Operative treatment in postthrombotic syndrome: an update
2. Department of Cardiovascular Surgery, Hesperia Hospital Modena, Italy.
3. Associate Professor of Surgery Grenoble and for Institution Unité de Pathologie Vasculaire Jean Kunlin, Clinique du Grand Large, Chassieu, Lyon, France.
ABSTRACT
Postthrombotic deep vein obstruction and/or incompetence can lead to severe chronic venous insufficiency in a significant number of cases. Postthrombotic lesions are essentially of two types: (i) obstruction of various degrees; and (ii) valve destruction with subsequent reflux. These two elements are variously present at different levels. The most frequent combination is proximal occlusion or obstruction associated with subinguinal reflux. The leading technique for treating proximal obstruction is stenting. Conversely, the leading technique for treating obstruction located below the inguinal ligament is endophlebectomy mainly in the common femoral vein. The treatment of deep venous reflux in postthrombotic syndrome is based on a precise strategy deriving from an accurate diagnostic evaluation, based on several investigations. This phase should give us useful information for addressing the treatment. Various procedures can be used including valve transfer, neovalve according to investigation data. When treating conditions characterized by proximal obstruction and distal reflux, the treatment should be divided into two actions and usually venous stenting addresses the first action. This allows us to check the degree of improvement obtained after the first treatment.
INTRODUCTION
Postthrombotic deep vein incompetence can lead to severe chronic venous insufficiency (CVI) in 20% to 60% of cases.1 However, a postthrombotic deep vein lesion is not only related to valve incompetence, but also to various degrees of obstruction including occlusion. Moreover, the postthrombotic syndrome (PTS) may comprise four hemodynamic disorders: the two mentioned above, as well as superficial vein insufficiency and perforator incompetence. The deep venous lesions (obstruction and valve destruction) are variously combined at different levels. The increase in resistance to blood flow is due to stenosis and rigidity of the venous wall. Stenosis is caused by endoluminal processes (endoluminal fibrosis and synechiae) (Figure 1) or exoluminal processes (extrinsic fibrotic compression). Both are inflammatory postthrombotic sequelae. The reflux is determined by the absence of valvular competence as a result of lesions extending, to a greater or lesser degree, to the valvular apparatus itself. Actually, although obstruction and reflux can be associated with various combinations such as to sites and severity, the obstructive lesions are usually located at the proximal iliac and common femoral site, while the reflux involves the femoral-popliteal crural segment.
Postthrombotic recanalization is more complete at the subinguinal area while it is less complete at the iliac area. The reasons for this difference in recanalization are not well known.
Physiopathology
The isolated proximal venous obstruction is the principal cause of PTS in approximately one-third of cases, while in two-thirds of all cases, CVI is caused by the association of obstruction and reflux following the above-mentioned lesions: iliac proximal obstruction and distal reflux. CVI with deep venous disease and without perforator and superficial venous system incompetence, can be divided into four main types according to the A and the P of the CEAP (Clinical; Etiological, Anatomical, Pathophysiological) classification Table I.2
1. Ad, Po 6-7- 9
2. Ad, Po 6-7-9-11
3. Ad, Po 6-7-9-11-13
4. Ad, Po 6-7-9-11-13-14-15
Given that each type can be associated with Ad, Pr 11-12-13-14-15, we can find scenarios characterized by an obstructive process at vena cava, iliac, and common femoral level and/or at femoral, popliteal, and tibial level and/or an associated reflux. However, the more frequent scenario will be Ad, Po 7-9 Pr 11-13-14-15.
When two hemodynamic elements are associated, it is advisable to distinguish the more significant to correct it first. Consequently, it is important to establish which of them, an iliac obstruction or a femoral-popliteal reflux, is the most significant. Investigative procedures to separately evaluate the two components are currently not discriminant and the only parameter at our disposal is the cumulative result of both on venous function In other words, the severity and extent to which reflux and obstruction, and their interaction, impact venous function can only be known by their global combined impact.
Venous physiology theory states that venous pressure detected distally in the standing position and after exercise are essential parameters. In reality, venous physiology is founded on more complex phenomena rather than venous pressure alone. The venous system has a variable capacity: variations in caliber affect variations in flow, which influences pressure. Another crucial factor in venous physiology is the valve function.3 Its role is evident as can be seen by observing the clinical signs and symptoms correlated to reflux. Even if a correlation between deep venous reflux and clinical severity has not yet been established, we know that axial reflux is more permissive than segmental. In addition, other factors play a role in venous function such as the extrinsic contraction of the lower limb muscles. Anatomical damage being equal, the muscular force acts as a counter force, which means that the same pathology may be more or less severe. In other words, the same pathology can manifest as a C6 or C3 class.
