Phlebolymphedema: is it a new concept?
Professor of Surgery and Director,
Center for the Lymphedema and Vascular
Malformations, George Washington
University, Washington DC, USA
Abstract
Venous and lymphatic systems are inseparable “dual” outflow systems with mutually complimentary function so that such mutual interdependence between these two systems causes a new condition to affect both systems simultaneously when one of the two systems should fail to provide sufficient compensation to the other system, known as “phlebolymphedema” (PLE): combined condition of chronic venous insufficiency (CVI)/chronic venous hypertension and chronic lymphatic insufficiency (CLI)/chronic lymphedema. PLE is, therefore, an unavoidable outcome of the joint failure of this “inseparable” venous-lymphatic circulation system, presenting as a combined condition of venolymphatic edema caused by CVI and CLI due to various etiopathogenesis. PLE can be managed more effectively when open and/or endovascular therapy is added to basic compression therapy to control the CVI and CLI together. Primary PLE is caused mostly by vascular malformation components of Klippel-Trenaunay syndrome as the combined condition of CVI attributed to marginal vein (MV)/venous malformation and CLI attributed to primary lymphedema/lymphatic malformation. CVI attributed to reflux of MV can be treated with MV resection, whereas CVI attributed to deep vein dysplasia can usually be treated with conventional compression therapy alone. Secondary PLE is usually the outcome of deep-vein thrombosis (DVT)/ postthrombotic syndrome (PTS). CVI attributed to PTS can be further improved with correction of the venous outflow obstruction with angioplasty and stenting, especially when the DVT sequalae is involved at multiple levels of the iliacfemoral-popliteal vein system.
Introduction
Phlebolymphedema is not a new concept! Indeed, this “combined” condition of venolymphatic disorders has been well recognized and reported all along for many decades, but the term “phlebolymphedema” has not been properly defined, partly due to ignorance.1-4
Phlebolymphedema is an unavoidable outcome of joint failure of the “inseparable” venous-lymphatic circulation system. These two systems are a mutually interdependent “dual” outflow system of the circulation to transport used blood away from the tissue; these two systems maintain a hemodynamically unique relationship that means they share the same destiny. When one system goes bad, the other follows.
Indeed, the more we learned about this unique relationship between the venous circulation and the lymphatic circulation over the last three decades, the more we realized how “overlooked and underappreciated” phlebolymphedema is.5-8
Although these two systems’ hemodynamic principles, venodynamics and lymphodynamics, are based on totally different rheodynamics, both systems are mutually complimentary. Furthermore, the crucial role of the lymphatic system in comparison with the venous system was “underestimated” for many decades and recently reevaluated through a revised Starling principle based on the glycocalyx model of transvascular fluid exchange.9-12
The venodynamic is based purely on a passive lowpressure system of 10 mm Hg during an average run, via heart, diaphragm/breathing, and muscle contraction, etc; however, the “normal” lymphodynamic is based on “self-propelled” peristalsis via chains of multiple units of “lymphangion” to overcome the pressure gradient in excess of 30 mm Hg (~40 cm H2O) in human legs. Indeed, the lymphangion system reduces the pressure downstream before additional fluid arrives from upstream segments through coordinated contractility.13,14 However, a critical aspect of lymphodynamics is that should this normal peristaltic function of lymphangion be lost, due to various conditions, the “abnormal” lymphodynamic essentially becomes the same as the venodynamic.
When one of these two systems is overloaded, the second/ other system is able to play an auxiliary role to assist the return of fluid to the circulation system to compensate for the insufficiency/failure of one system. However, this role/ function of the two systems to help each other in case of overload is possible only when they are in a normal functional state.
Indeed, when the failure of one system contributes to overloading the other system and this exceeds the limit of compensatory function, resulting in the long-term failure of one system, it eventually results in “total” failure of this “inseparable” dual system altogether, so-called phlebolymphedema.15-18
Such mutual interdependence between the venous and lymphatic systems causes a new condition to affect both systems simultaneously when one of the two systems fails in its normal function to provide sufficient compensation to the other system. Thus, phlebolymphedema is the combined condition of chronic venous insufficiency (CVI)/chronic venous hypertension and chronic lymphatic insufficiency (CLI)/chronic lymphedema.
Pathophysiology
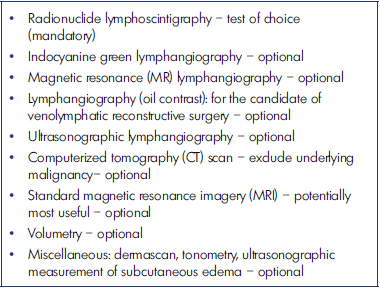
Phlebolymphedema represents a clinical condition of limb swelling as the result of the accumulation of excess intercellular/interstitial fluid in the legs and feet by the “combined” conditions of phlebogenic leg edema (ie, phleboedema) and lymphogenic leg edema (ie, lymphoedema) by various etiologies. Hence, this unique condition can be defined as “lymphaticovenous edema” caused by CLI and CVI (Figure 1).
