Phlebolymphedema: an up-to-date review
Sebastian CIFUENTES,2 MD;
Valentin FIGUEROA,2 MD;
Emelyn Van UDEN,2 MD;
Javier A. BRAVO,2 MD;
Miguel AMORE,3 MD;
Jairo RAMÍREZ,1 MD, FACS
1Vascular Surgery Department, Fundación
Santa Fe de Bogotá University Hospital;
Universidad de los Andes Medical School.
Bogotá, Colombia;
2Universidad de los Andes Medical
School, Fundación Santa Fe de Bogotá
University Hospital. Bogotá, Colombia;
3Vascular Surgery Department, Fundación
Favaloro, Buenos Aires, Argentina
Abstract
Phlebolymphedema is a vascular condition in which there is damage of the venous and lymphatic systems in the lower limbs. It is secondary to chronic venous insufficiency, which results in venous hypertension causing fluid leakage into the interstitial space. Under normal conditions, this fluid would be drawn away by the lymphatic system. However, when the capacity of the lymphatic system is exceeded over time, the lymphatic vessels get damaged and the interstitial protein-rich fluid cannot be drained. Current information on the most effective way to diagnose and treat this pathology is incomplete and inconclusive. Nowadays, the diagnosis is based on clinical examination, duplex ultrasound to assess the venous component of the disease, and lymphoscintigraphy to evaluate the lymphatic damage. The treatment commonly consists of complete decongestive therapy and manual lymph drainage. Despite these being the most used management methods, new modalities of diagnosis and treatment are emerging and will be discussed in this article. The purpose of this review is to increase physician knowledge about the epidemiologic aspects, physiopathology, and management of phlebolymphedema. We intend to raise awareness of this disease, which has been underdiagnosed and poorly treated, and to show the need for new studies that compare treatment and diagnostic methods to establish a consensus about how to approach this disease.
Introduction
Phlebolymphedema is a pathology with damage of the venous and lymphatic systems in the lower limbs. Chronic venous insufficiency (CVI) promotes venous hypertension and fluid leakage that under normal conditions would be drained through the lymphatics; however, in this disease, it cannot be cleared away. This article includes the definition, epidemiology, pathophysiology, classification, diagnosis, and treatment of phlebolymphedema based on up-to-date evidence. This narrative review aims to increase physician knowledge and awareness of this commonly neglected disease.
Methods
We used the MeSH terms “Phlebolymphedema,” “Chronic Venous Insufficiency,” “Phlebolymphedema AND Chronic Venous Insufficiency” and “Lymphedema” to search in PubMed, Google Scholar, and Embase.
Inclusion criteria:
1. “Phlebolymphedema” as a keyword.
2. “Chronic Venous Insufficiency” with “Lymphatic system” and/or “Lymphedema” in the abstract or as a keyword.
3. Articles published in the last 10 years.
Exclusion criteria:
1. Articles not written in English or French.
2. Case reports, case series.
In total, 20 691 articles were found, out of which 53 met the inclusion criteria. To ensure the quality of the article, the Scale for the Assessment of Narrative Review Articles (SANRA)1 was followed.
Definition
Phlebolymphedema consists in damage of the venous and lymphatic systems, resulting in limb edema. These systems play complementary roles in fluid return from the extremities; hence, if one is overloaded, the other one compensates as long as it can. When one fails (eg, in CVI or lymphedema), their interdependence leads to overloading of the other, leading to a dual system failure, known as phlebolymphedema.2,3
Epidemiology
Phlebolymphedema is an underdiagnosed condition, and its epidemiology is not clear.4 A study in Poland in 2003 calculated the incidence of phlebolymphedema as 10%, in 40 095 patients with CVI.5 Moreover, Dean et al showed that out of 440 patients with lymphedema, 41.8% of cases were caused by phlebolymphedema, followed by cancer in 33.9% and lipedema in 11.8%.6 The lymphatics play a major role in any kind of edema,2,7,8 with >300 million people suffering from edema worldwide.4 Szyber et al reported that there were approximately 6 million cases of lower-limb lymphedema, and 60% of them had CVI.9 Conversely, a more recent study showed that out of 26 902 patients with lymphedema, 10.4% presented phlebolymphedema.10
Phlebolymphedema is the most important cause of secondary lower-limb lymphedema in industrialized countries. However, the main etiology of lymphedema worldwide is filariasis; a parasitic infection caused by Wuchereria bancrofti. It is the second cause of chronic disability worldwide, affecting around 120 million people, 15 million with severe illness.11 Although this infection is eradicated in the United States (US), it is endemic in tropical and subtropical developing countries.12
Pathophysiology
