Pelvic vein incompetence: a review of diagnosis and treatment
Skåne University Hospital,
Malmö, Sweden
ABSTRACT
Pelvic vein incompetence is often associated with typical clinical signs of congestion as well as pelvic pain. This clinical entity is often underestimated and patients suffering from pain related to pelvic varicosities undergo a long and inconclusive diagnostic workup before the exact cause of symptoms is recognized. Besides the typical chronic pelvic pain, signs such as vulvar varicosities are not always present. Because of the wide variation of clinical and radiological presentations, there is a general consensus that diagnostic and therapeutic approaches should be patient-tailored.
To date, noninvasive diagnostic techniques including ultrasound, computed tomography, and magnetic resonance imaging have been used to identify patients who are candidates for treatment. Venous embolization is now accepted worldwide as the treatment of choice, because of its promising results in terms of clinical success and its limited invasiveness. This article reviews currently available diagnostic and therapeutic options.
INTRODUCTION
The important role of incompetent pelvic and ovarian veins in the etiology of pelvic congestion syndrome has emerged in recent decades thanks to the increasing number of reports dealing with this peculiar clinical condition.1-4 Over the years, many investigators have suggested that pelvic varicosities may be responsible for the otherwise unexplained symptoms5,6 that characterize pelvic congestion syndrome, and venographic studies in women following normal laparoscopy for chronic pelvic pain have revealed dilatation of the major pelvic veins and congestion in the ovarian plexuses and broad ligaments in more than 80% of cases.7 Furthermore, ovarian vein dilatation has been observed in up to 10% of asymptomatic women and up to 60% of them can develop pelvic congestion syndrome.1,8,9
Most patients with pelvic vein incompetence (PVI) have undergone a wide range of diagnostic examinations, but despite this, few receive a correct diagnosis because of limited appreciation of this condition among practicing physicians. Patients who are likely to have PVI often present with atypicalvaricose veins arising at the buttocks or upper posteromedial thigh and extending to the vulvar and perivulvar regions. Many cases are found incidentally during lower limb venous duplex for recurrent varicose veins. Perrin reported a 17% incidence of PVI in female patients with recurrence after surgical treatment of varicose veins.10
In recent years, advances in technology have led to the use of noninvasive imaging methods such as ultrasound (US), computed tomography (CT), and magnetic resonance venography (MRV), which can differentiate cases of primary PVI from alternative causes of pelvic pain and at the same time provide a plan for minimally invasive treatment options for this not uncommon condition.
Due to its encouraging results,4,11 percutaneous embolization is now accepted as first-choice treatment of symptomatic PVI.
This article reviews the imaging options for establishing diagnosis of PVI, and the emerging minimally invasive treatments.
ETIOLOGY
The precise etiology of PVI is poorly understood. The underlying mechanism is reflux of blood in the pelvic and/or ovarian veins. The primary defect is the absence of functioning valves, resulting in retrograde blood flow and eventual venous dilatation.12 Valves are absent from the orifices of the gonadal veins in 15% of women, and, in those where valves are present, they are incompetent in 40% on the left and 35% on the right.13
Furthermore, during pregnancy, the vascular capacity of the ovarian veins may increase 60-fold and remain this way for months after delivery.14 Moreover, dilated veins are more frequently observed with increased parity.4,14,15 Dilatation of ovarian veins causes valvular incompetence and retrograde venous flow.
Some cases of pelvic varicosities have been associated with mechanical compressive causes, such as uterine malposition causing kinking of the ovarian veins,16 and the nutcracker syndrome, where the left renal vein is compressed between the aorta and the superior mesenteric artery.3
The often associated worsening of congestion symptoms during intercourse, the higher prevalence in multiparous women, the positive therapeutic effects of hormonal substitution on symptoms17 as well as the higher concentration of sexual hormones in blood refluxing to the groin18 suggest that hormonal factors could play a crucial role in determining this peculiar clinical entity.
CLINICAL ASPECTS
Besides being a potential cause of symptomatic leg varicosities, PVI is often associated with a typical clinical pattern. Taylor12 in 1949 identified a tetrad of symptoms consisting of pelvic pain, dysmenorrhea, dyspareunia, and pelvic varicosities. The important role of incompetent pelvic veins is underlined by the fact that the intensity of pain is higher in patients with lower limb varicosities and PVI compared with those with isolated lower limb varicosities.19 The symptoms may be exacerbated by postural changes, walking, prolonged standing, or other activities that increase abdominal pressure, such as lifting. Urinary symptoms are also common.
