though not all valve structures have been restored to continence.18 In postthrombotic syndrome, valves cannot be repaired, and even if repair were not possible in one segment, it would have scant hemodynamic significance. Preventing reflux in a principal axis does not mean resolving the patient’s problem since he or she may also be affected by a more significant reflux in the deep femoral axis.19 Before undertaking any therapy on the deep venous system, it is thus necessary to carry out a close study in which, unlike in studies of the superficial venous system, phlebography is still the benchmark. From screening of a selection of patients, a percentage will emerge who can benefit from surgery to correct deep venous reflux.
Kistner’s transposition technique resolves the problem ingeniously by transposing the femoral vein to a position above a continent valve in the deep femoral vein or the saphenous vein. It should be observed, however, that only a small percentage of cases present the anatomical situation that makes this operation feasible.
Valve transplantation is another operation worthy of note: it entails the removal of a segment containing a valve from the brachial vein and inserting it at popliteal level.
This operation also has its limits, principally where the donor vein has a noncontinent valve or where the popliteal vein is not anatomically suitable for the procedure.
Various surgeons have begun experimental work: valvecusp transplants; invagination of a saphenous vein segment to create an antireflux mechanism; the implant of a heterologous valve inserted into stents. Some attempts have failed and other techniques are still in the research stage.
Our contribution resulted from constant observation in the course of operations such as femoral transposition. We were struck by the frequent thickening of the vein wall, which was sometimes uniform in texture and distributed around the vein’s circumference, at other times patchy. This thickening suggested the idea of creating a parietal flap by dissection in order to obtain an antireflux mechanism entirely similar to a venous valve. Not infrequently, it is necessary to combine with endophlebectomy,20 or to ablate a pseudovalve against the flow. The flap is tailored to individual requirements, but based on general principles. It is still unclear exactly how the flap works, but it probably differs from the delicate action of the natural human valve,21 even if the antireflux mechanism proves effective. By contrast, other procedures, such as perforator ligation, yield results that are not lasting. Several techniques combined will probably produce a definitive solution to invalidating diseases with a high social cost.


References
2. Sethia KK, Darke SG. Long saphenous incompetence as a cause of venous ulceration. Br J Surg. 1984;71:754-755.
3. Partsch U. Compression therapy of venous ulcers. Hemodynamic effects depend on interface pressure and stiffness. EWMA Journal. 2006;6:16-20.
4. Kistner RL. From serendipity to practicality. Fundamental questions generated by the success of the first valvuloplasty 1968. Paper presented at: The Artic Fjords Conference and Workshop on chronic venous disease; October 2-6, 2007; Hurtigruten, Norway.
5. Kistner RL. Surgical repair of a venous valve. Straub Clin Proc. 1968;24:41-43.
6. Kistner RL, Sparkuhl MD. Surgery in acute and chronic venous disease. Surgery. 1979;85:31-43.
7. Taheri SA, Lazar L, Elias S, Marchand P, Heffner R. Surgical treatment of postphlebitic syndrome with vein valve transplant. Am J Surg. 1982;144:221- 224.
8. Raju S, Fredericks R. Valve reconstruction procedures for nonobstructive venous insufficiency: rationale, techniques, and results in 107 procedures with two- to eight-year follow-up. J Vasc Surg. 1988;7:301-310.
9. Dalsing MC, Raju S, Wakefield TW, Taheri S. A multicenter, phase I evaluation of cryopreserved venous valve allografts for the treatment of chronic deep venous insufficiency. J Vasc Surg. 1999;30:854-856.
10. Neglén P, Raju S. Venous reflux repair with cryopreserved vein valves. J Vasc Surg. 2003;37:552-557.
11. Plagnol P, Ciostek P, Grimaud JP, Prokopowicz SC. Autogenous valve reconstruction technique for postthrombotic reflux. Ann Vasc Surg. 1999;13:339-342.
12. Karagoz HY, Dogan N, Kocailik M, Sungun M, Duran E. Treatment of congenital venous avalvulosis using a surgically created autogenous vein valve. Cardiovasc Surg. 1993;1:131-133.
13. Maleti O. Venous valvular reconstruction in postthrombotic syndrome. A new technique. J Mal Vasc. 2002;27:218-221.
14. Maleti O. Lugli M. Neovalve construction in postthrombotic syndrome. J Vasc Surg. 2006;43:794- 799.
15. Christopoulos D, Nicolaides AN, Cook A, Irvine A, Galloway JM, Wilkinson A. Pathogenesis of venous ulceration in relation to the calf muscle pump function. Surgery. 1989;106:829-835.
16. Perrin M, Gillet JL. Insuffisance valvulaire non post-thrombotique du système veineux profond des membres inférieurs. In: Angéiologie. Encycl Med Chir 2003:19-2020, Paris.
17. Raju S, Owen S, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35:8-15.
18. Perrin M. La chirurgie des reflux veineux profonds des membres inférieurs. J Mal Vasc. 2004;29:73-87.
19. Eriksson I, Almgren B. Influence of the profunda femoris vein on venous hemodynamics of the limb. Experience from thirty-one deep vein valve reconstructions. J Vasc Surg. 1986;4:390-395.
20. Puggioni A, Kistner RL, Eklof B, Lurie F. Surgical disobliteration of postthrombotic deep veins – endophlebectomy – is feasible. J Vasc Surg. 2004;39:1048-1051.
21. Lurie F, Kistner RL, Eklof B, Kessler RVT. Mechanism of venous valve closure and role of the valve in circulation: a new concept. J Vasc Surg. 2003;38:955-961.