The physiology of fluid movement through the body has three major components, the venous, arterial, and lymphatic systems. Normally, the hydrostatic pressure of the precapillary end of the arteriole pulls the fluid out to the interstitial space, and the oncotic pressure at the venule site takes it back to the circulation. Ten percent of the ultrafiltrate is reabsorbed by the lymphatics, which capture, drain, filter, and concentrate this protein-rich fluid in the lymph nodes and take it to the circulation.4 Lymphatic flow varies widely according to cardiovascular hemodynamics in exercise or resting state. The lymphatics have a large capacity and usually work at about 10% of their capacity in a healthy individual.13,14
The important function of lymphatic drainage consists of removing proteins, macromolecules, cytokines, and blood elements from the interstitium. Then, the lymph gets concentrated and filtered in the lymphatic nodes. Lymph’s flow and impulse are possible owing to mechanisms like the lymphatic pumps, driven by external factors such as muscular contraction, arterial pulsations or stretch bandaging, and internal mechanisms like the lymphangions. These are microscopic one-way bicuspid-valve micropumps, located from the capillary throughout the whole lymphatic system. Lymphangions have their own rhythmic contractility with 1 to 30 contractions per minute preventing fluid backflow.4
On the other hand, when there is alteration of the venous return mechanisms, CVI can occur. This might be attributable to age-related loss of valvular competence, surgery, or deep venous thrombosis (DVT). The severely increased venous pressure produces massive hyperemia and accumulation of interstitial fluid that must be drained by the lymphatics. DVT produces a proinflammatory state that leads to vessel fibrosis and long-term destruction of the valve leaflets. The postphlebitic syndrome is characterized by both valvular incompetence and fibrosis of the vessels, resulting in long-term venous hypertension.15
In phlebolymphedema, the chronic interstitial accumulation of proteins and proinflammatory cytokines such as transforming growth factor beta (TGF-ß), tumor necrosis factor alpha (TNF-α), immunoglobulins, and macromolecules with a size <1μm, lead to tissue fibrosis and damage of the skin, promoting the entrance of bacteria capable of generating severe cellulitis episodes.16 Another important function of the lymphatic system is the control of local immune responses. Phlebolymphedema may lead to a particular clinical condition of a locally altered immune response: an immunocompromised district,17 a skin zone previously damaged by events such as chronic lymphedema, herpes zoster infections, radiation, burns, and trauma that developed unusual immune behavior.18 These areas remain vulnerable to frequent opportunistic infections, tumors, and immunity-related disorders.19
The weakened immune control predisposes patients with phlebolymphedema to suffer from recurrent bacterial and fungal infections (eg, erysipelas and tinea pedis respectively) and to malignancy of the chronic venous ulcers, with squamous cell carcinoma as the most common type.20 Even a multicentric neoplasm like lymphoma could display preferential localization on a chronic venous ulcer.21 Moreover, recurrent infections lead to increased scarring and trauma of the vessels, causing further damage and resulting in a worsening lymphedema.4
Classification
This disease is classified according to the etiology; primary phlebolymphedema usually presents at birth or during early ages,17 is caused by congenital malformations like deepvein dysplasia, lymphatic aplasia or hypoplasia, or defect of both the venous and lymphatic systems, such as Klippel- Trenaunay syndrome (KTS), characterized by hemolymphatic malformations,22,23 capillary abnormalities, and hemorrhagic lymphangiectasia. It might be accompanied by adipose or osseous tissue overgrowth, becoming part of the PIK3CA-related overgrowth syndromes (PROS). The proportion of patients that present lymphedema is low, and is usually mistaken for overgrowth.24,25 Primary pure lymphatic malformations represent less than 30% of the causes, and approximately 28 mutations have been described, with a familial component.26
On the other hand, secondary phlebolymphedema is acquired, mostly as a result of CVI but also because of lymphatic injury in vein stripping (saphenectomy), vein-harvesting procedures for coronary bypass, vascular or orthopedic surgery (Figures 1 and 2), or penetrating trauma.17,27 All these causes can lead to chronic lymphatic insufficiency (CLI).28 Therefore, secondary phlebolymphedema is limited to the affected portion of the limb and usually takes a long time to debut, whereas primary phlebolymphedema has a diffuse presentation along the whole territory of the truncular malformation.
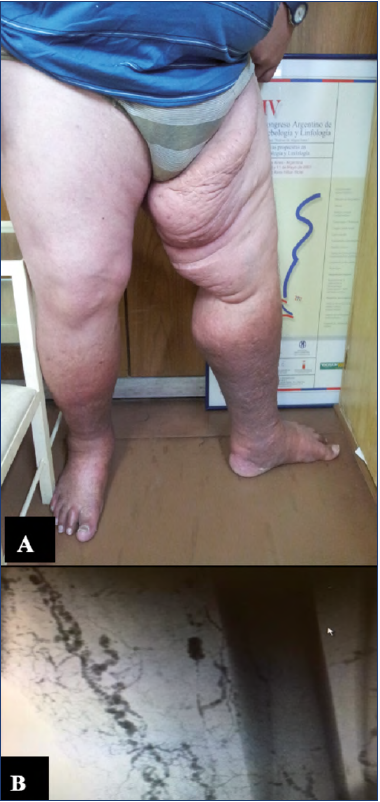
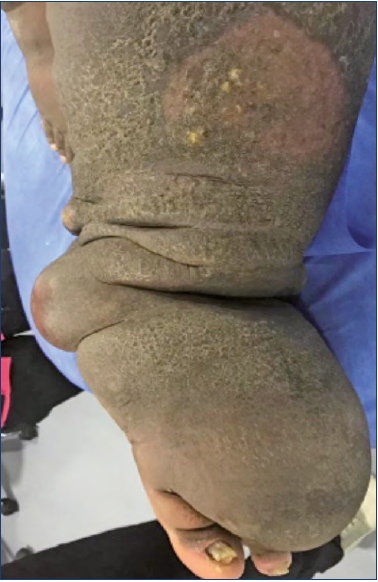
Figure 1. 52-year-old male patient with morbid obesity and chronic venous insufficiency (CVI), with previous history of greater saphenous vein stripping. He has a direct lesion in the canalicular and nodal lymphatics.
A) Predominant thigh lymphedema secondary to the procedure.
B) Contrast lymphography shows ectasia and ruptured lymphatic vessels.
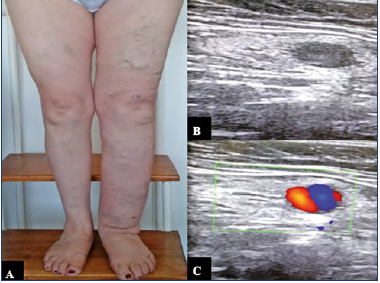
Figure 2. 61-year-old female patient with prior history of great saphenous vein stripping, three months after the procedure the patient started showing clinical signs of lymphedema.