PVI is also associated with a typical pattern of varicosities. Scultetus et al20 described three different clinical presentations:
• vulvar varicosities without signs of pelvic congestion;
• varicose veins at the medial and posterior aspect of the thigh, usually caused by incompetent ovarian veins;
• gluteal as well as vulvar varicosities which are often caused by reflux in the internal iliac veins.
Besides clinically manifest varicosities, tenderness may be elicited by deep palpation of the ovarian point, which, if associated with dyspareunia, may be 94% sensitive and 77% specific for pelvic congestion.21
IMAGING
Typical pelvic symptoms in combination with the distinctive clinical pattern of varicosities help to identify patients who need to undergo a further diagnostic workup. Since anatomical venous variations in the pelvis are common,22,23 it is important to know the anatomy ofthese vessels for treatment planning. Imaging is critical in the evaluation of pelvic varices, both to differentiate them from other conditions and also because pelvic varices may be secondary to serious underlying pathologies, such as inferior vena cava obstruction, portal hypertension, and vascular malformations.
Furthermore, in a large number of cases, incompetence is present in more than one of the pelvic veins.24 This underscores the importance of selectively examining the ovarian as well as the internal iliac venous system in every patient in order to identify adequately any existing reflux pattern.
Transabdominal and transvaginal US is minimally invasive and as such is the tool most often used in gynecological practice. The presence of dilated pelvic veins and retrograde flow on color duplex Doppler examination is predictive of PVI. Besides its limited cost and high reproducibility, US allows dynamic examination of the blood flow through tortuous pelvic veins in patients with PVI. Furthermore, US is not associated with exposure to radiation or to iodinated contrast agents and can identify primary abdominal disorders that can cause pelvic varicosities.
Although several authors2,14,15 consider that patients with clinical signs of PVI should first undergo transabdominal or transvaginal US, Park et al8 could identify pelvic varicocele in only 53% of a group of 139 patients using US, which underlines the importance of further diagnostic workup.
An accurate duplex US examination of the groin may also provide evidence of PVI. A typical ultrasound finding is that of refluxing epigastric, pubic, and pudendal veins entering the groin with the reflux coming from above the inguinal ligament and not from the saphenofemoral junction. This reflux pattern in combination with typical clinical signs yielded a positive predictive value of 74% in a series of 101 patients.25
CT and magnetic resonance venography (MRV) can be used as noninvasive methods to diagnose pelvic and ovarian varices. These appear as tortuous, dilated structures in the uterine adnexa or besides the ureter. The advantage of these techniques is their ability to provide information about any coexisting abdominal or pelvic disorder.
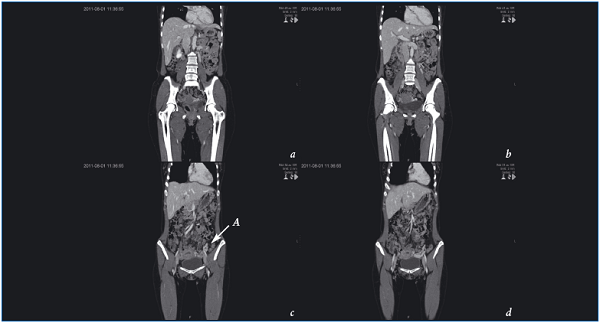
Figure 1. Computed tomography (a,b,c) shows incompetent left ovarian vein (A) filling dilated ovarian plexus (d).
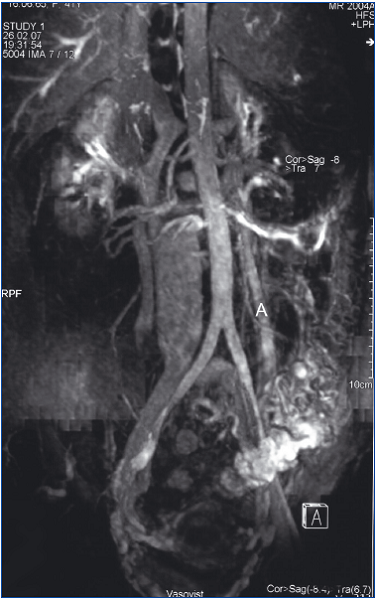
Figure 2. Magnetic resonance imaging signs of pelvic reflux from
the left renal vein to the left gonadal vein (A). Maximum intensity
projection image demonstrates dilated parauterine varices filled due
to passive reflux of contrast.
