Overcoming the diagnostic challenges in deep venous obstruction: the imperative integration of morphological and hemodynamic testing in clinical practice
Nicos Labropoulos, PhD
Anisha Javvaji, BS
Renaissance School of Medicine at
Stony Brook University, Division
of Vascular and Endovascular
Surgery, Department of Surgery
ABSTRACT
Deep vein obstruction (DVO) in the lower limbs stems from a range of underlying causes, encompassing both thrombotic and nonthrombotic origins. In some cases, preexisting nonthrombotic obstructions may evolve into thrombotic ones, a phenomenon exemplified in rare conditions like iliac vein compression aplasia or hypoplasia. The complex nature of DVO leads to differences in its clinical presentation and hemodynamic impact. The diverse clinical manifestations of DVO, ranging from asymptomatic cases to venous ulcers, may present challenges in the evaluation of this condition. In this article, we address limitations of common diagnostic methods such as ultrasound, intravascular ultrasound, venography, computed tomography venography, and magnetic resonance venography for DVO. Furthermore, we emphasize the need for a combined morphological and hemodynamic testing approach, as relying solely on one method often provides an incomplete picture of the obstruction’s nature and symptom severity. Morphological testing focuses on visualizing the physical structure of the veins to identify any obstructions using factors such as stenosis, occlusions, collaterals, etc. Hemodynamic testing, on the other hand, examines the functional aspects of blood flow within the affected veins, providing information on blood pressure, velocity, and flow characteristics. By combining the 2 assessments, we can use an integrated approach to enhance diagnostic accuracy and develop personalized and efficacious treatment plans.
Introduction
Deep vein obstruction (DVO) associated with the lower limbs is common. The DVO pathophysiology is most often due to a previous episode of thrombosis, nonthrombotic causes, or the combination of the two. Nonthrombotic DVO or extraluminal venous obstruction is often attributed to venous compression.1 Additionally, congenital anomalies, characterized by structural irregularities present since birth, can also contribute to nonthrombotic DVO. The incidence of congenital abnormalities among patients with DVO is less than 10%, and nonthrombotic cases typically manifest during the second decade of life.2 A hemodynamically significant vein obstruction reduces blood flow, leading in some cases to ambulatory venous hypertension, which, in turn, causes the development of signs and symptoms related to chronic venous disease (CVD).
Postthrombotic obstruction arises from past episodes of deep venous thrombosis (DVT)
Postthrombotic obstruction is easier to diagnose and is more often associated with signs and symptoms. Nonthrombotic obstruction has a less clear association and is frequently asymptomatic.2 Typically, postthrombotic lesions are longer than nonthrombotic lesions and the wall is less compliant. This causes the outflow resistance in patients with postthrombotic lesions to be higher, so they are more likely to be symptomatic. Nonthrombotic lesions are usually less symptomatic and have tighter lesions if they are symptomatic.3
In some cases, DVO can result from preexisting nonthrombotic obstructions but subsequently some individuals develop DVT. The latter can be the cause to unravel the nonthrombotic obstruction as many patients prior to DVT have no symptoms. Those that could have been symptomatic prior to DVT get worse when they develop thrombosis. Only 2% to 3% of DVT cases are associated with iliac vein compression (IVC).2 An example of this case is IVC aplasia or hypoplasia, which are congenital abnormalities or are induced by a catheter insertion at younger ages that can also trigger thrombus formation. These conditions are rare and typically affect 0.5% of the general population.4 Such lesions may be asymptomatic and are incidentally found through imaging. They could also cause the development of recurring thrombosis of the lower extremities and pelvic veins. IVC aplasia or hypoplasia should be suspected in patients younger than 30 years who present with proximal DVT. The following images (Figure 1A and 1B) demonstrate how obstruction presents in patients with such abnormalities.