A) Phlebolymphedema as a consequence of intranodal venous hypertension.
B,C) Duplex ultrasound that shows ectasia of the subcapsular sinus and intranodal venous hypertension.
Diagnosis
The diagnosis of phlebolymphedema is based on a detailed clinical history and physical examination, looking for signs of both venous and lymphatic malfunction.
Stasis dermatitis
Venous hypertension transmits hydrostatic pressure to the dermal capillaries, increasing their permeability and leading to fibrinogen leakage, producing ulcers and stasis dermatitis.29 Patients report pruritus of insidious onset, followed by a reddish-brown skin discoloration and hyperpigmentation due to the hemosiderin deposits after the extravasation of erythrocytes (hemosiderosis). It often affects the medial ankle but can extend circumferentially.4
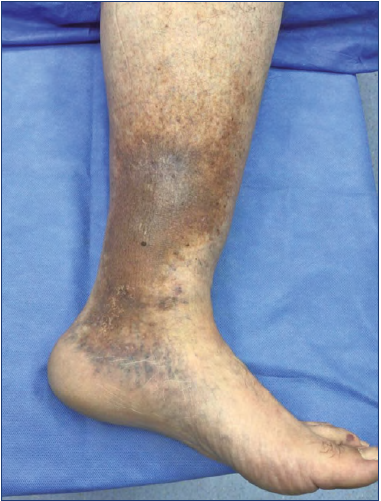
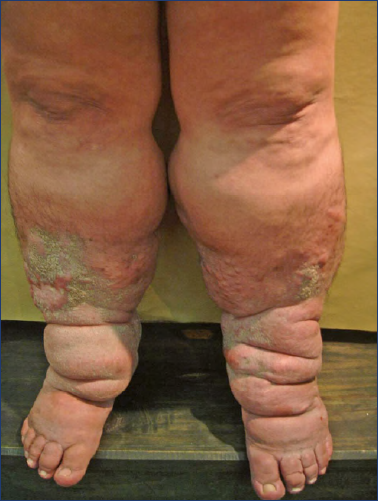
Figure 3. Lipodermatosclerosis. C4 patient (according to CEAP classification [Clinical, Etiologic, Anatomic Pathophysiologic]), with reddish-brown skin discoloration and hyperpigmentation.
Lipodermatosclerosis
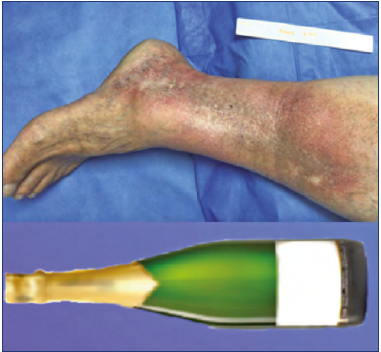
Lipodermatosclerosis (LDS) corresponds to skin induration, fibrosis, and hyperpigmentation caused by panniculitis (Figures 3 and 4),30 which may give the leg the “inverted champagne bottle” sign (Figure 5).4 Up to 70% of LDS is associated with CVI. Initially, it can mimic cellulitis; however, biopsies would show fibrosis on the dermal layers and thick capillaries surrounded by fibrin and siderophages, but further invasive diagnosis is discouraged because of tissue fragility. Localized patches of atrophy and thickened skin with papillomatosis can also be found.31
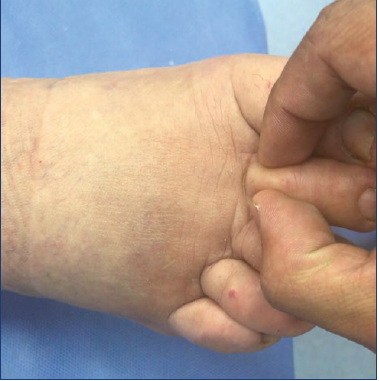
Figure 6. Kaposi-Stemmer sign. Skin of the patient’s toes cannot be pinched due to the high protein accumulation.
Kaposi-Stemmer Sign
The Kaposi-Stemmer Sign is pathognomonic of lymphedema and occurs due to the high content of proteins in the interstitium, preventing fluid redistribution. It is positive when it is not possible to pinch the skin fold, typically in the second toe (Figure 6).
‘‘Sausage Toes’’
The dorsum of the foot is classically involved in lymphedema. Patients may develop large, swollen, sausage-appearing toes (Figure 7).
Lymphedema rubra
Lymphedema rubra is caused by inflammatory hyperemia, given the proinflammatory changes from protein-rich fluid, and characterized by a blanching redness of affected skin that can mimic cellulitis.4 However, it has no signs of infection or response to antibiotics.
After the clinical assessment, the physician should identify modifiable risk factors that can contribute to the edema, like some systemic drugs (nonsteroidal anti-inflammatories, antihypertensives, calcium channel blockers, beta blockers, clonidine, hydralazine, and corticosteroids among others), myxedema, nephrotic syndrome, congestive heart failure, cirrhosis, etc. Additionally, obesity should be treated because it increases abdominal venous pressure, transmitted to the lower-limb veins and worsening CVI.4,32
Imaging tools can be used to confirm the disease and plan an efficient treatment. Duplex ultrasonography (DUS) is the test of choice to confirm CVI, identifying reflux and occlusions.3 In primary phlebolymphedema, plethysmographic studies are required to identify the congenital venous malformations,33 and computed tomography or magnetic resonance venography can be useful to identify iliofemoral occlusions. On the other hand, to assess lymphatic dysfunction, more options are available.