On CT the varicosities are isodense to other abdominal veins on post-contrast imaging (Figure 1), while on MRV (Figure 2) they show no signal on T1-weighted sequences and are hyperintense on T2, but can also be hypointense or isointense, depending on the velocity of blood flow.26 Gradient echo sequences show high signal intensity within the varices.
Contrast-enhanced MRV is likely to become the initial noninvasive investigation of choice in the diagnosis of PVI. It allows a complete examination of the pelvic anatomy because of its multiplanar imaging capability. Dynamic subtraction MR angiograms can provide an overview of the vessels and demonstrate abdominal vascular anatomy and vessel occlusions.27-29
Although the safety of MR procedures during pregnancy has not been definitively proven,30,31 the risk of exposing the developing fetus to any radiological diagnostic imaging technique that uses ionizing radiation is probably greater than the theoretical risk of MRV.32
Furthermore, the isotonic nature of gadolinium eliminates the risk of thrombosis associated with conventional iodinated contrast agents.33 The low risk of anaphylactic reactions inherent to extracellular paramagnetic agents is an additional factor contributing to the attractiveness of MRV. However, because of its low specificity MRV may underestimate venous disease.34 This is because conventional cross-sectional imaging studies are generally performed in the supine position in which ovarian and pelvic varices may not be as prominent.
In recent decades, catheter-directed venography (Figure 3a) has become more a “therapeutic” than a diagnostic tool in the management of patients with PVI.
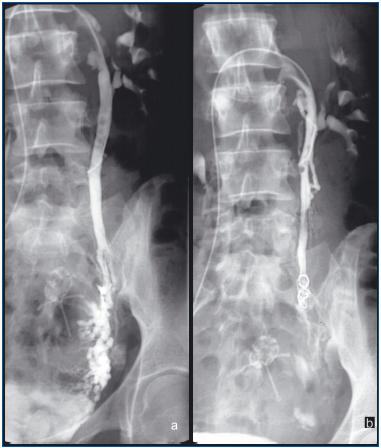
Figure 3. (Same patient as seen in Figure 2) Anteroposterior view
during left ovarian phlebography with patient in supine position
shows significant stagnation of contrast material in the dilated vein
(a). After embolization of the dilated left ovarian vein with 4
platinum coils (3 9×60 mm, 1 7×40 mm), no reflux was detected (b).
Although related mortality and morbidity are low, patient discomfort and costs make this an unattractive method of diagnosis. Furthermore, though effective, this method is invasive and also exposes patients to ionizing radiation of the pelvis. The latter is a particular concern because many of these women are of childbearing age. In addition, complications associated with the use of iodinated contrast material are reported to occur in 2% to 5% of patients.33
Venographic diagnostic criteria for PVI are ovarian vein diameter >10 mm or dilatation throughout the course of the vessel, uterine venous enlargement, congestion of the ovarian plexus, retrograde filling of the main stem of the internal iliac vein and at least one side branch (gluteal, ischiatic, or obturator veins), filling of the pelvic veins across the midline and/or filling of vulvovaginal and thigh varicosities.25,35 The advantage of selective catheter-directed venography, besides its excellent visualization of incompetent pelvic veins, is the option of performing interventional treatment if needed.
As CT and MRV need the use of contrast agents and are still costly, researchers’ attention has turned to alternative diagnostic methods that can guarantee high sensitivity and specificity as well as low costs. High sex hormone levels in venous blood sampled at lower leg varicosities have shown high sensitivity and specificity in identifying patients with phlebographic signs of PVI.18 Tests on a larger number of patients are needed before routine use of blood sampling to identify patients with typical symptoms who can directly undergo interventional catheter-directed venography.
TREATMENT
Since venous valves are found in only about 10% of internal iliac veins and their tributaries,36 there might be some degree of reflux in these veins even in healthy subjects. The clinical relevance of pelvic reflux not feeding varicose veins nor causing typical symptoms is still unclear.