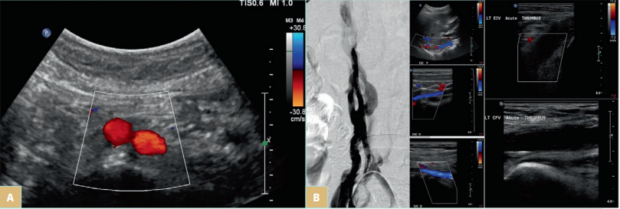
Figure 1. A) Iliac vein compression (IVC) aplasia in a young patient who presented with bilateral lower-limb edema. The infrarenal IVC and both common iliac veins were absent. The patient had no catheterization, and this is a case of congenital absence. In the image above, the aortic bifurcation is seen but the IVC is absent. The common iliac veins were absent too. There were many collateral veins in the subcutaneous space from the groin to the abdomen and many pelvic collaterals through both internal iliac veins. B) IVC hypoplasia in a young female who presented with deep venous thrombosis (DVT) from left calf to the common iliac veins. The venogram demonstrates the collateral veins around the hypoplastic IVC that has a diameter of 2-5 mm (first panel). The ultrasound images show the patent but hypoplastic IVC proximal, middle, and distal (center panel from top to bottom) and acute thrombosis in the left external iliac (top image on the third panel) and common femoral veins (bottom image).
Clinical presentation and hemodynamics of DVO
Patients with DVO have a wide range of clinical presentations, from being asymptomatic to venous claudication and ulceration. Many factors can affect clinical presentation, such as CVD from primary reflux, obesity, efficiency of the foot and calf muscle pumps, and other conditions. In regard to DVO alone, the severity and the extent of obstruction play a major role.3 Several papers have reported the clinical signs and symptoms of patients with DVO. Iliofemoral obstruction has the highest association with the development of signs and symptoms (Figure 2). However, it is not always the case as patients with infrainguinal obstruction may also have reflux and other factors that contribute to the severity of clinical presentation. Venous claudication is almost exclusively seen in patients with postthrombotic iliofemoral obstruction.5 It has also been reported in patients with nonthrombotic obstruction, but this, in our experience, is rare.
The significance of an iliac vein lesion is determined by its impact on blood flow. There may be a flow reduction across the lesion at rest or during physical activity. Collateral veins may often bypass the obstruction. If the collateral veins provide adequate drainage, the patients are asymptomatic. The collateral veins may be adequate at rest but not be enough during physical activity. Patients with DVO can develop signs and symptoms due to ambulatory venous hypertension from inadequate venous return. IVC is a prevalent finding in the general population. Unfortunately, there is no robust diagnostic criteria for defining hemodynamically significant obstruction. In fact, in a study in which 20 healthy individuals were tested for obstruction by venography, 80% (16 out of 20 of the volunteers) had at least 2 venographic signs indicative of IVC.6 This shows that diagnosis of true iliac vein obstruction is quite challenging. Clearly, the morphologic and hemodynamic changes seen in venography do not translate into disease severity, as all of them were normal individuals. Therefore, it is better to focus on treating the patient based on their symptoms. It is not clear why and when nonthrombotic obstruction leads to symptoms. It may be that with the obstruction getting worse over time, the venous wall becomes less compliant or, in a more obvious case, symptoms arise after development of ipsilateral thrombosis. Furthermore, nonthrombotic stenosis can occur in other areas and not just in the left common iliac vein (CIV). It has been described in the right CIV, in both external iliac veins (EIVs), and in the common femoral vein (CFV). Patients may present with more than one lesion or a combination of nonthrombotic and postthrombotic obstruction. It is important to diagnose all the areas and types of venous obstruction.
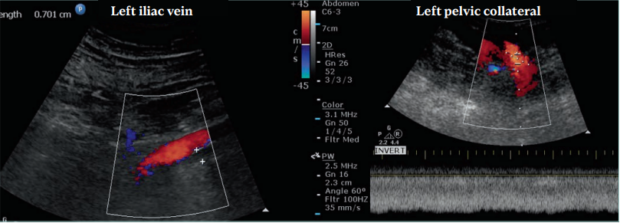
Figure 2. This patient had a chronic postthrombotic occlusion. The patient presented with edema, pain, and skin damage. The left external iliac vein (EIV) was compressed by the left external iliac artery and the patient developed ipsilateral iliofemoral deep venous thrombosis (DVT) leading to skin damage. The left EIV was occluded with the diameter measuring 7 mm. The vein had a focal stenosis prior to DVT but after it retracted throughout its length. The common femoral, femoral, and popliteal veins had partial recanalization with reflux. The ipsilateral common iliac vein (CIV) was patent, receiving flow from the left internal iliac vein. A large pelvic collateral vein (12 mm in diameter) is seen with nonphasic, high-velocity flow. No flow augmentation is seen during thigh compression. Other collateral veins were found connecting to the internal iliac vein.