Lymphography
Lymphography consists of intradermally injecting a patent blue dye that outlines the superficial lymphatics for cannulation with a needle under an operating microscope and infusion of an oil soluble iodinated contrast medium for visual lymphography.34 Due to the technical difficulty and the morbidity associated with the contrast agent, the original technique is rarely used.17
Duplex ultrasound
DUS is a noninvasive, simple, and widely available tool. It provides indirect signs of severity of the affection based on the degree of thickness and echogenicity of the tissues, and information about the etiology of the edema characterizing the tissue layers (Figure 8).35 It is useful to determine response to therapy by comparing the thickness of tissues before and after interventions. Unfortunately, there is a lack of studies comparing DUS to the gold-standard diagnostic test for lymphedema, lymphoscintigraphy (LSG).17
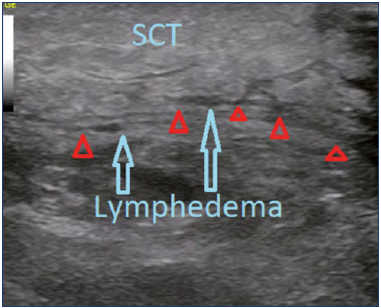
Figure 8. Lower-limb lymphedema Doppler ultrasound. Subcutaneous cellular tissue (SCT). Red triangles show the fascia, blue arrows show the areas with lymphedema.
Lymphoscintigraphy
First described in 1953 by Sherman and Ter-Pogossian,36 LSG is the examination of choice for functional assessment of lymphedema, as recommended by the American Venous Forum guidelines.17 However, the International Society of Lymphology Consensus emphasizes that the diagnosis of lymphedema is clinical, and LSG is only required when there is doubt about the lymphatic compromise.37 It consists of intradermal injection of radionuclides in the interdigital space, with examination of the lymphatic transport of the labeled substance. LSG provides qualitative information like presence and caliber of the lymphatic vessels, lymph nodes, collateral networks, and delay in the radionuclide uptake,38 and quantitative interpretation measures such as radionuclide transit and clearance time.
Despite the usefulness of this tool, there is no standardization for the type of radionuclide, dosage, and injection site, and it requires long waiting times.17 Also, LSG can be negative in early stages of the disease, and give false negative results in lymphedema with dynamic insufficiency (impaired fluid drainage due to exceeded lymphatics maximum transport capacity).4 LSG assesses the transport capacity of the vessels; in phlebolymphedema, the whole lymphatic system is overwhelmed; therefore, the transport capacity will not be directly affected.39
Computed tomography and magnetic resonance imaging
Providing direct and indirect signs of lymphatic dysfunction, computed tomography (CT) and magnetic resonance imaging (MRI) are useful for surveillance. Non-contrast MRI lymphography can identify the location of the edema, seen as subcutaneous soft tissue fibrosis and fluid accumulation, exhibiting a honeycomb distribution, pathognomonic of lymphedema.17,40 According to MRI lymphography findings, lymphedema can be classified as aplasia, hypoplasia, and hyperplasia.40 Enlarged lymphatic nodules present in neoplasias are more precisely shown with MRI than with LSG.41
Newer lymphatic imaging modalities
The injection of fluorescent intradermal contrast agents is a radiation-free alternative to visualize the lymphatics. Fluorescein sodium (excited by visible light) and indocyanine green (ICG; excited by near-infrared fluorescence light [NIRF]) are commonly used contrast agents with immediate uptake.17 Visible light has a wavelength between 300 nm and 760 nm, being easily absorbed by primary tissue constituents, such as blood, water, and melanin, allowing visualization of subepidermal lymphatics in contexts such as microsurgery.42,43
On the other hand, NIRF has a wavelength of 780 nm to 900 nm, penetrating about 3 cm under the tissues with no confounding autofluorescence. NIRF with ICG is useful in peripheral lymphatics with high and immediate sensitivity.44 C5 and C6 patients typically exhibit a diffuse dermic reflux pattern in the distal portion of the affected limb, which correlates with the expected signs of CVI and not with the classic lymphedema. Probably, the deep lymphatic circulation compensates the superficial lymphatic hypertension produced by venous hypertension. (Figure 9).
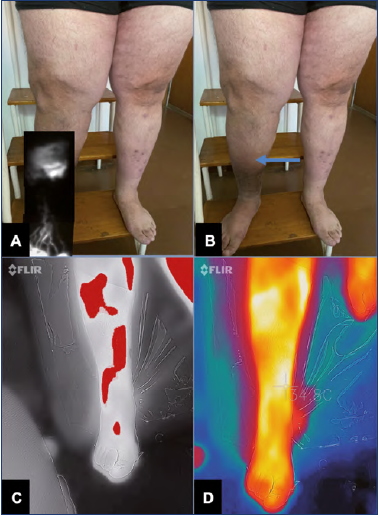
Figure 9. 48-year-old patient with chronic venous insufficiency (CVI).
A) Indocyanine green (ICG) fluorescence lymphography, which shows diffuse dermic reflux pattern in the zone labeled with the arrow in B with normal linear pattern in the distal portion.
B) No visible clinical signs of lymphedema, only trophic skin changes secondary to CVI.
C,D) Thermography of phlebolymphedema indicating greater inflammation in the zones of lymphatic damage, comparable with the findings of A.