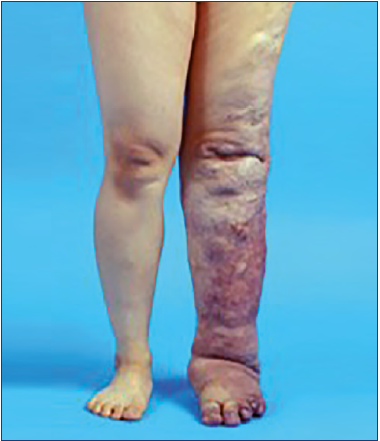
Figure 1. Phlebolymphedema of Klippel-Trenaunay syndrome origin. This clinical condition of limb swelling represents “lymphaticovenous edema” caused by chronic lymphatic insufficiency and chronic venous insufficiency.
When CVI19-22 results in an excessive fluid load within tissue, it disrupts the “checks and balance” function of the capillary system, allowing an additional load to the lymphatic system as well. If this overloading exceeds the maximum capacity of the normal lymphatic compensatory function, the lymphatics themselves are also damaged following an initially enhanced function to compensate for the insufficient venous system.
Indeed, the lymphatic system is a dynamic system, with limits in the volume of capillary ultrafiltrate that it can handle varying considerably. The fragile vessels of the lymphatic system can be easily damaged by infection, trauma, tissue inflammation, or radiation.
Lymphatic overloading caused by CVI can lead to valvular failure in lymphatic vessels, and increased tissue edema stretches the skin to cause the lymphatic capillaries to be pulled open by anchoring elastic fibers/filaments, widening interendothelial junctions; extreme distention causes rupture of these filaments, damaging the lymphatic capillary walls. Greater permeability of blood capillaries leads to further extravasation of proteins into the interstitial space, with their critical oncotic pressure to hold water molecules, creating a swollen extremity. Subsequently, safety valve insufficiency of the lymphatic system becomes inevitable, allowing the “lymphostasis” that results in CLI.23-26
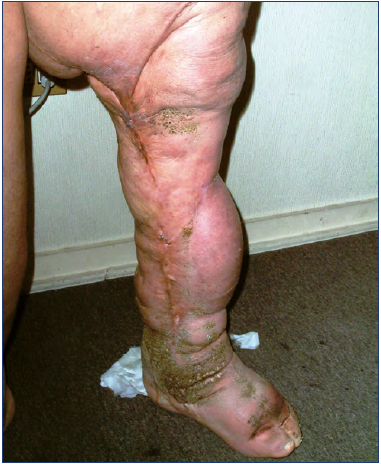
Figure 2. Phlebolymphedema with lipodermatosclerosis. This clinical condition known as lipodermatosclerosis is the inevitable outcome of lymphostasis caused by chronic lymphatic insufficiency. Accumulation of protein-rich fluid in the interstitial space initiates a cascade of events of a major fibrosis.
Accordingly, when venous stasis/phleboedema exceeds this maximum lymphatic compensatory capacity, the insufficiency becomes “phlebolymphatic,” and the inability of the lymphatic system to drain interstitial fluids and macromolecules effectively results in accumulation of protein-rich fluid in the interstitial space, initiating a cascade of events leading to major fibrosis of the interstitial tissue, called lipodermatosclerosis (Figure 2).
Accumulation of these proteins and proinflammatory cytokines in the interstitial space creates a proinflammatory state that leads to a complicated inflammatory process resulting in tissue fibrosis.
Protein-rich fluid causes collagen deposition and a scarring process (lipodermatosclerosis) that impedes absorption of the interstitial fluid by lymph vessels and reduces permeability of lymph vessels, permanently compromising the microvascular and lymphatic systems. Indeed, increased protein concentration in the tissue further reduces the cellular oxygen and nutrients, interfering with wound healing and accelerating the degenerative phlebolymphatic process, which results in dystrophic ulcers and skin infections, etc.27-30
This CLI becomes more prominent when the lymphatic drainage condition is “compromised” by various etiologies (eg, surgery/radiotherapy associated with cancer treatment). Depending upon etiology (primary and secondary) and the degree/extent of the CVI and CLI, the clinical manifestation of phlebolymphedema varies greatly and, infrequently, multiple factors are involved, including systemic disease (eg, congestive heart failure, cirrhosis, or nephropathy), compounding the inability to drain interstitial fluids and macromolecules by the lymphatic system.
Definition
For the last two decades, lymphedema that develops along with advanced CVI has been called phlebolymphedema. However, this condition represents only one form of phlebolymphedema, with lymphedema as the outcome of valvular failure of lymphatic vessels due to lymphatic overloading to compensate for venous insufficiency caused by CVI.
For example, chronic “indolent” venous stasis ulcers on the distal lower leg are a typical model of phlebolymphedema that represents a “combined” condition of CVI and CLI despite having for many decades been considered a hallmark of “advanced” CVI as the sequalae of deep-vein thrombosis (DVT). Although these ulcers might have started as a single venous condition of the CVI, they no longer remain a sole venous condition when the local condition changes/advances to a “combined” condition of venous and lymphatic insufficiency, becoming incalcitrant and resistant to healing (Figure 3).27-30
Figure 3. Phlebolymphedema with chronic “indolent” ulcers. This clinical condition often starts as a single chronic venous insufficiency (CVI) condition as the sequalae of deep-vein thrombosis but becomes a “combined” condition of CVI and chronic lymphatic insufficiency (CLI) when the local condition changes/advances to precipitate lymphatic insufficiency, with CLI becoming incalcitrant, resistant to healing.
If ulcers become incalcitrant, resisting healing, they are considered a new condition—secondary phlebolymphedema— caused by the lymphatic failure/CLI that was precipitated by the initial CVI.