Investigations
The goals are to precisely identify each lesion, which means that the venous system must be investigated from the ankle to the vena cava. Several investigations are available: (i) Duplex; (ii) B-Flow; (iii) Plethysmography; (iv) Venous Pressure; (v) CT; (vi) RMI; (vii) venography; and (viii) intravascular ultrasound (IVUS).
None of the above-mentioned investigations provides the full information, but by using different investigations, valuable data can be obtained to correct the hemodynamic damage. They can also supply information on PTS evolution. This phase should give us morphologic and hemodynamic information in order to address the treatment.
The crucial information for determining ideal operative procedures is as follows:
1. Presence and/or absence of proximal obstruction including occlusion.
2. Presence of axial reflux below the inguinal ligament, from groin to calf, via femoro-popliteal axis or by superficial or profunda transfer as well as their combination.
3. Presence and/or absence of proximal competence of the profunda vein.
4. I n case of profunda vein incompetence, to identify single or multiple re-entry points into the popliteal vein, it may be useful to determine what is the easiest access and way to occlude the re-entry point by endovascular procedure.
5. Presence and competence of great and small saphenous vein.
6. Popliteal vein feature (single or multiple channels) and their competence and/or incompetence.
7. Caliber of femoral and popliteal vein.
8. Femoro-popliteal flow during exercise.
9. Caliber and competence of the axillary vein.
10. Presence of endoluminal fibrosis, which determines a double channel at femoro-popliteal level.
Surgical indications
Although improvement can be achieved by wearing elastic stockings, in a significant number of patients, using compression therapy may be insufficient or not well tolerated.4 The C6 class, in presence of an active ulcer, is supposed to be the most severe CEAP class, however, it should be stressed that class C4b is sometimes just as invalidating as class C6. It should also be noted that class C4b is less tolerant to compression.
Actually, PTS determines a severe structural change in the leg to such an extent that variations in the structure of collagen also intervene in the extracellular matrix of the skin, as well as in the matrix of the venous tissue. This is what underlies the chronic and inflammatory processes that characterize the pathology. What is surprising in PTS is its extreme variability and the less-than-constant correspondence between anatomic damage and its hemodynamic effects. We know that a slight alteration in venous flow can lead to major hemodynamic consequences and so, from the therapeutic point of view, partial correction of a venous defect can drastically modify venous hemodynamics, leading to a compensated and acceptable clinical state.5 It has been asserted that the extensive damage produced by PTS was not entirely correctible and that it was impossible to restore the homeostatic conditions.
This is undoubtedly true, but it should also be remembered that our purpose is to achieve better hemodynamics in order to improve the patient’s quality of life. This improvement does not mean restoring normal anatomy and physiology of the damaged system, but acting in a strategic and progressive way on particular hemodynamic lesions. That means that a leg, which, even though pathological, can allow the patient to lead a normal life, such as to perform normal activities and engage socially, in other words to improve quality of life.
Given that ulcers, lipodermatosclerosis, pain, burning, itching, and permanent edema are all signs or symptoms impeding quality of life, it is against these that we must act. The aim of treatment in PTS is to improve either sign or symptoms and finally quality of life. This can be achieved by methods less incapacitating than the pathology itself. Wearing elastic bandages or high-compression elastic stockings daily, particularly in high temperatures, cannot be defined as a therapeutic approach that improves the patient’s quality of life.
Any envisaged surgical procedure deserves serious consideration, provided it is a low-risk operation. Though it may cause inconvenience, this can hardly compare with decades of poor quality of life.
We do not hesitate to submit a patient to a hip-replacement operation – which is not without risks – in order to relieve chronic pain and improve their ability to walk. By the same token, there is no reason why we should discourage deep venous system surgery, thus condemning the patient to wear compression stockings for the rest of their life. Hesitations of this nature probably stem from a taboo that deep venous system surgery is dangerous and unadvisable. What does the literature tell us about the series of patients who undergo such treatment? Mortality and complications are nil or negligible, and improvement can be achieved in many cases.
Operative treatments
As is mentioned above, it is opportune to continue step by step, starting with more simple actions and progressing, if necessary, to more sophisticated ones. Nowadays, deep venous endovascular treatment has benefited from a very efficient technique: recanalization of the occluded or obstructed vein with angioplasty and stenting. This has allowed us to treat one of the principal obstacles to lower-limb blood flow, (the iliac-cava obstruction syndrome), without the need for open surgery.