A normal NIRF image shows well-defined linear lymphatics, whereas, in disease, it exhibits tortuous and dilated lymphatics, dermal backflow, and extravasation (Figure 9A). NIRF also shows the lymphangion function; contractile frequencies of 0.4 ± 0.3 propulsions/min in legs of healthy adults are considered normal, whereas lymphedema shows contractile frequencies of 0.2 ± 0.2 propulsions/min in legs.45
Treatment
There are no treatment guidelines or consensus for phlebolymphedema. If possible, the treatment should be etiology specific, eg, manual drainage, neutralization of proinflammatory cytokines, surgical restoration of the lymphatic drainage through lymphovenous anastomosis,17 or correction of a malformation.
In secondary phlebolymphedema, especially due to CVI, both problems have to be addressed. Treating one system prevents the other from continuing to fail. Occluded veins can be permeated; however, in postthrombotic syndrome, venous reflux without occlusion predominates. In this case, foam sclerotherapy or laser ablation of refluxing segments can be considered.3
Once the venous problem is treated, lymphedema must be handled, aiming to redistribute the proteins and the fluid to the proximal zones of the limb, where reabsorption can take place via the remaining functional lymphatics. The use of diuretics in these patients is contraindicated, as it increases the concentration of proteins and macromolecules in the interstitial space, producing more severe tissue damage.4 Furthermore, compression therapy is effective; however, it should not be used alone, as water remotion will keep the proteins more concentrated and eventually increase water retention when the compression is released.4
Some specialists consider the standard of care to be complete decongestive therapy (CDT).16 CDT has an acute phase to reduce the lymphedema, and a lifelong maintenance phase to prevent recurrence.4 It is based on manual lymph drainage (MLD) by specialized therapists, with gentle limb massages that push the fluid to the competent lymphatic zones. This strategy should be accompanied by skin care and hydration to prevent infections, and careful ambulation to prevent contact lesions. Exercise programs and short-stretch compression bandaging should also be considered, ensuring low pressures in the limb while resting and high pressures as soon as the contraction begins.4 Due to high cost and low availability, MLD has been replaced with new treatment modalities such as pneumatic intermittent compression (PIC).
A study conducted in Poland in 2015 in phlebolymphedema patients compared MLD performed in rehabilitation units versus PIC at home with 120 mm Hg for 45 minutes daily. Both interventions were applied for 4 weeks. The results showed that PIC was more effective, with marked diminishment of limb diameters and improved mobility.46 Additionally, long-term safety and efficacy of PIC was evaluated in patients with chronic lymphedema who were treated for 3 years with an 8-chamber sleeve at 120 mm Hg of pressure applied 50 minutes per day. Results were satisfying and no complications, such as genital edema, were found.47
PIC is effective, though whereas this type of compression is suitable for patients with a long history of phlebolymphedema with high presence of fibrotic tissue, it is not as effective for those with a predominant vein component; such patients have better results with lower-pressure compression. Also, the adequate pressure remains to be established. A study in 2015 including 81 patients with phlebolymphedema found that after 4 weeks, patients treated with multilayer bandaging combined with a pressure of 120 mm Hg showed significant improvement compared with patients that received bandaging alone or 60 mm Hg pressures.48
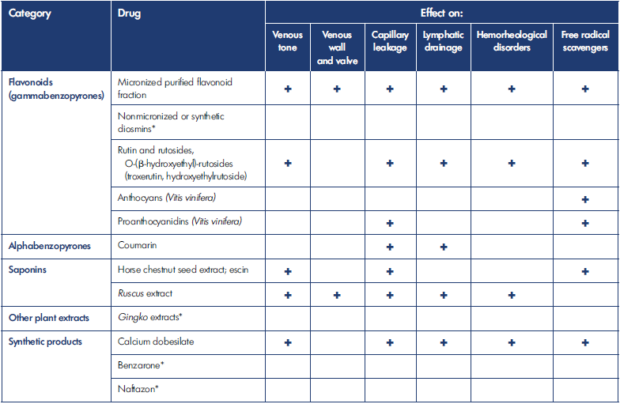
*No data available
Table I. Evidence-based modes of action of the main vasoactive drugs.
After reference 57: European Venous Forum, International Union of Angiology, Cardiovascular Disease Educational and Research Trust (UK), Union Internationale de Phlébologie; Nicolaides et al, eds. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181-254. Reprinted by permission of Edizioni Minerva Medica.
Olszewski recommends pressures ranging from 80 to 150 mm Hg for 45 to 60 minutes per day,49 because the physiologic hydrostatic lymphatic pressure in the lower limbs is near 12 mm Hg, and the pressure exerted externally toward the skin is 40 mm Hg. Only pressures greater than this value will be enough to stimulate lymph drainage. PIC significantly reduces the costs of treatment, occupational therapy, sequela, and the incidence of cellulitis in up to 75% of patients, as compared with conservative management and hospitalizations due to cellulitis.50-52
At the same time, studies have strongly demonstrated that patients with phlebolymphedema secondary to CVI may benefit from venoactive drug therapy (VAD). A number of VADs have been shown to increase lymphatic drainage in animal models (Table I).53-57 Micronized purified flavonoid fraction (MPFF), an oral VAD (composed of 90% diosmin and 10% active flavonoids), is one of the drugs for which we have more evidence worldwide.