Therefore, this is a unique condition of “safety valve insufficiency” as the combined effect of increased lymph flow and reduced drainage capacity in the diseased lymphatic system, often having the outcome of advanced postthrombotic syndrome (PTS).
In other words, CLI involved with phlebolymphedema is not simply a condition that represents mechanical insufficiency only, with low lymph flow due to defects in the lymph system, or that represents dynamic insufficiency only, with high lymph flow overwhelming the maximum load-carrying capacity of the lymphatic system, but both conditions.
Classification
Primary phlebolymphedema
A combined form of congenital vascular malformation, known as Klippel-Trenaunay Syndrome (KTS),31-34 represents the unique condition of primary phlebolymphedema35-38 as a combined condition of CVI and CLI caused by its two vascular malformation components: venous malformation39-42 and lymphatic malformation (Figure 4).43-46,47
Among many different types of venous malformation as vascular malformation components of KTS, marginal vein (MV) is the most common lesion to cause CVI with venous reflux/hypertension. However, other forms of deep-vein dysplasia (eg, iliac vein agenesis, hypoplastic femoral vein) or defective vein (eg, web, stenosis, aneurysm, ectasia) would also cause CVI with various degrees of venous outflow obstruction/hypertension, either alone as an independent lesion or combined with MV.48-51
MV is a relatively common venous malformation lesion in KTS patients. However, MV is not like other truncular lymphatic malformation lesions; it is an embryonic vein remnant, a birth defect that failed to involute after developmental arrest during the vein trunk formation period in the “later stage” of embryonic development. Therefore, MV has a defective vessel wall and, though they look similar, is not like a varicose vein with matured vascular structure.
Indeed, MV often runs along the lateral aspect of the lower extremity, very superficially beneath the skin, with minimum soft tissue coverage for the most part and so looks similar to ordinary varicose veins (Figure 5).
Nevertheless, MV accompanies a special condition called “avalvulosis/avalvulia” with a congenital absence/lack of venous valves so that it allows severe reflux, resulting in chronic venous hypertension/stasis with subsequent CVI. Besides, abnormal vessel wall structure due to defective/ deficient media of the vein wall, often with a lack of smooth muscle layers (cf. varicose vein), accompanies a high risk of intravascular thrombosis resulting in venous thromboembolism (VTE) in addition to severe CVI, which then precipitates CLI.
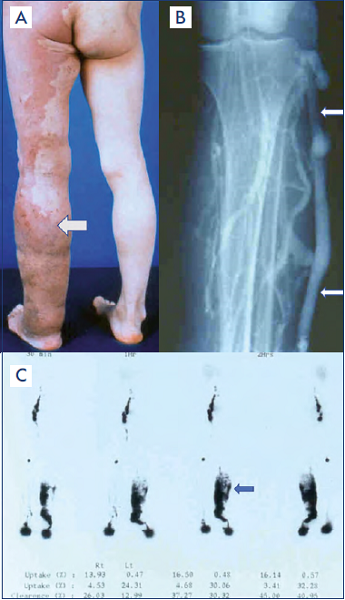
Figure 4. Primary phlebolymphedema.
A) A clinical condition of primary phlebolymphedema along the left lower extremity (arrow) caused by the vascular malformation components of Klippel-Trenaunay syndrome: chronic venous insufficiency by venous malformation and lymphatic malformation.
B) Marginal vein, embryonic vein remnant/ vascular malformation, along the left lower extremity (arrow) with defective development of normal deep-venous system, to cause chronic venous insufficiency/phlebolymphedema.
C) Radionuclide lymphoscintigraphic findings of massive dermal backflow (arrow) resulting from chronic lymphatic insufficiency. It was caused by primary lymphedema as the outcome of defective development of the lymphatic system along the later stage of lymphangiogenesis known as truncular lymphatic malformation.
After reference 47: Lee and Villavicencio. Figure 170- 2 Hemo-lymphatic malformations. A–I, Klippel-Trénaunay syndrome (KTS). Chapter 171. General considerations. Congenital vascular malformations. Section 26. Vascular Malformation. In: Sidawy AN, Perler BA, eds. 9th ed. Rutherford’s Vascular Surgery and Endovascular Surgery. Philadelphia, PA, USA: Saunders Elsevier; 2019:2236-2250.
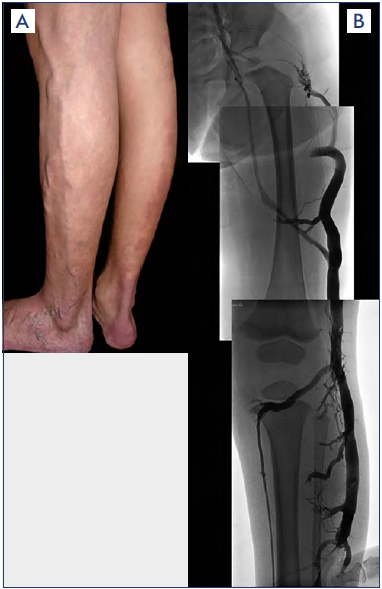
Figure 5. Marginal vein.
A) Clinical findings of marginal vein (MV) running along the lateral aspect of the lower extremity very superficially to mimic common varicose veins. However, MV is not a varicose vein but rather an embryonic vein remnant remaining as a birth defect after it failed to involute.