Diagnosing DVO
There are many ways to diagnose DVO such as ultrasound, intravascular ultrasound (IVUS), venography, computed tomography venography (CTV), and magnetic resonance venography (MRV). Plethysmography has been seen in several reports, but nowadays is not so common in clinical practice. Vein pressures have been reported but not used routinely.
Typically, DVO can be evaluated through 2 distinct approaches: morphological testing and hemodynamic testing. Morphological testing focuses on visualizing the physical structure of the veins to identify any obstructions. Specific factors such as stenosis, occlusion, length, collaterals, and flow patterns are assessed. However, relying solely on morphological evaluation may lead to incomplete diagnoses or misdiagnoses due to potential discrepancies with real-life conditions. In contrast, hemodynamic testing examines the functional aspects of blood flow within the affected veins, encompassing information about blood pressure, velocity, and flow characteristics. Additionally, the assessment of positional differences and blood flow during rest and exercise is beneficial. Various methods, including plethysmography and measurement of pressure differences, can facilitate hemodynamic testing. Combining information from both types of testing allows health care professionals to perform a comprehensive assessment, facilitating the development of an appropriate treatment plan tailored to the individual patient’s condition. This integrated approach ensures a more accurate and holistic evaluation of DVO, enhancing patient care and outcomes.
The current method used routinely in first line for diagnosing DVO morphologically is ultrasound. It is cheap, readily available, and can give both morphologic and dynamic information. Direct and indirect criteria for diagnosing DVO with ultrasound are used, as follows:
Direct criteria
• Planimetric diameter stenosis.
• Peak vein velocity ratio >2.5.
• Luminal changes.
Indirect criteria
• Evaluation of flow patterns of the veins in the groin area and most often the common femoral vein.
› Nonphasic flow at rest and particularly during the Valsalva maneuver.
› Low or no velocity augmentation in CFV during thigh compression or dorsi/plantar flexion.
› Asymmetrical flow pattern between the left and right.
› Reversed flow in the ipsilateral internal iliac and deep external pudendal veins.
› Cephalad flow in the ipsilateral inferior epigastric vein.
• Presence of collateral veins.
• Difficulty in compressing CFV (high venous pressure).
Although these measures offer valuable information, they also have limitations. Planimetric evaluation relies on vein diameter and area measurements, which can be influenced by image quality, vessel shape irregularities, and operator variability. Noncircular or tortuous veins can also complicate precise measurements. The presence of collaterals, whereas suggestive of chronic obstruction, does not rule out an acute or recent DVO, and some patients may have naturally occurring collaterals. Flow patterns can be impacted by patient positioning and body movement, making it challenging to distinguish true flow alterations from artifacts when relying solely on morphological-based diagnostic tools.
Inflow veins are usually evaluated by ultrasound and venography. The evaluation of inflow veins is crucial for diagnosing DVO. Inflow veins can be evaluated by monitoring the flow velocity and flow patterns. It is very important to evaluate inflow veins before performing any procedures. They can help determine what type of procedure is required. If the inflow veins have little to no flow, stenting would not be the method of choice as the failure rate is high. Evaluating inflow veins is useful but still has some limitations, adding another challenge to diagnosing DVO. Flow rates during venography are not standardized, and values for ultrasound evaluation are not yet established. More work is needed to establish robust criteria for the inflow to improve the management of such patients.
Another challenge with using only morphologic testing such as ultrasound is that individuals with nonthrombotic obstruction have positional stenosis. All routine testing is done in supine position. Postural changes dramatically affect the cross-sectional area of the left CIV and the left renal vein and thus the degree of stenosis in women diagnosed with pelvic venous disorders (Figure 3A). Stenosis found in patients while supine often disappears when the position is changed to lying on the left side or to standing.7 Symptoms of venous obstruction are present and more pronounced during physical activity. This is important to consider because therapeutic decisions made when patients are in a supine position are more likely to be ineffective. We should identify patients with a fixed stenosis and appropriate symptoms (Figure 3B). Patients with postthrombotic obstructions typically do not experience positional stenosis. The obstruction is longer and most often there is intraluminal material. In few cases (work in progress) patients with nonthrombotic stenosis who develop DVT may have positional changes or the lesion can become fixed due to postthrombotic changes.
