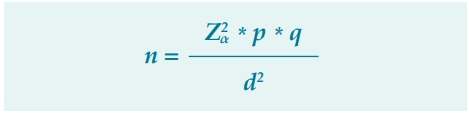Prevalence of neuropathic pain in patients with venous disease in the Guatemalan population
ABSTRACT
Chronic venous disease changes the hemodynamics of venous return, causing intraluminal hydrostatic hypertension and congestion. All of this triggers inflammation, tissue damage, and possible nerve damage due to low oxygen levels. Prevalence of neuropathy was identified in patients with chronic venous insufficiency. In a transversal descriptive study, a nonprobability sample of 370 adult patients with chronic venous insufficiency classified as CEAP 3 to 6 (according to clinical, etiological, anatomical, pathophysiological classification) were evaluated in 4 clinics in Guatemala City where they were assessed for neuropathy via the DN4 scale (douleur neuropathique questionnaire). The prevalence of neuropathy in patients with chronic venous insufficiency (CEAP 3 to 6) was 44.6% [95% CI, 28.9-39.9]. No significant differences were observed in the prevalence of neuropathy by sex and age (P=0.655 and P=0.463, respectively); but according to CEAP classification (P<0.001), the higher the degree of CEAP, the higher the prevalence of neuropathy. It was concluded that more than two-fifths of patients with chronic venous insufficiency may develop neuropathy, with this prevalence being higher in patients with more advanced venous disease.
Introduction
For many years, the term chronic venous insufficiency (CVI) has been used generically, but nowadays we prefer to talk about chronic vein disease (CVD) when referring to any morphological or functional alteration of long evolution of the venous system (especially in the lower limbs). And we reserve CVI for advanced clinical stages (C3-C6).1
CVI is a very common public health problem worldwide, causing a significant impact on the quality of life and economic impact on both patients and the health system. In the United States, up to 7 million people are reported to have chronic venous disease (in France, an estimated 12 million people have varicose veins) at a cost of $40 000 over the lifetime of each of these patients,2-4 and if such patients develop ulceration, the cost to the health care system would be even higher, eg, in the United Kingdom, it can be as much as £100 to £400 million per year. Also, in the United Kingdom, more than 200 000 hospitalizations per year are estimated due to the problem.4,5 The DETECT-IVC study (an epidemiological survey on CVI in Spain) found that 71% of people reported some symptom or sign dependent on CVI.6
There are several symptoms that are described in patients with CVD but none of these alone are pathognomonic of the disease and therefore could have a different origin and cause as they are nonspecific.1,3,4
The symptoms of CVD are so uncertain that even some authors, as in the Edinburgh Vein Study, question whether the symptoms described in surgical textbooks really correspond only to venous disease.7
Most of the venous symptoms such as the heaviness described in large population-based studies, for example, the Edinburgh Vein Study or Bonn Vein Study3,4,7,8 have been investigated in depth. However, there is an understudied range of symptoms that are neuropathic.9
The symptoms described as cramps, dysesthesia, pins and needles sensations, and paresthesia10 seem to correspond more to peripheral neuropathies such as those found in diabetes mellitus; also the ones caused by neurotoxic drugs and vitamin B-12 deficiency and alcohol-related neuropathies, peripheral arteriopathies with microangiopathy, among others.10-13 Peripheral arteriopathies with microangiopathy can produce ischemic polyneuropathy mainly due to tissue hypoxia with consequent neurological damage.10
In CVD there is a sustained ambulatory venous hypertension with capillary distension and extravasation of fluid into the interstitial space. This causes decreased oxygen diffusion and endothelial edema with consequent release of interleukins and free radicals, which can cause damage to all peripheral tissues and may include nerve fibers.2,5,10,14,15
Although neuropathic symptoms are sometimes not adequately recognized in CVD, there is scientific evidence that neurological damage occurs in venous disease at the molecular level where oxidative stress and angiogenesis play the primary role in the development of perfusion-dependent peripheral neuropathy.13
Clinical and electrophysiological methods can be used to evaluate neuropathic pain, the latter being the most accurate for evaluating peripheral nerve, radicular, and plexus lesions, but their cost and complexity limit their use. Among the clinical methods, the most accepted is the DN4 (douleur neuropathique questionnaire), developed in France and the only test validated in Spanish. This questionnaire contains 10 questions and 3 elements of physical examination. It is useful to differentiate neuropathic pain from nociceptive pain with a sensitivity of 83% and specificity of 90%.16
The aim of this study was to determine, in the Guatemalan population, the presentation of neuropathic symptoms in patients with CVI and without suffering from neurodegenerative diseases such as diabetes mellitus or rheumatic disease. Also, whether there was a correlation between the severity of CVI and the neuropathic symptoms.