B) Angiographic finding of MV revealing its extent to compensate for a deficient deep-vein system.
CLI in KTS causes phlebolymphedema together with the venous malformation lesions, mostly due to primary lymphedema by truncular lymphatic malformation lesion alone (eg, lymphatic dysplasia: aplasia, hypoplasia, or hyperplasia) and extratruncular lymphatic malformation (lymphangioma) seldom involved with CLI.52-55
When these two conditions of CVI attributed to MV and CLI due to primary lymphedema are combined, they exert a synergistic impact on the mutually interdependent and inseparable venous-lymphatic system, worsening the limb swelling and making its management much more difficult.
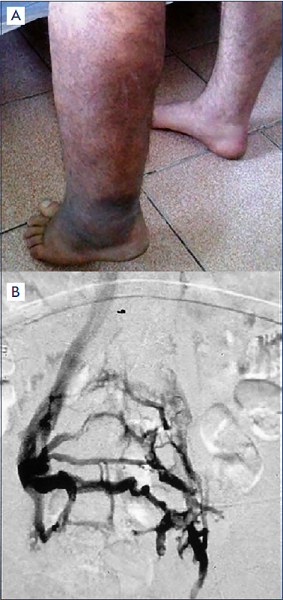
Figure 6. Secondary phlebolymphedema.
A) Depiction of a clinical condition of secondary phlebolymphedema along the left lower extremity representing the end-stage of chronic venous insufficiency (CVI) as the sequalae of postthrombotic syndrome (PTS) following deep-vein thrombosis. Steady progress of the local tissue damage (eg, ulcer) by the CVI/PTS caused chronic lymphatic insufficiency, resulting in secondary regional/local lymphedema.
B) Angiographic findings of thrombosed left iliac vein with extensive collaterals to contralateral/right iliac veins through pelvic veins to illustrate the cause of this secondary phlebolymphedema.
Secondary phlebolymphedema
Secondary phlebolymphedema develops along the end stage of CVI, mostly as the sequalae of PTS after DVT, as explained previously. After steady progress of local tissue damage (eg, ulcer) resulting from CVI/PTS, CLI generally begins as a secondary regional/local lymphedema showing a visibly strained lymphatic system as a victim of abnormal venous condition. CLI of secondary phlebolymphedema often makes the condition more complicated to manage, as a newly added condition of local/regional lymphedema; it becomes a major obstacle to clinical management due to the complexity of the local circulation (Figure 6).56-59
Occasionally, however, the clinical/subclinical condition of primary lymphedema exists as the cause of CLI, unnoticed until it accelerates the deterioration of the underlying benign primary venous disorder (eg, reflux), resulting in CVI.
Diagnosis – general strategy
Appropriate appraisal of CVI and of CLI should start with differential diagnosis between primary phlebolymphedema of congenital origin and secondary phlebolymphedema with various backgrounds. However, the diagnostic evaluation of phlebolymphedema should be initiated with the appraisal of these two closely linked conditions (CVI and CLI) together regardless of the etiology, either primary or secondary.15-17,20-23,33-37,40,46,50,53,57
All chronic venous conditions that carry suspicion of further progression to phlebolymphedema, including stasis ulcers that resist conventional care, should undergo assessment not only of the venous system itself but also together with the lymphatic system as a two-system assessment.
Similarly, for chronic lymphedema assessment among those with clinically suspected phlebolymphedema, the evaluation of the venous system should also be part of a mandatory evaluation of these two inseparable systems. In other words, if phlebolymphedema is suspected, such simultaneous evaluation of the two systems is essential as for any two-system assessment.
Nevertheless, the evaluation of limb swelling/edema should exclude or confirm the involvement of systemic causes of edema: (eg, cardiac failure, renal failure, hepatic failure, hormonal disturbances, malignant tumors) as a basic appraisal; other iatrogenic causes for edema should be investigated, including the use of calcium antagonists, vasodilators, and anti-inflammatory drugs.
The evaluation of CVI as the venous component of phlebolymphedema should start with a thorough evaluation of the anatomy of the entire venous system, including evaluation of the presence, size, and extension of refluxes both in the deep and superficial veins, and also evaluation of the presence of an obstruction of congenital or acquired origin.
Such basic information is essential for proper diagnosis of phlebolymphedema, and it has to be completed before proceeding to more specific investigation on chronic DVT/ PTS resulting in CVI in secondary phlebolymphedema, and truncular venous malformation lesions, such as marginal vein/lateral embryonic vein, in primary phlebolymphedema as mentioned previously.
CLI as the lymphatic component of phlebolymphedema is equally important for determining primary, as well as secondary nature, but additional investigation on the lymphatic malformation, other than in primary lymphedema (eg, lymphangioma), is extremely important when primary phlebolymphedema is attributed to KTS with the risk of coexistence of additional vascular malformations.60-63
Diagnosis – laboratory assessment
Clinical diagnosis of primary as well as secondary phlebolymphedema should be confirmed based on proper combination of various available laboratory tests, mostly on the basis of being noninvasive to less invasive, so that proper treatment strategy can be formulated by the multidisciplinary team on the basis of the laboratory-assessed extent of CVI and CLI.
A limited exam for laboratory evaluation of the venous system (Table I)41-46,48-60 for phlebolymphedema to assess the extent/severity of the CVI is generally sufficient, with tests to determine noninvasive to less-invasive status based on duplex ultrasonography (DUS), magnetic resonance imaging (MRI), and/or computerized tomography (CT).