A pilot study on the evolution of microlymphatic parameters in patients with severe CVI showed an increase in the number of functional lymphatic capillaries and decrease in their diameter along with a decrease in endolymphatic pressures, which would improve microlymphatic drainage, after 4 weeks of treatment with MPFF. The benefit was maintained even 2 weeks after treatment was stopped.58
More evidence that MPFF contributes to lymphatic drainage is found in its lymphagogue effect, increasing lymphatic flow, as demonstrated in a dose-dependent manner in thoracic duct fistula in mongrel dogs. Increased lymph flow was also described in the hind limb of ewes administered MPFF.53,59 Moreover, increased lymph flow could be attained by increasing pulsatility of the lymph vessels. MPFF was shown to increase the frequency of spontaneous isometric contractions of isolated rings of sheep mesenteric lymphatics,60 and in isolated bovine lymphatic vessels as well in a concentration related manner.53
In CVI, venous hypertension triggers initial inflammation, with subsequent red-blood-cell extravasation and release of inflammatory mediators. MPFF works by decreasing the immune response to blood extravasation.61 There is some evidence that in an experimental inflammatory model in rat, MPFF reduces prostaglandin and thromboxane availability by inhibiting their synthesis. As these are involved in edema formation, this could contribute to a beneficial effect of MPFF on the formation of edema.58 A study in hamsters with surgical vein ligation (simulation of DVT) and subsequent inflammatory response and microvascular changes showed significant decrease in leukocyte rolling and adherence and increased venular diameter when treated with MPFF, as compared with the control group; indicating that MPFF could prevent CVI or slow the disease progression.62
A small pilot study of 10 patients with secondary phlebolymphedema following breast cancer treatment demonstrated that with 6-months MPFF treatment, relative edema volume, compared to pretreatment measures, decreased continuously through the study and a decrease in intensity of skin hardness and heaviness were observed.63 A larger controlled trial followed, including 104 women with upper limb lymphedema. In patients with more severe lymphedema, MPFF treatment significantly improved lymphatic migration speed over placebo and improved the half-life of the colloidal compound used as tracers in this procedure over time. Whereas the change in colloidal clearance over time approached significance in those treated with MPFF (and not in the placebo group), this larger study found no significant difference in evolution of lymphedema volume.64,65
Recent evidence suggests that MPFF improves symptomatology with a single dose of 1000 mg daily or 500 mg twice daily after 2 weeks of treatment.66,67 This treatment led to recovery from up to 9 different leg symptoms, including swelling, cramps, pain, heaviness, and clinical signs of CVI. For instance, a recent observational study, The VEIN ACT Program (chronic VEnous dIsorders maNagement and evaluAtion of Chronic venous disease treatment effecTiveness), included subjects with leg pain and at least one sign of CVI. Treatment with combined VAD, including MPFF, exhibited at least a 50% improvement in symptoms. Similarly, a study conducted in Russia, using only MPFF, evidenced up to a 50% decrease in symptom intensity and frequency, with 95% rate of patient satisfaction.68 As well as that, MPFF demonstrated effectiveness in the early stages of the disease by lowering great saphenous vein (GSV) reflux, and it even led to elimination of evening GSV reflux in 76% of patients with C0s or C1s, appearing to be a promising drug.69
Finally, the effectiveness of MPFF-based conservative treatment in patients with chronic venous edema (CVE) was assessed as part of a prospective observational program that evaluated the management of patients with CVE caused by the primary forms of CVD in real clinical practice in Russia with a mean study duration of 2.5±0.5 months. MPFF-based conservative treatment, irrespective of addition of surgical intervention, was associated with a significant reduction in ankle volume in patients with CVD of CEAP class C3EpAsPr.70
Conclusions
PIC has been shown to be effective, affordable, and reliable for patients with phlebolymphedema. It should be offered as the first-line treatment, together with management of reflux and/or occlusion, keeping in mind that proper pressure will determine the success of the intervention. In addition to a mechanical approach, VADs such as MPPF can help to reduce symptomatology while suggesting a potential prevention for the disease progression.
The treatment strategy should be individualized, keeping in mind special needs, comorbidities, and the etiology of the phlebolymphedema (venous or lymphatic) in each patient. Conservative management should be combined with PIC or VAD in order to achieve best results for the patients. However, there is a need for a consensus in order to create guidelines for physicians to choose the most effective and safe diagnostic and therapeutic management. Thus, more comparative studies on efficacy are needed from which to develop solid recommendations.
REFERENCES
1. Baethge C, Goldbeck-Wood S, Mertens S. SANRA- a Scale for the quality Assessment of Narrative Review Articles. Res Integr Peer Rev. 2019;4(1).
2. Lee B. Phlebolymphedema is the ultimate comorbidity/outcome of lymphedema. J Vasc Surg Venous Lymphat Disord. 2019;7(5):731.
3. Bunke N, Brown K, Bergan J. Phlebolymphedema: usually unrecognized, often poorly treated. Perspect Vasc Surg Endovasc Ther. 2009;21(2):65-68.
4. Farrow W. Phlebolymphedema–a common underdiagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec. 2010;2(1):14-23.
5. Jawien A, Grzela T, Ochwat A. Prevalence of chronic venous insufficiency in men and women in Poland: multicentre cross-sectional study in 40,095 patients. Phlebology. 2003;18(3):110-122.
6. Dean S, Valenti E, Hock K, Leffler J, Compston A, Abraham W. The clinical characteristics of lower extremity lymphedema in 440 patients. J Vasc Surg Venous Lymphat Disord. 2020;8(5):851- 859.
7. Lee BB, Andrade M, Antignani PL, et al; International Union of Phlebology. Diagnosis and treatment of primary lymphedema. Consensus document of the International Union of Phlebology (IUP)–2013. Int Angiol. 2013;32:541-574.
8. Lee BB, Antignani PL, Baroncelli TA, et al. IUA- ISVI consensus for diagnosis guideline of chronic lymphedema of the limbs. Int Angiol. 2015;34:311-332.
9. Szyber P, Szyber P. Lower limb lymphoedema – therapeutic problem. Therapy. 2008;2(1):70-72.
10. Son A, O’Donnell T, Izhakoff J, Gaebler J, Niecko T, Iafrati M. Lymphedema associated comorbidities and treatment gap. J Vasc Surg Venous Lymphat Disord. 2019;7(5):724-730.