These results may provide the basis for further research to verify the improvement of neuropathic symptoms following treatment aimed at correcting venous disease.
Objectives
To determine, by means of a validated neuropathic pain scale, the frequency with which patients with CVI (CEAP 3 to 6) present neuropathy.
To determine the frequency of neuropathy according to sex, age, and CEAP classification.
Patients and methods
Patients and methods A prospective cross-sectional descriptive study was carried out from April to September 2023.
The study was carried out in 4 clinical centers in Guatemala City dedicated to the study and treatment of venous pathologies.
Patients were evaluated clinically, and subsequently venous echo-Doppler examination was performed. Patients were evaluated in a standing position and the presence of venous reflux was determined in any segment of the path of the great or small saphenous veins of both lower limbs. The latter was defined as a return of blood greater than 0.5 seconds after performing a Valsalva maneuver or distal compression of the ipsilateral calf.
Patients over 18 years of age with chronic venous disease (CVD) classified as CEAP C3 to C6 were included: C3 corresponding to edema, C4 to skin changes, C5 to closed ulcer, and C6 to active ulcer.
After meeting the above criteria, an interview was performed using the approved form for neuropathies, DN4. Developed by the French Neuropathic Pain Group, it has a sensitivity of 83% and a specificity of 90%. It consists of 10 questions. If the patient answered yes to at least 4 of them, then the pain was considered to have neuropathic characteristics.
Patients with concomitant neurodegenerative diseases such as diabetes mellitus, rheumatic disease, or alcohol-related neuropathies, or polyradiculopathies caused by neurotoxic drugs, vitamin B-12 deficiency or peripheral arteriopathies10-13 were excluded from the study.
The data were tabulated and analyzed in jamovi software version 2.3.28. Variables were described by frequency and percentage. The population prevalence of neuropathy was estimated with a 95% confidence interval of proportions. The evaluation of the statistical association was done with Pearson’s chi-square tests and the estimation of the size of the association with the odds ratio (OR) and their 95% confidence intervals.
To calculate the sample size, the formula for estimating a population proportion was chosen:
Where
Z2α = Standardized z-value, for a confidence level of 95% (1.96)2
p = Proportion of the event of interest, approximately 40% of patients with chronic venous disease are expected to have neuropathic symptoms (0.40).
q = Complement of the proportion of the event of interest obtained by subtracting the value of the event proportion from 1 (1-0.4 = 0.6).
d2 = Sampling error of 5% (0.05)2
n = Minimum sample size of 369 patients
The minimum size calculated was 369 patients, who were randomly selected in a stratified proportional allocation manner from the 4 centers in which the individuals were collected.
Results
In this study, patients with CVI more frequently were female (74.3%), aged between 61 and 70 years (43.2%), and classified as CEAP 3 (60.8%), as shown in Table I.
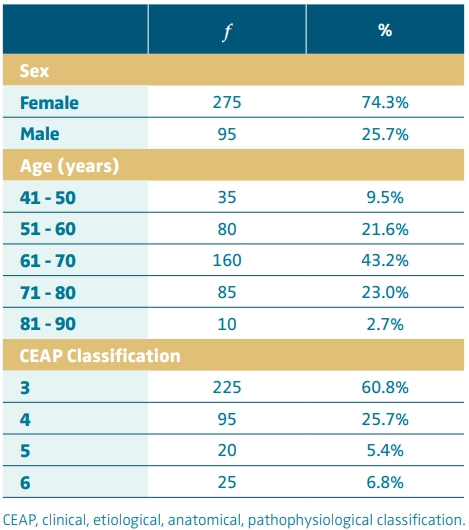
Table I. Characteristics of patients with chronic venous insufficiency, April to September 2023, n = 370.