Indeed, DUS remains a gold standard of morphofunctional studies for the evaluation of the venous system, especially for primary phlebolymphedema with MV as the cause of CVI. This technique makes possible the visualization of the entire length/course of the MV, which is located supra- and subfascially, together with the perforators, and also simultaneously provides crucial information on the hemodynamic status of the MV and the deep-vein system (eg, extent and severity of the reflux and outflow resistance).
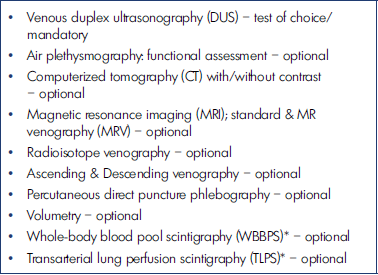
Table I. Laboratory evaluation for the venous system.41,60* For the congenital vascular malformation assessment
Since more than one-third of KTS patients with MV are known to have an additional defective deep-venous system (eg, hypoplasia of femoral vein, aplasia of iliac vein) that makes CVI more complicated, such DUS information remains crucial to the management plan.
However, various plethysmographic assessments, as listed, would also be needed as additional tests for further accurate evaluation to set up a management plan especially for secondary phlebolymphedema caused by DVT/PTS. Aside from that, ascending/descending phlebography is also needed together with direct puncture phlebography as a roadmap, especially for the primary phlebolymphedema involved in MV/venous malformation in KTS.
Laboratory evaluation for the lymphatic system (Table II)23,25 for phlebolymphedema to assess functional status in CLI is a bit more complex. Morphological evaluation based on the DUS or CT scan with contrast medium makes it possible to visualize the edema with respect to size and depth, as well as lymph nodes for possible neoplastic pathology. However, the functional test remains crucial, and radionuclide lymphoscintigraphy64-67 is still the examination of choice although a few other advanced tests are now available with limited usage/indication.
Clinical management
Depending upon the type of phlebolymphedema, the long-term care strategy will have to be organized to meet specific needs to control its pathoetiology, namely venous malformation/lymphatic malformation for the group of primary phlebolymphedema, and DVT/PTS for the secondary phlebolymphedema group, as previously pointed out above.
However, the basic approach for the management of phlebolymphedema, of either primary or secondary origin, should aim at maximum control of both conditions of CVI and CLI together since this unique condition is the outcome of simultaneous failure of two inseparable (dual) outflow systems.17,20,27,29,35,36,58,59
As the principles of management of CLI for primary phlebolymphedema is basically the same as those for secondary phlebolymphedema, the baseline therapy for both conditions is compression therapy, according to the manual lymphatic drainage (MLD)-based complex decongestive therapy (CDT) principle regardless of its etiology, which effectively controls the CVI as well.68-71 Also, additional care with palliative and/or reconstructive surgical treatment modalities can be effectively accommodated to improve management of CLI in phlebolymphedema patients as well.72,73
However, CVI for the group of primary phlebolymphedema is mandated for further specific management, especially when MV becomes the cause of CVI, precipitating DVT, with the risk of pulmonary embolism. Therefore, contrary to deep-vein dysplasia—another cause of CVI in primary phlebolymphedema—MV should be aggressively controlled, preferably via resection whenever possible and, if not, embolosclerotherapy, as long as the deep-vein system is able to tolerate the sudden influx of the diverted blood volume from the MV once it’s excised.48-51
In other words, ablation of the MV to relieve CVI can be done safely only when the deep system is in normal condition. If not, it would accompany a high risk of acute venous stasis to precipitate acute venous gangrene and cause further exacerbation of CLI via overloading of the lymphatic system, which would already be in jeopardy. In such case, prophylactic anticoagulation with weightadjusted low-molecular-weight heparin (LMWH) is generally recommended (eg, DVT/pulmonary embolism).74,75
However, deep-vein dysplasia (eg, aplastic or hypoplastic iliac-femoral venous system) as a common truncular venous malformation that causes CVI/phlebolymphedema can usually be controlled with conventional compression therapy alone and seldom requires more than conservative management unless there is clear evidence for hemodynamic gain via bypass surgery of hypoplastic/ aplastic iliac/femoral veins.
Finally, CVI in secondary phlebolymphedema caused by DVT/PTS should be treated more aggressively to relieve the cause of obstruction/reflux with open surgical (eg, bypass) and/or endovascular therapy (eg, angioplasty and stenting) as much as possible.20,76-78 When CVI is caused by multilevel DVT sequalae (eg, indolent ulcer), even minimal correction of the obstruction/stenosis is able to provide significant relief of venous hypertension to improve the efficacy of compression therapy–based conservative management.
Conclusion
• Phlebolymphedema is a joint failure of the “inseparable” venous-lymphatic system as a mutually interdependent dual-outflow circulation system.
• Phlebolymphedema is, therefore, a combined condition of CVI and CLI caused by various etiopathogeneses.
• Phlebolymphedema can be managed more effectively when open surgical and/or endovascular therapy is added to basic compression therapy to control CVI and CLI together. Primary phlebolymphedema is caused mostly by vascular malformation components of KTS: CVI by MV/venous malformation, and CLI by primary lymphedema/lymphatic malformation. CVI attributed to reflux of MV can be treated with MV resection, whereas CVI attributed to deep-vein dysplasia can usually be treated with conventional compression therapy alone.