Treatment failure is explained by the complex anatomy of the pelvic veins, which show a wide variation in terms of trunks, venous valves, duplications, and crossover connections.22,36 This aspect combined with the fact that reflux often affects more than one pelvic vein makes it difficult to identify and treat all patterns of reflux and, on the other hand, facilitates the development of alternative reflux pathways once one incompetent vein has been successfully treated.
Medical suppression of ovarian function and hysterectomy with or without bilateral salpingooophorectomy have been described as potential alternatives7,17,37 but are not widely used. Open surgical division of ovarian veins is infrequently performed due to the associated surgical trauma. Even laparoscopic division has been described.38
The continuous development of endovascular techniques offered a minimally invasive and very effective alternative to the above mentioned treatments. It was Edwards39 who described the first case of successful embolization of an incompetent ovarian vein. Endovascular treatment can be carried out with coils (Figure 3b), glue, foam, or a combination thereof through a jugular or femoral approach, with local anesthesia and as a day-case procedure.
In the last two decades there has been wide variation in clinical success rates (40% to 93%) with short-term follow-up in relatively small patient cohorts.1,4,11,40 This variation occurs because of the various definitions used for PVI and the use of different outcome measures. In particular, Creton11 reported a high rate of technical success and a clinical improvement in 80% of cases 3 years after embolization in a group of 24 patients.
Similar results were achieved by Kim et al4 in a larger cohort of patients (131) with a more aggressive approach consisting of embolization of all incompetent vessels throughout the ovarian and internal iliac venous system. In the eyes of Kim et al, this was necessary in order to eliminate all reflux pathways and to prevent recurrence.
Although endovascular treatment is minimally invasive, complications such as coil migration and local thrombophlebitis have been observed.
Due to the spread of endovascular techniques, the number of reports is constantly increasing and clinical trials have already been started in order to assess their long-term results using different sclerosing agents. Radiofrequency- and laser-based approaches have also been taken in consideration.
CONCLUSIONS
PVI is an often underestimated clinical entity that can be extremely debilitating. Its identification is based on the presence of typical symptoms and reflux patterns at dynamic diagnostic examinations. The diagnostic and therapeutic approach to PVI must be tailored to the individual patient’s needs and must take into account the severity of symptoms. Recent experience shows encouraging technical and clinical success rates for selective embolization of incompetent veins. However, there is still a need for studies to address the long-term outcome and to clarify which patient populations benefit from which treatment approaches.

REFERENCES
1. Venbrux AC, Chang AH, Kim HS, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Interv Radiol. 2002;13:171-178.
2. Maleux G, Stockx L, Wilms G, et al. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol. 2000;11:859-864.
3. Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001;34:812-819.
4. Kim HS, Malhotra AD, Rowe PC, et al. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiol. 2006;17:289-297.
5. Topolanski-Sierra R. Pelvic phlebography. Am J Obstet Gynecol. 1958;76:44-52.
6. Tarazov PG, Prozorovskij KV, Ryzhkov VK. Pelvic pain syndrome caused by ovarian varices. Treatment by transcatheter embolization. Acta Radiol. 1997;38:1023-1025.
7. Beard RW, Highman JH, Pearce S, et al. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2:946-949.
8. Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182:683-688.
9. Ganeshan A, Upponi S, Hon LQ, et al. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol. 2007;30:1105-1111.
10. Perrin MR, Labropoulos N, Leon LR Jr. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43:327-334; discussion 334.
11. Creton D, Hennequin L, Kohler F, et al. Embolisation of symptomatic pelvic veins in women presenting with nonsaphenous varicose veins of pelvic origin – three-year follow-up. Eur J Vasc Endovasc Surg. 2007;34:112-117.
12. Taylor HC, Jr. Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; etiology and therapy. Am J Obstet Gynecol. 1949;57:654-668.
13. Kaufman JA, Waltman AC, Rivitz SM, et al. Anatomical observations on the renal veins and inferior vena cava at magnetic resonance angiography. Cardiovasc Intervent Radiol. 1995;18:153- 157.
14. Hodgkinson CP. Physiology of the ovarian veins during pregnancy. Obstet Gynecol. 1953;1:26-37.
15. Adams J, Reginald PW, Franks S, et al. Uterine size and endometrial thickness and the significance of cystic ovaries in women with pelvic pain due to congestion. Br J Obstet Gynaecol. 1990;97:583-587.