11. Dreyer G, Addiss D, Gadelha P, Lapa E, Williamson J, Dreyer A. Interdigital skin lesions of the lower limbs among patients with lymphoedema in an area endemic for bancroftian filariasis. Trop Med Int Health. 2006;11(9):1475-1481.
12. Centers for Disease Control and Prevention. Parasites – lymphatic filariasis: epidemiology & risk factors. Updated March 5, 2019. Accessed May 20, 2020. Available from: https://www.cdc.gov/ parasites/lymphaticfilariasis/epi.html
13. Guyton AC. Textbook of Medical Physiology. 8th ed. Philadelphia, PA: WB Saunders; 1991.
14. Földi M, Földi E, Kubik S. Deficiency and insufficiency of the lymphatic system. In: Textbook of Lymphology for Physicians and Lymphedema Therapists. Munich, Germany: Urban and Fisher; 2003:209- 215.
15. Földi E, Földi M. Chronic venous insufficiency and venous-lymphostatic insufficiency. In: Földi’s Textbook of Lymphology. 2nd ed. Munich, Germany: Elsevier; 2006:434-447.
16. O’Donnell T, Rasmussen J, Sevick-Muraca E. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord. 2017;5(2):261- 273.
17. Ruocco E, Brunetti G, Brancaccio G, Lo Schiavo A. Phlebolymphedema: disregarded cause of immunocompromised district. Clin Dermatol. 2012;30(5):541-543.
18. Ruocco V, Brunetti G, Puca RV, Ruocco E. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
19. Ruocco V, Ruocco E, Brunetti G, Sangiuliano S, Wolf R. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
20. Smith J, Mello LF. Nogueira Neto NC, et al. Malignancy in chronic ulcers and scars of the leg (Marjolin’s ulcer): a study of 21 patients. Skeletal Radiol. 2001;30:331-337
21. Cendras J, Sparsa A, Bedane C, Delage M, Touati M, Bonnetblanc JM. Lymphome B primitif à grandes cellules CD20-, CD79a+ apparu sur un ulcère veineux chronique de jambe. Ann Dermatol Venereol. 2007;134:357-361.
22. Klippel M, Trenaunay J. Du noevus variqueux et osteohypertrophique. Arch Gén Méd. 1900;3:641-672.
23. Gloviczki P, Driscoll DJ. Klippel–Trenaunay syndrome: current management. Phlebology. 2007;22:291-298.
24. Vahidnezhad H, Youssefian L, Uitto J. Klippel-Trenaunay syndrome belongs to the PIK3CA-related overgrowth spectrum (PROS). Exp Dermatol. 2015;25(1):17-19.
25. Mirzaa G, Timms A, Conti V, et al. PIK3CAassociated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight. 2016;1(9):e87623.
26. Jones G, Mansour S. An approach to familial lymphoedema. Clin Med (Lond). 2017;17(6):552-557.
27. Brodell JD, Brodell RT. Recurrent lymphangitic cellulitis syndrome. Contemp Orthop. 1992;25:461-468.
28. Szuba A, Razavi M, Rockson SG. Diagnosis and treatment of concomitant venous obstruction in patients with secondary lymphedema. J Vasc Interv Radiol. 2002;13(8):799-803.
29. Flugman SL, Clark RA. Stasis dermatitis. Medscape. Updated March 27, 2020. Accessed January 16, 2020. Available at: http://emedicine.medscape.com/ article/1084813-overview
30. Falanga V, Moosa HH, Nemeth AJ, Alstadt SP, Eaglstein WH. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch Dermatol. 1987;123(5):620-623.
31. Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MDP. Lipodermatosclerosis: review of cases evaluated at the Mayo Clinic. J Am Acad Dermatol. 2002;46(2):187-192.
32. Goldman MP. Lipodermatosclerosis: review of cases evaluated at the Mayo Clinic. J Am Acad Dermatol. 2002;46:187-192.
33. Weber J, Daffinger N. Congenital vascular malformations: the persistence of marginal and embryonal veins. Vasa. 2006;35:67- 77.
34. Kinmonth JB. Lymphangiography in man; a method of outlining lymphatic trunks at operation. Clin Sci. 1952;11:13-20.
35. Suehiro K, Morikage N, Murakami M, Yamashita O, Samura M, Hamano K. Significance of ultrasound examination of skin and subcutaneous tissue in secondary lower extremity lymphedema. Ann Vasc Dis. 2013;6:180-188.
36. Sherman AI, Ter-Pogossian M. Lymph-node concentration of radioactive colloidal gold following interstitial injection. Cancer. 1953;6:1238-1240.
37. Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology. 2016;49(4):170-184.
38. Patel KM, Manrique O, Sosin M, Hashmi MA, Poysophon P, Henderson R. Lymphatic mapping and lymphedema surgery in the breast cancer patient. Gland Surg. 2015;4:244-256.
39. Mihara M, Hara H, Araki J, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One. 2012;7:e38182.
40. Arrivé L, Derhy S, El Mouhadi S, Monnier- Cholley L, Menu Y, Becker C. Noncontrast magnetic resonance lymphography. J Reconstr Microsurg. 2016;32(1):80-86.
41. Liu N, Zhang Y. Magnetic resonance lymphangiography for the study of lymphatic system in lymphedema. J Reconstr Microsurg. 2016;32:66-71.
42. Bollinger A, Jager K, Sgier F, Seglias J. Fluorescence microlymphography. Circulation.1981;64:1195-1200.
43. Ayestaray B, Bekara F. Fluorescein sodium fluorescence microscope-integrated lymphangiography for lymphatic supermicrosurgery. Microsurgery. 2015;35:407-410.