• Secondary phlebolymphedema is usually the outcome of DVT/PTS. CVI attributed to PTS can be further improved with correction of the venous outflow obstruction via angioplasty and stenting, especially when the DVT sequalae is involved at multiple levels of the iliacfemoral- popliteal vein system.

REFERENCES
1. Mortimer PS. Evaluation of lymphatic function: abnormal lymph drainage in venous disease. Int Angiol. 1995;14(3 suppl 1):32-35.
2. Bull RH, Gane JN, Evans JE, Joseph AE, Mortimer PS. Abnormal lymph drainage in patients with chronic venous leg ulcers. J Am Acad Dermatol. 1993;28(4):585-590.
3. Friedli S, Mahler F. Venous and lymphatic reasons for edema–the swollen leg from the angiologist’s point of view. Ther Umsch. 2004;61(11):643-647.
4. Szuba A, Razavi M, Rockson SG. Diagnosis and treatment of concomitant venous obstruction in patients with secondary lymphedema. J Vasc Interv Radiol. 2002;13(8):799-803.
5. Laredo J, Lee BB. Venous physiology and pathophysiology. In: Ochoa Chaar CI, ed. Current Management of Venous Diseases. Springer International Publishing AG; 2018:23-35.
6. Lee BB, Nicolaides AN, Myers K, et al. Venous hemodynamic changes in lower limb venous disease: the UIP consensus according to scientific evidence. Int Angiol. 2016;35(3):236-352.
7. Partsch H, Lee BB: Phlebology and lymphology – a family affair. Editorial. Phlebology. 2014;29(10):645-647.
8. Lee BB. Phlebolymphedema is the ultimate comorbidity/outcome of lymphedema. J Vasc Surg Venous Lymphat Disord. 2019;7:731. Invited commentary for: Lymphedemaassociated comorbidities and treatment gap. J Vasc Surg Venous Lymphat Disord. 2019;7(5):724-730.
9. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87(2):198-210.
10. Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesthesia. 2012;108(3):384-394.
11. Piller N. Phlebolymphoedema/chronic venous lymphatic insufficiency: an introduction to strategies for detection, differentiation and treatment. Phlebology. 2009;24:51-55.
12. Farrow W. Phlebolymphedema-a common under diagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec. 2010;2:14-23.
13. Eisenhoffer J, Kagal A, Klein T, Johnston MG. Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc Res. 1995 ;49(1):97- 110.
14. Unno N, Nishiyama M, Suzuki M, et al. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg. Elsevier. 2010;52(4):946-952.
15. Lee BB. Phlebolymphedema: neglected outcome of combined venous and lymphatic insufficiency. Editorial. Vascular Specialist Int. 2020;36(1):1-3. doi:10.5758/vsi.2020.36.1.1.
16. Michelini S, Failla A, Sterbini GP, Micci A, Santoro A, Valle G. Limb phlebolymphoedema: Diagnostic noninvasive approach and therapeutic implications. Eur J Lymphology Related Problems. 1995;5(20):103-108.
17. Cavezzi A. Diagnosis and management of secondary phlebolymphedema. In: Lee BB, Stanley G, Bergan J, eds. Lymphedema: a Concise Compendium of Theory and Practice. 2nd ed. Springer International Publishing AG; 2018:913- 924.
18. Bunke N, Brown K, Bergan J. Phlebolymphemeda: usually unrecognized, often poorly treated. Perspect Vasc Surg Endovasc Ther. 2009;21(2):65-68.
19. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-346. doi:10.1161/ CIRCULATIONAHA.113.006898.
20. Raju S, Furrh JB 4th, Neglen P. Diagnosis and treatment of venous lymphedema. J Vasc Surg. 2012;55(1):141-149. doi:10.1016/j.jvs.2011.07.078.
21. Rasmussen JC, Aldrich MB, Tan IC, et al. Lymphatic transport in patients with chronic venous insufficiency and venous leg ulcers following sequential pneumatic compression. J Vasc Surg Venous Lymphat Disord. 2016;4(1):9-17.
22. Foldi E, Foldi M, Strossenreuther RHK, Kubik S. Chronic venous insufficiency and venous-lymphostatic insufficiency. In: Foldi E, Foldi M, Strossenreuther RHK, Kubik S, eds. Foldi’s Textbook of Lymphology for Physicians and Lymphedema Therapists. 2nd ed. Elsevier; Munich, Germany: 2006:434- 447.
23. Lee BB, Antignani PL, Baroncelli TA, et al. IUA-ISVI Consensus for diagnosis guideline of chronic lymphedema of the limbs. Int Angiol. 2015;34(4):311-332.
24. Kirkman R, Sawdon M. Capillary dynamics and interstitial fluid lymphatic system. Anaesth Intensive Care Med. 2004;6(1):179-183.
25. Lee BB, Andrade M, Antignani PL, et al. Diagnosis and treatment of primary lymphedema. Consensus Document of the International Union of Phlebology (IUP)-2013. Int Angiol. 2013;32(6):541- 574.
26. Silva JH, Janeiro-Perez MC, Barros N, Vieira-Castiglioni ML, Novo NF, Miranda F. Venouslymphatic disease: lymphoscintigraphic abnormalities in venous ulcers. J Vasc Bras. 2009;8(1):33-42.