IVUS is very useful in making accurate diagnoses as it is inside the vein and offers 360-degree views. It is best to characterize the stenosis, wall, and intraluminal changes. It is also used to guide procedures and offer immediate results on the effect of interventions. In nonthrombotic patients, it was shown that a threshold of >61% of diameter stenosis by IVUS may better predict clinical improvement.8 Positioning of the patient can also be an issue in making an accurate diagnosis. However, performing the Valsalva maneuver and hydrating the patient well improves the diagnosis.
Axial imaging with CTV and MRV using appropriate protocols provides accurate imaging and offers great differential diagnosis. The large field of view, 3D reconstructions, and intravascular and extravascular images are advantages of these methods. MRV can also provide dynamic flow that can help in determining the hemodynamic patterns. Positioning, motion artifacts, metallic structures, and poor hydration can pose significant problems for accurate diagnosis. It has been demonstrated that placing the patient in a prone position may overcome the positional stenosis.9
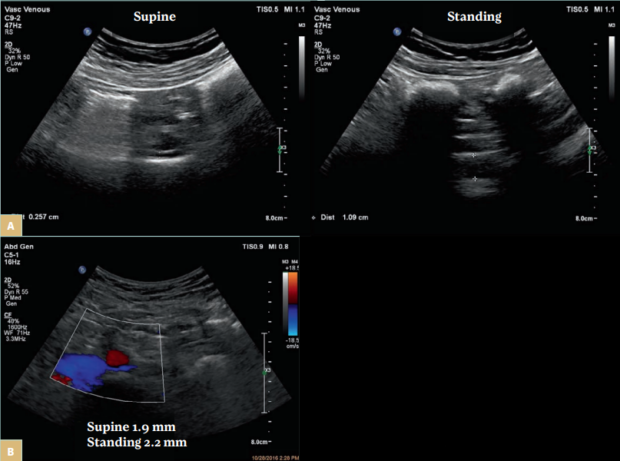
Figure 3. A) A female patient with tight stenosis in the left common iliac vein (CIV) in the supine position (left panel). The vein is compressed by the right common iliac artery over the fifth lumbar vertebra. The remaining lumen is 2.6 mm. In the standing position, the vein at the same location measured 10.9 mm (right panel). The contralateral CIV measured 12.4 mm. B) A female patient presenting with pelvic pain and fullness. The left CIV fixed stenosis as the diameter is similar in both the supine (1.9 mm) and standing positions (2.2 mm). The ipsilateral distal CIV measured 14.3 mm and the contralateral CIV, 12.2 mm. The ipsilateral internal iliac vein had reversed flow.
Venography is used most often in treatment. It offers both morphologic and dynamic information. It is a good method that provides a large field of view and flow patterns in the areas of obstruction, collaterals, and inflow veins. It’s not as good as IVUS for characterizing the severity of the obstruction and luminal changes, and it also cannot be used to visualize the wall and extraluminal structures. Thus, it is often used together with IVUS. Similarly with the other methods, venography is performed in the supine position and has issues with positional stenosis. Venography can be misleading as shown by van Vuren et al,6 and it can over- and underestimate the disease.
One of the ways we can overcome some of the limitations of morphologic testing alone is by combining the strengths of different methods. By integrating morphologic testing, we gain precise insights into the obstruction’s shape and dimensions. Simultaneously, employing hemodynamic tools empowers us to assess the obstruction’s impact on blood flow dynamics, completing a comprehensive picture of the condition.
In many patients, the symptoms become more apparent during exercise, and therefore hemodynamic evaluation before and after exercise provides valuable insights. In a study involving 50 patients with postthrombotic disease, venography and bilateral femoral vein pressure measurements were carried out. The severity of the obstruction was best evaluated by observing the pressure elevation and difference after exercise and the time required for the parameters to return to baseline.
Interestingly, after exercising, 12 out of the 50 patients showed pressure changes like those with normal iliac veins, indicating improved blood flow and less-severe obstructions. Their pressures returned to pre-exercise levels within 20 seconds. In this instance, phlebography played a morphological role, whereas femoral vein pressure measurements provided clinically significant information before and after exercise for postthrombotic iliac vein disease.10 The use of pressure measurements completes the picture and may provide better guidance for treating patients. The presence of a venous pressure gradient increases the confidence for performing an intervention. The absence of pressure gradient cannot always exclude the contribution of obstruction in the occurrence and severity of signs and symptoms.