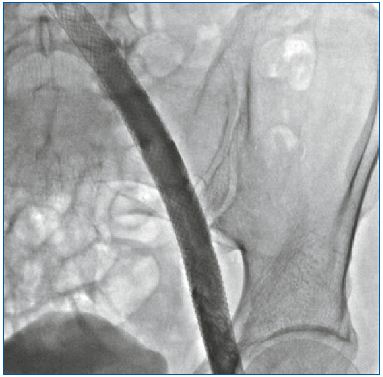
The venous endoluminal technique has proved superior in reducing mortality and morbidity, but above all, in terms of the results achieved, results that were never achieved with open surgery. This has meant that the type Ad, Po 6-7-9 could be treated successfully only with endovascular procedures (Figure 2). In the case of an obstructed and not occlusive process, the success of the treatment is so demonstrable that the procedure has received a A grade of recommendation. When the patient is Ad, Po 6-7-9 Pr 11-12-13-14-15 the result may be the same but in a more restricted number of patients. In the Raju series,5 half the patients obtained healing of trophic lesions reducing the need for compression support, leaving the subinguinal refluxes unchanged. This is a clear demonstration of the fact that we can improve the patient by partially correcting PTS sequelae.
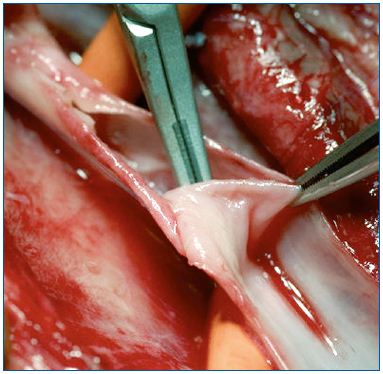
In Ad, Po 7-9-11 o, Ad, Po 11 patients, open surgery such as endophlebectomy may be necessary. The technique was described by Kistner and Puggioni6 and consists of removing the endoluminal fibrotic tissue in the common femoral vein. This also supports the idea that a limited action can improve the leg. Indeed, although the endoluminal fibrosis is more extensive in the distal vein, restoring a normal lumen in the common femoral vein by opening the tributaries’ termination creates a re-entry point that increases inflow. At the inguinal level, the open technique is preferable to stenting since it is an area that is easily accessible to surgery, and it undergoes extrinsic compression due to the movement of the leg. Stenting yields excellent results with a minimum level of morbidity and a restenosis less than 5%; it leads to a significant improvement in pain, edema, and quality of life.
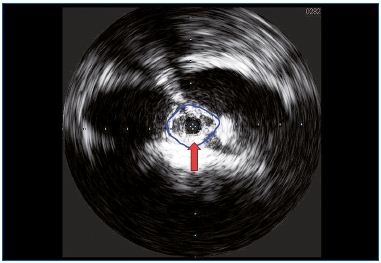
In PTS, iliac venous obstruction is broadly represented, and this explains why in half of the cases we obtain very good results simply by treating this lesion. However, we do not yet have pretreatment methods to determine whether an iliac stenosis is hemodynamically significant or not, and the presence of collateral pathways shown by venography does not give us sufficient information. Intraprocedural IVUS (Figure 3) yields useful information on the degree of venous stenosis. Nevertheless, IVUS provides morphologic information, but no hemodynamic ones. Unlike in the case of arteries, the transstenotic pressure gradient is meaningless, given that its system differs in the way it fills, yielding very low-pressure values and misleading scenarios produced by collateral pathways.