27. Momin TA, Neville RF. Management of phlebolymphedema ulcers. In: Lee BB, Bergan J, Rockson SG, eds. 1st ed. Lymphedema: a Concise Compendium of Theory and Practice. Springer-Verlag, London, UK; 2011:557-561.
28. Lee BB. Stasis ulcer is a chronic condition of combined venous and lymphatic insufficiency: phlebo-lymphedema (PLE). The Saint Peregrine Project. J Theor Appl Vasc Res. 2019;4(2):33-38.
29. Gianesini S, Menegatti E, Zamboni P. Management of phlebolymphedema ulcer. In: Lee BB, Stanley G, Bergan J, eds. 2nd ed. Lymphedema: a Concise Compendium of Theory and Practice. Springer International Publishing AG; 2018:913-924.
30. Eliska O, Eliskova M. Morphology of lymphatics in human venous crural ulcers with lipodermatosclerosis. Lymphology. 2001;34:111-123.
31. Lee BB, Laredo J. Hemo-lymphatic malformation: Klippel-Trenaunay Syndrome. Review. Acta Phlebologica. 2016;17(1):15-22.
32. Gloviczki P, Stanson AW, Stickler GB, et al. Klippel-Trenaunay syndrome: the risks and benefits of vascular interventions. Surgery. 1991;110(3):469-479.
33. Noel AA, Gloviczki P, Cherry KJ Jr, Rooke TW, Stanson AW, Driscoll DJ. Surgical treatment of venous malformations in Klippel-Trenaunay syndrome. J Vasc Surg. 2000;32(5):840-847.
34. Lee BB. A stepwise daily guide to the KTS. Vasculab Debate. J Theor Appl Vasc Res. 2016;1(2):33-38.
35. Lee BB, Laredo J, Neville R, Loose D. Diagnosis and management of primary phlebolymphedema. In: Lee BB, Bergan J, Rockson SG, eds. 1st ed. Lymphedema: a Concise Compendium of Theory and Practice. Springer-Verlag, London, UK; 2011:537-546.
36. Endicott K, Laredo J, Lee BB. Diagnosis and management of primary phlebolymphedema. In: Lee BB, Stanley G, Bergan J, eds. 2nd ed. Lymphedema: a Concise Compendium of Theory and Practice. Springer International Publishing AG; 2018:913-924.
37. Lee BB, Laredo J, Neville R, Mattassi R. Primary lymphedema and Klippel- Trenaunay syndrome. In: Lee BB, Rockson J, Stanely G, eds. 1st ed. Lymphedema: a Concise Compendium of Theory and Practice. Springer-Verlag, London, UK; 2011:427-436.
38. Cavezzi A, Michelini S. Phlebolymphoedema, from Diagnosis to Therapy. Bologna, Italy: Edizioni PR; 1998. ISBN 88-900300-1-1.
39. Lee BB. Venous malformation and haemangioma: differential diagnosis, diagnosis, natural history and consequences. Phlebology. 2013;28(suppl 1):176-187.
40. Lee BB, Baumgartner I. Contemporary diagnosis of venous malformation. J Vasc Diagn. 2013:1:25-34.
41. Lee BB, Baumgartner I, Berlien P, et al. Diagnosis and treatment of venous malformations consensus document of the International Union of Phlebology (IUP): updated 2013. Int Angiol. 2015;34(2):97-149.
42. Lee BB. Current concept of venous malformation (VM). Phlebolymphology. 2003;43:197-203.
43. Lee BB, Kim YW, Seo JM, et al. Current concepts in lymphatic malformation (LM). Vasc Endovasc Surg. 2005;39(1):67-81.
44. Lee BB, Laredo J, Seo JM, Neville R. Treatment of lymphatic malformations. In: Mattassi R, Loose DA, Vaghi M, eds. Hemangiomas and Vascular Malformations. Milan, Italy: Springer- Verlag Italia; 2009:231-250.
45. Michelini S, Paolacci S, Manara E, et al. Genetic tests in lymphatic vascular malformations and lymphedema. J Med Genet. 2018;55(4):222-232. doi:10.1136/jmedgenet-2017-105064.
46. Lee BB, Amore M. Defective development of peripheral lymphatic system In: Gavins FNE, Alexander JS, eds. Lymphatic Structure and Function in Health and Disease. San Diego, California: Elsevier Inc/Academic Press; 2020:109-125.
47. Lee BB, Villavicencio. Figure 170- 2 Hemo-lymphatic malformations. A–I, Klippel-Trénaunay syndrome (KTS). Chapter 171. General considerations. Congenital vascular malformations. Section 26. Vascular Malformation. In: Sidawy AN, Perler BA, eds. 9th ed. Rutherford’s Vascular Surgery and Endovascular Surgery. Philadelphia, PA, USA: Saunders Elsevier; 2019:2236- 2250.
48. Lee BB. Marginal vein is not a varicose vein; it is a venous malformation. Veins Lymphatics. 2014;3(4050):64-70.
49. Mattassi R, Vaghi M. Management of the marginal vein: current issues. Phlebology. 2007;22:283-286.
50. Lee BB. Marginal vein is not a simple varicose vein: it is a silent killer! Review. Damar Cer Derg. 2013;22(1):4-14.
51. Kim YW, Lee BB, Cho JH, Do YS, Kim DI, Kim ES. Haemodynamic and clinical assessment of lateral marginal vein excision in patients with a predominantly venous malformation of the lower extremity. Eur J Vasc Endovasc Surg. 2007;33(1):122-127.