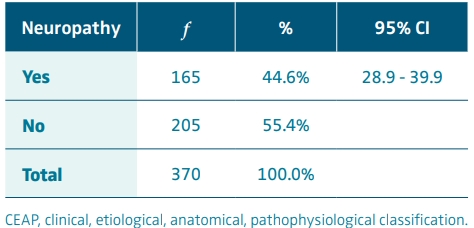
Of the 370 patients with CVI (CEAP 3-6), 165 had neuropathy according to the standardized neuropathic pain scale; therefore, the prevalence corresponded to 44.6%. The 95% CI for the population prevalence of neuropathy ranged from 28.9% to 39.9% (Table II).
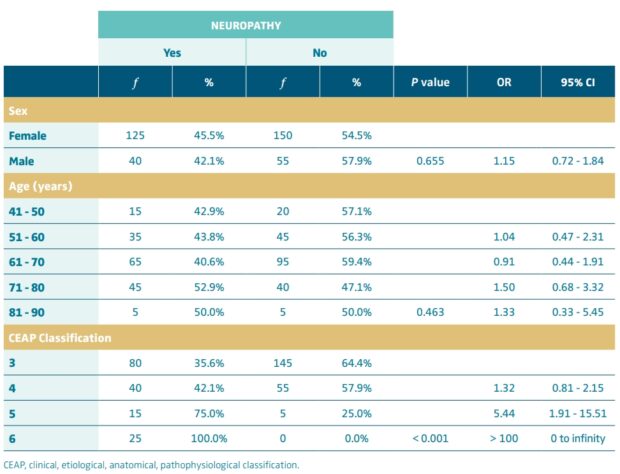
The specific prevalence of neuropathy did not vary significantly by sex or age (P=0.655 and P=0.463, respectively), but did vary significantly by CEAP classification, it was determined that as one had a higher grade in the CEAP classification, the prevalence was higher (P<0.001). Patients with a classification of CEAP 4 were 32% more likely to have neuropathy than those classed as CEAP 3; those with CEAP 5 were 5.44 times more likely to have neuropathy than those with CEAP 3, and those with CEAP 6 were more than 100 times more likely to have neuropathy than those with CEAP 3 (Table III).
The higher the CEAP classification, the higher the prevalence of neuropathy, 35.6% in CEAP 3, 42.1% in CEAP 4, 75.0% in CEAP 5 and 100.0% in CEAP 6 (Figure 1).
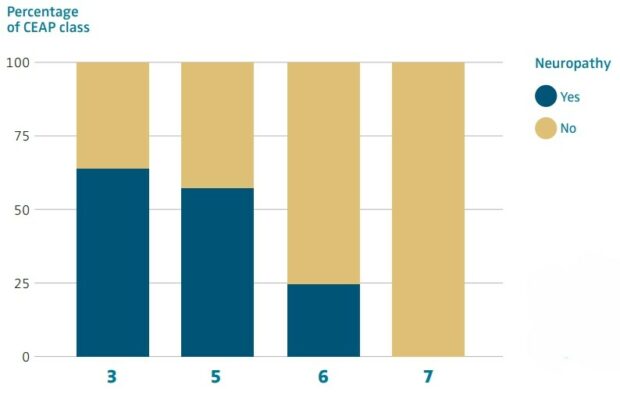
Figure 1. Specific prevalence of concomitant neuropathy according to CEAP classification. CEAP, clinical, etiological, anatomical, pathophysiological classification.
Conclusions
The prevalence of neuropathy in patients with CVI (CEAP 3 to 6) according to the approved DN4 neuropathic pain scale was 44.6% (95% CI, 28.9 to 39.9).
The prevalence of neuropathy in patients with CVI did not vary according to sex and age (P=0.655 and P=0.463, respectively), but did vary according to CEAP classification (P<0.001). The higher the CEAP grade, the higher the prevalence of neuropathy.