The criterion used to evaluate and treat an iliac stenosis is still more morphological than hemodynamic, and this explains why it proves impossible in a global evaluation of the lower limb to evaluate the various lesions. Therefore, the criterion to first treat an iliac stenosis combined with below inguinal reflux relies on a pragmatic principle, to start by the most simple and less invasive procedure. When the reflux is the main cause of signs and symptoms, and this is evident in Ad, Po 7-9 Pr 11-13-14-15 patients that become after stenting Ad, Po 7-9-11-13-14-15 and in isolated cases of Ad, Pr 11-13-14-15, we must take into account the treatment of the reflux itself. We know that axial reflux is directly correlated with CVI.7
Paradoxically, deep venous axial reflux seems to be tolerated better than superficial reflux, but this is true only in the short term; the persistence of deep venous reflux leads to particularly severe CVI in the long term, besides contributing to recurrence of treated varices. The correction of postthrombotic reflux is obtained by applying various techniques: Kistner valvuloplasty,8 venous transposition,9 venous transplant,10 and neovalve creation.11
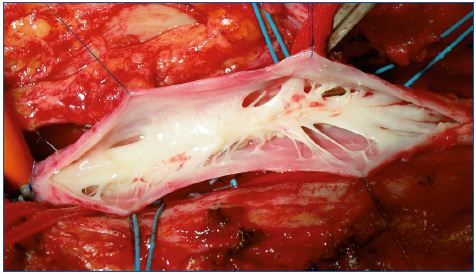
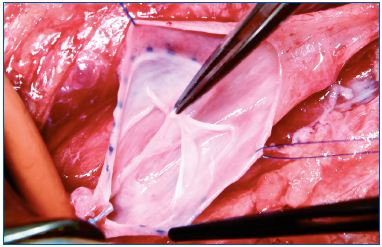
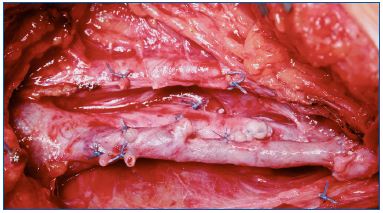
Each of these techniques has its specific application. Valvuloplasty (Figure 4) is a procedure aimed at restoring the competence of valves. In selected PTS a proximal valve is reparable allowing valvuloplasty to be performed. By shortening the valvular cusps free borders, the valve regains competence that segments the refluxing hydrostatic column. When reparable valves are not detectable in the femoralpopliteal segment, but a competent valve is present in the proximal portion of the profunda vein, a femoral transposition might be considered and the femoral vein is transposed into the profunda vein below the competent valve. When we have a still competent great saphenous vein, a saphenous transposition is performed, creating a new deep competent axis. (Figure 5).
When the profunda and great saphenous veins are incompetent, a transplant taken from the axillary vein and inserted into the popliteal vein can be used. A possible alternative, when the above-mentioned operations are not technically performable in a conspicuous number of cases, a neovalve construction is the only option (Figure 6). The neovalve can be performed with various techniques, and it has proven to be a reliable procedure in the long term.
Discussion and Conclusions
The surgical treatment of CVI has frequently addressed perforators. The reduced morbidity offered by subfascial endoscopic perforator surgery (SEPS) compared with open surgery has considerably increased its frequency of use. How should we now consider the perforators? In other words, is ablation of incompetent perforating veins recommended when deep venous obstruction and reflux are present? We know that incompetent perforators play a different role in primary superficial vein incompetence in combination with primary superficial and deep venous reflux and in combination with superficial reflux and secondary deep venous reflux and obstruction.
Unfortunately, the role of perforators has not yet been established, neither whether the incompetent perforators are the cause of superficial venous incompetence, nor whether the saphenous venous reflux is the cause of dilatation and subsequent incompetence of the perforators. Studies related to this problem provide data that suggest that there is no additional hemodynamic improvement after treatment of the superficial system. When the deep venous system is competent, the treatment of perforators associated with superficial venous treatment doesn’t improve the results and the perforators can regain their competence after saphenous vein surgery alone.
When deep venous insufficiency is associated, we have no data about the regained competence of perforators after treating the superficial system or deep venous system or both. We know that SEPS in PTS is not justified, due to the high percentage (56%) of ulcer recurrence after five years in postthrombotic limbs.12 In 40 to 50 years of deep vein surgery, no severe complications have been reported by the authors13-15 who have practiced it, and the fear of pulmonary embolisms have proven unfounded, but for rare exceptions. Nowadays, PTS severity commences when an acute phlebothrombosis develops, and thus restoring iliac patency during the acute phase forms the basis for preventing postthrombotic disease. The second point worth making is that early treatment of a symptomatic patient can achieve better results and probably impedes the progression of the PTS. Angioplasty and stenting in proximal lesions have shown their efficacy, and given the low risk of open surgery, the surgical correction of PTS should be considered every time the conservative treatment proves either inefficient or not well tolerated, especially in young patients. The purpose of these procedures is to improve the quality of life, given that PTS and its correlated complications are poorly tolerated.16
Before the stenting era, the role of reflux as a principal cause of CVI was probably overestimated. Nowadays, we know that proximal venous obstruction and occlusion play a crucial role and this concept does not derive from hemodynamic evaluation, but from the clinical improvement of the patient after treating obstruction. Therefore, given that both obstruction and reflux are subsumed in a single hemodynamic scenario, the strategy of correction will occur before treating the proximal obstruction and this for three principal reasons: (i) we can improve the patient’s condition without the need to perform open surgery; (ii) endovascular technique is less invasive; and (iii) a deep vein reconstruction can fail if associated with a poor inflow or outflow.