These findings underscore that relying solely on venography would not be sufficient to accurately assess the severity of obstructions in patients with postthrombotic disease. A comprehensive evaluation is essential to gain a complete understanding of each patient’s condition. Different patients may exhibit varying degrees of blood flow improvement, which could influence treatment decisions. Some patients might require less aggressive interventions, whereas others may need more intensive treatments to manage their condition effectively. Moreover, using invasive pressure measurements as a stand-alone diagnostic tool may not always be indicative of a DVO diagnosis. Therefore, a hemodynamic diagnostic approach should not be used in isolation, and integrating both hemodynamic and morphologic evaluation ensures a more comprehensive and accurate assessment of thrombosis or obstruction. Patients with CVD symptoms and signs where both morphologic and hemodynamic assessment are associated with the clinical presentation are the best candidates for intervention. However, patients may still have signs and symptoms without hemodynamic changes. In absence of anything else, such patients may benefit from treatment, but this needs to be further studied.
Finally, current diagnostic tests need to be optimized and improved criteria need to be developed for DVO diagnosis. Since signs and symptoms are more apparent during physical activity, tests need to be modified at least in those patients where the contribution of obstruction is not clear. Together with advances in DVO diagnosis, the findings need to be considered in context with the clinical presentation, other contributing factors, and history of the patients.
Conclusion
To address challenges for DVO diagnosis and improve patient outcomes, it is imperative to adopt a more comprehensive diagnostic approach. Presently, the standard diagnostic method primarily relies on only morphologic testing; however, this approach may not provide a complete understanding of the obstruction and its characteristics. Thus, it is necessary to improve by incorporating both morphologic and hemodynamic tests when an obstruction is suspected. By combining these diagnostic modalities, clinicians can gain comprehensive insights into the nature of the obstruction, enabling them to develop more personalized and effective treatment plans. Further work is needed to develop more rigorous tests and robust criteria for optimizing the care of patients with DVO.

CORRESPONDING AUTHOR
Nicos Labropoulos, PhD
Division of Vascular and Endovascular
Surgery, Department of Surgery,
Health Sciences Center. Stony Brook,
NY, United States
email: nlabrop@yahoo.com
References
1. Zucker EJ, Ganguli S, Ghoshhajra BB, Gupta R, Prabhakar AM. Imaging of venous compression syndromes. Cardiovasc Diagn Ther. 2016;6(6):519-532.
2. Esposito A, Charisis N, Kantarovsky A, Uhl JF, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60:118-125.
3. Labropoulos N, Volteas N, Leon M, et al. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg. 1997;132:46-51.
4. Kim H, Labropoulos N, Blake AM, Desai K. Prevalence of inferior vena cava anomalies and their significance and impact in clinical practice. Eur J Vasc Endovasc Surg. 2022;64(4):388-394.
5.Tsouknidas I, Charisis N, Eklof B, Labropoulos N. Venous claudication: a scoping review of the pathophysiology and clinical importance. Eur J Vasc Endovasc Surg. 2022;64(5):535-543.
6. van Vuuren TMAJ, Kurstjens RLM, Wittens CHA van Laanen JHH, de Graaf R. Illusory angiographic signs of significant iliac vein compression in healthy volunteers. Eur J Vasc Endovasc Surg. 2018;56:874-879.
7. Krzanowski M, Partyka L, Drelicharz L, et al. Posture commonly and considerably modifies stenosis of left common iliac and left renal veins in women diagnosed with pelvic venous disorder. J Vasc Surg Venous Lymphat Disord. 2019;7(6):845-852.e2.
8. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6:48-56.
9. Behzadi AH, Khilnani NM, Zhang W, et al. Pelvic cardiovascular magnetic resonance venography: venous changes with patient position and hydration status. J Cardiovasc Magn Reson. 2019;21(1):3.
10. Albrechtsson U, Einarsson E, Eklöf B. Femoral vein pressure measurements for evaluation of venous function in patients with postthrombotic iliac veins. Cardiovasc Intervent Radiol. 1981;4(1):43-50.