16. Giacchetto C, Catizone F, Cotroneo GB, et al. Radiologic anatomy of the genital venous system in female patients with varicocele. Surg Gynecol Obstet. 1989;169:403-407.
17. Farquhar CM, Rogers V, Franks S, et al. A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. Br J Obstet Gynaecol. 1989;96:1153-1162.
18. Asciutto G, Mumme A, Asciutto KC, et al. Oestradiol levels in varicose vein blood of patients with and without pelvic vein incompetence (PVI): diagnostic implications. Eur J Vasc Endovasc Surg. 2010;40:117-121.
19. Asciutto G, Mumme A, Asciutto KC, et al. Pelvic vein incompetence influences pain levels in patients with lower limb varicosity. Phlebology. 2010;25:179-183.
20. Scultetus AH, Villavicencio JL, Gillespie DL, et al. The pelvic venous syndromes: analysis of our experience with 57 patients. J Vasc Surg. 2002;36:881-888.
21. Beard RW, Reginald PW, Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95:153-161.
22. Lechter A, Lopez G, Martinez C, et al. Anatomy of the gonadal veins: a reappraisal. Surgery. 1991;109:735-739.
23.Wishahi MM. Detailed anatomy of the internal spermatic vein and the ovarian vein. Human cadaver study and operative spermatic venography: clinical aspects. J Urol. 1991;145:780-784.
24. Asciutto G, Asciutto KC, Mumme A, et al. Pelvic venous incompetence: reflux patterns and treatment results. Eur J Vasc Endovasc Surg. 2009;38:381- 386.
25. Geier B, Barbera L, Mumme A, et al. Reflux patterns in the ovarian and hypogastric veins in patients with varicose veins and signs of pelvic venous incompetence. Chir Ital. 2007;59:481-488.
26. Coakley FV, Varghese SL, Hricak H. CT and MRI of pelvic varices in women. J Comput Assist Tomogr. 1999;23:429- 434.
27. Dohke M, Watanabe Y, Okumura A, et al. Comprehensive MR imaging of acute gynecologic diseases. Radiographics. 2000;20:1551-1566.
28. Lebowitz JA, Rofsky NM, Krinsky GA, et al. Gadolinium-enhanced body MR venography with subtraction technique. AJR Am J Roentgenol. 1997;169:755-758.
29. Watanabe Y, Dohke M, Okumura A, et al. Dynamic subtraction contrastenhanced MR angiography: technique, clinical applications, and pitfalls. Radiographics. 2000;20:135-52; discussion 52-53.
30. Amin RS, Nikolaidis P, Kawashima A, et al. Normal anatomy of the fetus at MR imaging. Radiographics. 1999;19 Spec No:S201-S214.
31. Levine D, Barnes PD, Edelman RR. Obstetric MR imaging. Radiology. 1999;211:609-617.
32. Murphy WD, Feiglin DH, Cisar CC, et al. Magnetic resonance imaging of a third trimester abdominal pregnancy. Magn Reson Imaging. 1990;8:657-659.
33. Bettmann MA, Robbins A, Braun SD, et al. Contrast venography of the leg: diagnostic efficacy, tolerance, and complication rates with ionic and nonionic contrast media. Radiology. 1987;165:113-116.
34. Asciutto G, Mumme A, Marpe B, et al. MR venography in the detection of pelvic venous congestion. Eur J Vasc Endovasc Surg. 2008;36:491-496.
35. Kennedy A, Hemingway A. Radiology of ovarian varices. Br J Hosp Med. 1990;44:38-43.
36. LePage PA, Villavicencio JL, Gomez ER, et al. The valvular anatomy of the iliac venous system and its clinical implications. J Vasc Surg. 1991;14:678- 683.
37. Soysal ME, Soysal S, Vicdan K, et al. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod. 2001;16:931-939.
38. Gettman MT, Lotan Y, Cadeddu J. Laparoscopic treatment of ovarian vein syndrome. JSLS. 2003;7:257-260.
39. Edwards RD, Robertson IR, MacLean AB, et al. Case report: pelvic pain syndrome—successful treatment of a case by ovarian vein embolization. Clin Radiol. 1993;47:429-431.
40. Cordts PR, Eclavea A, Buckley PJ, et al. Pelvic congestion syndrome: early clinical results after transcatheter ovarian vein embolization. J Vasc Surg. 1998;28:862-868.