Although the associations with gender and age were not statistically significant, it is important to consider that they could have clinical implications in specific contexts. As Dr Kathleen Ozsvath says “we have little understanding of the gender, socioeconomic, and ethnic disparities in both superficial and deep venous disease presentation.” On the other hand, the CEAP classification proved to be a robust and significant predictor of neuropathy, especially in its more advanced stages, which could be useful in risk stratification and clinical decision-making.
CORRESPONDING AUTHOR
Nery Estuardo Orozco Montenegro
6a. AV. 04-01 Edif. Medika 10, clinica 504, Guatemala City, Guatemala, 01010
e-mail: docnery@hotmail.com
References
1. Perrin M, Eklof B, Van Rij A, et al. Venous symptoms: the SYM Vein consensus statement developed under the auspice of the European Venous Forum. Int Angiol. 2016;35(4):374-398.
2. Shiman MI, Pieper B, Templin TN, Birk TJ, Patel AR, Kirsner RS. Venous ulcers: a reappraisal analyzing the effects of neuropathy, muscle involvement, and range of motion upon gait and calf muscle function. Wound Repair Regen. 2009;17(2):147-152.
3. Wrona M, Jöckel K-H, Pannier F, Bock E, Hoffmann B, Rabe E. Association of venous disorders with leg symptoms: results from the Bonn Vein Study 1. Eur J Vasc Endovasc Surg. 2015;50(3):360-367.
4. Priollet P. Venous edema of the lower limbs. Phlebolymphology. 2006;13(4):183-187.
5. Shami SK, Shields DA, Farrah J, Scurr JH, Coleridge Smith PD. Peripheral nerve function in chronic venous insufficiency. Eur J Vasc Surg. 1993;7(2):195-200.
6. Álvarez Fernández LJ, Lozano F, Marinello-Roura J, Masegosa-Medina JA. An epidemiological survey on chronic venous insufficiency in Spain: the DETECT-IVC 2006 study [article in Spanish]. Angiología. 2008;60:27-36.
7. Bradbury A, Evans CJ, Allan P, Lee AJ, Ruckley CV, Fowkes FG. The relationship between lower limb symptoms and superficial and deep venous reflux on duplex ultrasonography: the Edinburgh Vein Study. J Vasc Surg. 2000;32(5):921- 931.
8. Rabe E, Pannier F. What have we learned from the Bonn Vein Study? Phlebology. 2006;13(4):188-193.
9. Velasco M. Neuropathic pain. Rev Med Clin Condes. 2014;625-634.
10. Reinhardt F, Wetzel T, Vetten S, et al. Peripheral neuropathy in chronic venous insufficiency. Muscle Nerve. 2000;23(6):883-887.
11. Newland MR, Patel AR, Prieto L, Boulton AJM, Pacheco M, Kirsner RS. Neuropathy and gait disturbances in patients with venous disease: a pilot study. Arch Dermatol. 2009;145(4):485-486.
12. Padberg FT, Maniker AH, Carmel G, Pappas PJ, Silva Jr MB, Hobson 2nd RW. Sensory impairment: a feature of chronic venous insufficiency. J Vasc Surg. 1999;30(5):836-843.
13. Smith DI, Tran HT, Poku J. Hemodynamic considerations in the pathophysiology of peripheral neuropathy. In: Artis AS ed. Blood Pressure – From Bench to Bed [Preprint]. IntechOpen; 2018. https://doi. org/10.5772/intechopen.75872
14. Eusen M, Brenaut E, Schoenlaub O, Saliou P, Misery L. Neuropathic pain in patients with chronic leg ulcers. J Eur Acad Dermatol Venereol. 2016;30(9):1603-1605.
15. Yim E, Vivas A, Maderal A, Kirsner RS. Neuropathy and ankle mobility abnormalities in patients with chronic venous disease. JAMA Dermatol. 2014;150(4):385-389.
16. Pérez C, Gálvez R, Huelbes S, et al. Validity and reliability of the Spanish version of the DN4 (Douleur, Neuropathique 4 questions) questionnaire for the differential diagnosis of pain syndromes associated to a neuropathic or somatic component. Health Qual Life Outcomes. 2007;5:66.