The leading technique for treating proximal obstruction is stenting. Conversely, the leading technique for treating obstruction located below the inguinal level is endophlebectomy, performable at the femoral and popliteal level. When we treat a condition characterized by proximal obstruction and distal reflux the treatment should be divided into two separated actions. This allows us to check the degree of improvement obtained after the first treatment. When obstruction and reflux are associated below the inguinal ligament, endophlebectomy and correction of reflux should be performed in the same session, except for hybrid treatment in which iliac stenting and endophlebectomy are associated for technical reasons (ie, significant fibrosis in the stent’s landing area).
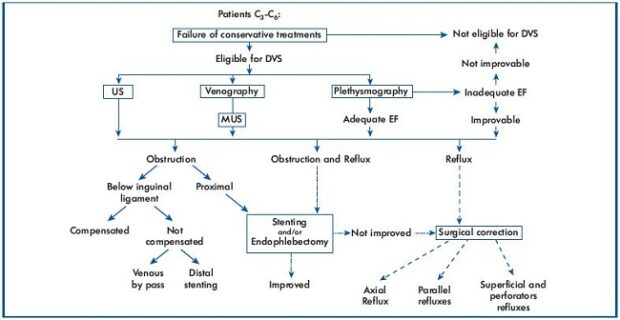
At the moment, the treatment of deep venous lesions in PTS is based on a precise strategy (Figure 7) coming from an accurate diagnostic phase.17 At present, four techniques for treating deep reflux are theoretically possible. We favor, in order, valvuloplasty, vein transposition, neovalve, and valve transplant. The treatment of perforators either before or after deep venous surgery is still a subject for debate.
References
1. Perrin M, Gillet JL, Guex JJ, et al. Syndrome post-thrombotique. Angéiologie. 2003;19(2040):12.
2. Eklof B, Rutherford RB, Bergan JJ, et al; American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248-1252.
3. Lurie F, Kistner RL, Eklof B, Kessler D. Mechanism of venous valve closure and role of the valve in circulation: a new concept. J Vasc Surg. 2003;38:955-961.
4. R aju S, Hollis K, Neglen P. Use of compression stockings in chronic venous disease: Patient compliance and efficacy. Annals of Surgery. 2007;21:790-795.
5. R aju S, Darcey R, Neglen P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51:401-409.
6. Puggioni A, Kistner RL, Eklof B, Lurie F. Surgical disobliteration of post thrombotic deep vein – endophlebectomy – is feasible. J Vasc Surg. 2004;39:1048-1052.
7. D anielsson G, Arfvidsson B, Eklof B, Kistner RL, Masuda EM, Satoc DT . Reflux from thigh to calf, the major pathology in chronic venous ulcer disease: surgery indicated in the majority of patients. Vasc Endovascular Surg. 2004;38:209-219.
8. K istner RL. Surgical repair of venous valve. Straub Clin Proc. 1968;24:41-43.
9. K istner RL, Sparkuhl MD. Surgery in acute and chronic venous disease. Surgery 1979;85:31-43.
10. Taheri SA, Lazar L, Elias S, Marchand P, Heffner R. Surgical treatment of postphlebitic syndrome with vein valve trans-plant. Am J Surg. 1982;144:221-224.
11. Maleti O, Lugli M. Neovalve construction in postthrombotic syndrome. J Vasc Surg. 2006;43:794-799.
12. K alra M, Gloviczki P, Noel AA et al. Subfascial endoscopic perforator vein surgery in patients with post-thrombotic venous insufficiency – is it justified? Vasc Endovascular Surg. 2002;36:41-50.
13. Masuda EM, Kistner RL. Long-term results of venous valve reconstruction: a four to twenty-one year follow-up. J Vasc Surg. 1994;19:391-403.
14. Perrin MR. Results of deep vein reconstruction. Vasc Surg. 1997;31:273-275.
15. Lugli M, Guerzoni S, Garofalo M, Smedile G, Maleti O. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49:156-62.
16. Lurie F, Kistner R, Perrin M, Raju S, Neglen P, Maleti O. Invasive treatment of deep venous disease. A UIP consensus. Int Angiol. 2010;29:199-204.
17. Maleti O, Lugli M, Perrin M. Syndrome post-thrombotique. EMC – Cardiologie 2013;8:1-12.