44. Sevick-Muraca EM, Kwon S, Rasmussen JC. Emerging lymphatic imaging technologies for mouse and man. JCI. 2014;124:905- 914.
45. Rasmussen JC, Tan IC, Marshall MV, et al. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl Oncol. 2010;3:362-372.
46. Śmiłowska A, Skorupińska A, Gromek K. Does intermittent compression therapy in home care program more reduce phlebolymphedema than manual lymph drainage applied in physical therapy outpatient unit? Practical reference guide for GPs. Fam Med Prim. 2015;3:219-224.
47. Zaleska M, Olszewski W, Durlik M. The effectiveness of intermittent pneumatic compression in long-term therapy of lymphedema of lower limbs. Lymphat Res Biol. 2014;12(2):103-109.
48. Dymarek R, Taradaj J, Rosinczuk J, Halski T, Schneider W. Comparison of efficacy of the intermittent pneumatic compression with a high- and low-pressure application in reducing the lower limbs phlebolymphedema. Ther Clin Risk Manag. 2015;11:1545-1554.
49. Olszewski WL. The “third” circulation in human limbs tissue fluid, lymph and lymphatics. Phlebologie. 2012;41(6):297- 303.
50. Lerman M, Gaebler J, Hoy S, et al. Health and economic benefits of advanced pneumatic compression devices in patients with phlebolymphedema. J Vasc Surg. 2019;69(2):571-580.
51. Karaca-Mandic P, Hirsch A, Rockson S, Ridner S. The cutaneous, net clinical, and health economic benefits of advanced pneumatic compression devices in patients with lymphedema. JAMA Dermatology. 2015;151(11):1187.
52. Tay EY, Fook-Chong S, Oh CC, Thirumoorthy T, Pang SM, Lee HY. Cellulitis Recurrence Score: a tool for predicting recurrence of lower limb cellulitis. J Am Acad Dermatol. 2015;72(1):140-145.
53. Labrid C. A lymphatic function of MPFF at a dose of 500 mg. Int Angiol. 1995;14(3 suppl 1):36-38.
54. Casley-Smith JR. Modern treatment of lymphoedema. II The bezopyrones. Australas J Dermatol.1992;33(2):69-74.
55. Casley-Smith JR, Morgan RG, Piller NB. Treatment of lymphedema of the arms and legs with 5,6-benzo-+AFsalpha+ AFO–pyrone. N Engl J Med. 1993;329(16):1158-1163.
56. Piller NB. The lymphogogue action of calcium dobesilate on the flow of lymph from the thoracic duct of anesthetized and mobile guinea pigs. Lymphology. 1988;21(2):124-127.
57. European Venous Forum, International Union of Angiology, Cardiovascular Disease Educational and Research Trust (UK), Union Internationale de Phlébologie. Nicolaides A, Kakkos S, Baekgaard N, et al, eds. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181-254.
58. Allegra C, Bartolo M Jr, Carioti B, Cassiani D, Besse Botti MG. Microlymphography: assessment of MPFF at a dose of 500 mg activity in patients with chronic venous insufficiency. Lymphology. 1998;31(suppl):12-16.
59. Labrid C. Pharmacologic properties of MPFF at a dose of 500 mg. Angiology. 1994;45(6 pt 2):524-530.
60. McHale NG, Hollywood MA. Control of lymphatic pumping: interest of MPFF at a dose of 500 mg. Phlebology. 1994(suppl 1):23-25.
61. Ulloa J. Micronized purified flavonoid fraction (MPFF) for patients suffering from chronic venous disease: a review of new evidence. Adv Ther. 2019;36(S1):20-25.
62. das Graças C, de Souza M, Cyrino FZ, Carvalho JJ, Blanc-Guillemaud V, Bouskela E. Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. Eur J Vasc Endovasc Surg. 2018;55(5):694-702.
63. Pecking AP. Evaluation by lyphoscintigraphy of the effect of a micronized flavonoid fraction (MPFF at a dose of 500 mg*) in the treatment of upper limp lymphedema. Int Angiol. 1995;14(3 suppl 1):39-43.
64. Pecking AP, Février B, Wargon C, Pillion G. Efficacy of MPFF at a dose of 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer). Angiology. 1997;48(1):93-98.
65. Pecking AP, Floiras JL, Rambert P, et al. Effect of MPFF at a dose of 500 mg on lymphedema secondary to breast cancer treatment. Lymphology. 1998;31(suppl):25-28.
66. Kirienko A, Radak D. Clinical acceptability study of once-daily versus twice-daily micronized purified flavonoid fraction in patients with symptomatic chronic venous disease: a randomized controlled trial. Int Angiol. 2016;35(4):399-405.
67. Carpentier P, van Bellen B, Karetova D, et al. Clinical efficacy and safety of a new 1000-mg suspension versus twice-daily 500-mg tablets of MPFF in patients with symptomatic chronic venous disorders: a randomized controlled trial. Int Angiol. 2017;36(5):402-409.
68. Lishkov DE, Kirienko AI, Larionov AA, Chernookov AI. Patients seeking treatment for chronic venous disorders: Russian results from the VEIN Act program. Phlebolymphology. 2016;23(1):44.
69. Tsukanov YT, Tsukanov AY. Diagnosis and treatment of situational great saphenous vein reflux in daily medical practice. Phlebolymphology. 2017;24(3):144-151.
70. Bogachev V. Effectiveness of MPFFbased conservative treatment in chronic venous edema. Phlebolymphology. 2020;27(2):70-80.