52. Lee BB. Lymphedema-angiodysplasia syndrome: a prodigal form of lymphatic malformation (LM). Phlebolymphology. 2005;47:324-332.
53. Lee BB, Villavicencio JL. Primary lymphedema and lymphatic malformation: are they the two sides of the same coin? Eur J Vasc Endovasc Surg. 2010;39:646-653.
54. Lee BB, Laredo J, Neville R. Primary lymphedema as a truncular lymphatic malformation. In: Lee BB, Bergan J, Rockson SG, eds. 1st ed. Lymphedema: a Concise Compendium of Theory and Practice. London, UK: Springer-Verlag; 2011:419-426.
55. Lee BB, Laredo J. Classification: venouslymphatic vascular malformation. In: Allegra C, Antignani PL, Kalodiki E, eds. News in Phlebology. Turin, Italy: Edizioni Minerva Medica; 2013:91-94.
56. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312-326.
57. Chen WYJ, Rogers A. Recent insight into the causes of chronic leg ulceration in venous diseases and implication on the other types of chronic wounds. Wound Repair Regen. 2007;15:434- 449.
58. Cavezzi A. Diagnosis and Management of secondary phlebolymphedema. In: Lee BB, Bergan I, Rockson SG, eds. 1st ed. Lymphedema: a Concise Compendium of Theory and Practice. London, UK: Springer-Verlag; 2011:547- 556.
59. Kahn SR, Galanaud JP, Vedantham S, Ginsberg JS. Guidance for the prevention and treatment of the post-thrombotic syndrome. J Thromb Thrombolysis. 2016;41(1):144-153. doi:10.1007/s11239-015-1312-5.
60. Lee BB, Antignani PL, Baraldini V, et al. ISVI-IUA consensus document – diagnostic guidelines on vascular anomalies: vascular malformations and hemangiomas. Int Angiol. 2015;34(4):333-374.
61. Aström KG, Abdsaleh S, Brenning GC, Ahlström KH. MR imaging of primary, secondary, and mixed forms of lymphedema. Acta Radiol. 2001;42(4):409-416.
62. Delis KT, Gloviczki P, Wennberg PW, Rooke TW, Driscoll DJ. Hemodynamic impairment, venous segmental disease, and clinical severity scoring in limbs with Klippel-Trenaunay syndrome. J Vasc Surg. 2007;45(3):561-567.
63. Lee BB, Laredo J, Lee SJ, Huh SH, Joe JH, Neville R. Congenital vascular malformations: general diagnostic principles. Phlebology. 2007;22(6):253- 257.
64. Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44(1):43-57.
65. Weissleder H. Diagnosis of lymphostatic edema of the extremities. Fortschr Med. 1997;115(22-23):32-36.
66. Raju S, Owen S Jr, Neglen P. Reversal of abnormal lymphoscintigraphy after placement of venous stents for correction of associated venous obstruction. J Vasc Surg. 2001;34(5):779-784.
67. Tiedjen KU. Detection of lymph vessel changes in venous diseases of the leg using imaging procedures. Z Lymphol. 1989;13(2):83-87.
68. Thompson B, Gaitatzis K, Janse de Jonge X, Blackwell R, Koelmeyer LA. Manual lymphatic drainage treatment for lymphedema: a systematic review of the literature. J Cancer Surviv. 2021;15(2):244-258.
69. Oliveira MMF, Gurgel MSC, Amorim BJ, et al. Long term effects of manual lymphatic drainage and active exercises on physical morbidities, lymphoscintigraphy parameters and lymphedema formation in patients operated due to breast cancer: a clinical trial. PLoS One. 2018;13(1):e0189176.
70. Hwang JH, Lee KW, Chang DY, et al. Complex physical therapy for lymphedema. J Korean Acad Rehab Med. 1998;22(1):224-229.
71. Hwang JH, Kwon JY, Lee KW, et al. Changes in lymphatic function after complex physical therapy for lymphedema. Lymphology. 1999;32:15- 21.
72. Lee BB. State of art in lymphedema. management: part 1. Phlebolymphology. 2018;25(2):160-171.
73. Lee BB. State of art in lymphedema. management: part 2. Phlebolymphology. 2018;25(3):189-200.
74. Lee BB, Laredo J. Coagulation issue in venous malformation and its management. In: Rao GHR, Kalodiki E, Leong WA, Fareed J, eds. Clinical Handbook of Management of Antithrombotic & Thrombolytic Therapy. New Delhi, India: Kontentworx; 2014:93- 127.
75. Dompmartin A, Acher A, Thibon P, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144(7):873-877.
76. Busuttil A, Lim CS, Davies AH. Post Thrombotic Syndrome. Adv Exp Med Biol. 2017;906:363-375. doi:10.1007/5584_2016_126. PMID: 27628001.
77. Pikovsky O, Rabinovich A. Prevention and treatment of the post-thrombotic syndrome. Thromb Res. 2018;164:116- 124. doi:10.1016/j.thromres.2017.07.008.
78. Rabinovich A, Kahn SR. The postthrombotic syndrome: current evidence and future challenges. J Thromb Haemost. 2017;15(2):230-241. doi:10.1111/jth.13569.