Prevention and treatment of venous disorders during pregnancy and the postpartum period
Slobodan TANASKOVIC1,2
Abstract
Chronic venous disease represents one of the most frequent medical conditions that could be observed in the general population. Pregnancy is one of the major predisposing factors for developing venous insufficiency due to an enlarged gravid uterus, which obstructs pelvic venous outflow, and an increase in hormone secretion, which weakens the vein wall. A clinical examination and Doppler ultrasound evaluation are used to diagnose venous insufficiency during pregnancy; these clinical findings can vary from insignificant telangiectases to severe varicose veins and skin damage. The relative risk of a venous thromboembolism (VTE) is increased by approximately 4 to 6 fold during pregnancy, and this risk is increased further during the postpartum period. In the first trimester, many fatal antenatal VTE events could occur; therefore, early prophylaxis for women with a previous VTE is necessary. In woman with a previous VTE, thromboprophylaxis should begin as early during pregnancy as practical, while women without a previous VTE, but with other risk factors, can start antenatal prophylaxis at 28 weeks of gestation. This article reviews and discusses the current guidelines for the diagnosis and treatment of chronic venous insufficiency during pregnancy and the prevention of a VTE. This article also discusses the current role of low molecular- weight heparin, warfarin, venotonic agents, and compression stockings in preventing a VTE and treating venous insufficiency during pregnancy.
Chronic venous insufficiency
Chronic venous disease represents one of the most frequent medical conditions that could be observed in the general population.1-3 The exact prevalence of chronic venous disease is difficult to determine due to differences in the selection criteria and the definition of the disease, but it is estimated to be present in >30% of adults in the Western population,1 affecting 10% to 15% of men and 20% to 25% of women.4 The American Venous Forum and Society for Vascular Surgery has classified chronic venous disease according to the clinical, etiological, anatomical, and pathological (CEAP) findings: C1, no visible or palpable signs of chronic venous disease; C2, telangiectasias or reticular veins; C3, varicose veins (>3 mm); C4, changes in skin or subcutaneous tissue; C4a, pigmentation or eczema; C4b, lipodermatosclerosis, white atrophy; C5, healed venous ulcer; and C6, active venous ulcer.5,6
Chronic venous disease and pregnancy
Predisposing factors for chronic venous insufficiency include age, sex, obesity, lifestyle, heredity, and physical inactivity. Pregnancy is also a major predisposing factor for developing venous insufficiency, and it can be observed in 28% of all pregnancies.7 Laurikka et al analyzed cohorts of 3284 men and 3590 women aged 40, 50, and 60 years, and they found that the prevalence of varicose veins in women with 0, 1, 2, 3, or ≥4 pregnancies was 32%, 38%, 43%, 48%, and 59%, respectively.8 Venous insufficiency occurs during pregnancy for two main reasons: (i) an enlarged gravid uterus with consequent hypertension in the lower extremity veins, distension, and valve damage, which obstructs pelvic venous outflow; and (ii) an increase in hormone secretion, which weakens the vein wall.9-12 Ciardullo et al13 showed that higher serum estradiol levels during pregnancy were significantly associated with venous distensibility and clinical evidence of varicose veins in menopausal women.
Diagnosing venous insufficiency during pregnancy
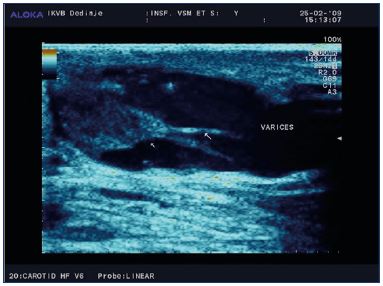
A clinical examination and a Doppler ultrasound evaluation are used to diagnose venous insufficiency during pregnancy. Pain, swelling, itching, burning, or numbness are some of the clinical signs and symptoms that could be observed during the clinical examination and anamnestic questioning; it is also important to determine whether or not the varicose veins were present before pregnancy. If the patient has a family history of chronic venous disease, these symptoms might be more pronounced. The clinical findings vary from insignificant telangiectasias to severe varicose veins and skin damage. Varicose veins can be observed in the great and small saphenous veins, collaterals, or perforating veins (Figure 1). These changes could involve one or both legs, while valvular and perineal varicose veins could be seen in 10% of the cases.13 Skin changes are very rare given the young age of pregnant women and the relatively short period with symptoms of venous insufficiency. However, if such changes occur (C5 or C6 classification), it is recommended to initiate the use of compression stockings immediately. In all patients who have complaints about any type of venous insufficiency, a color Doppler ultrasound of the lower extremities should be performed as early as possible. Changes should be carefully noted and followed up during regular check-ups.
Preventing a VTE during pregnancy
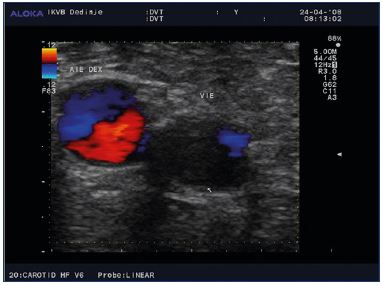
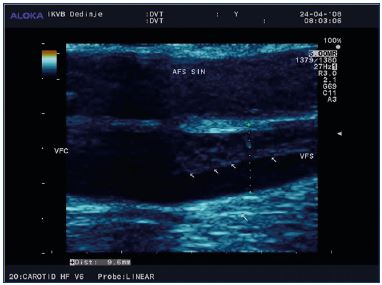
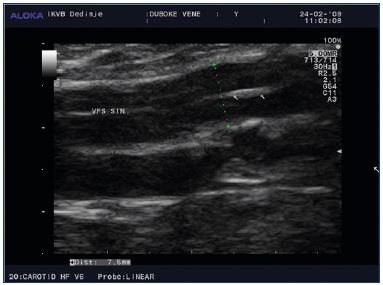
While the relative risk of a venous thromboembolism (VTE) is approximately 4- to 6-fold higher during pregnancy and increases more during the postpartum period, the absolute risk is low with an overall incidence of 1 to 2 per 1000 during pregnancy and the postpartum period.14-19 The absolute incidence of a VTE during pregnancy and the postpartum period is 107 per 100 000 person-years in the UK, 175 per 100 000 person-years during the postpartum period in Denmark, and 175 per 100 000 pregnancies in Canada.14,20,21 In the first trimester, many fatal antenatal VTE events could occur; therefore, prophylaxis for women with a previous VTE should begin early (Figure 2, Figure 3).22-24 The risk for a VTE increases with gestational age, with the maximum risk occurring right after delivery. While a cesarean section is a significant risk factor for a VTE, women having vaginal deliveries are also at risk.25-28 In the postpartum period, the risk of VTE is 5-fold higher heparin throughout the antenatal period (grade C) (Figure 4). For patients with heritable thrombophilia associated with antithrombin deficiency (these patients will often be on long-term oral anticoagulation) or antiphospholipid syndrome, thromboprophylaxis using a higher dose of low-molecular-weight heparin (50%, 75%, or full treatment dose) should be offered antenatally for 6 weeks postpartum or until returned to oral anticoagulant therapy after delivery (grade D). Antenatal prophylaxis should also be considered for all women with asymptomatic antithrombin, protein C or S deficiency, or >1 thrombophilic defect; these patients should be referred to a local expert. Even in the absence of additional risk factors, such patients are recommended to have 6 weeks of postnatal prophylaxis (grade D).
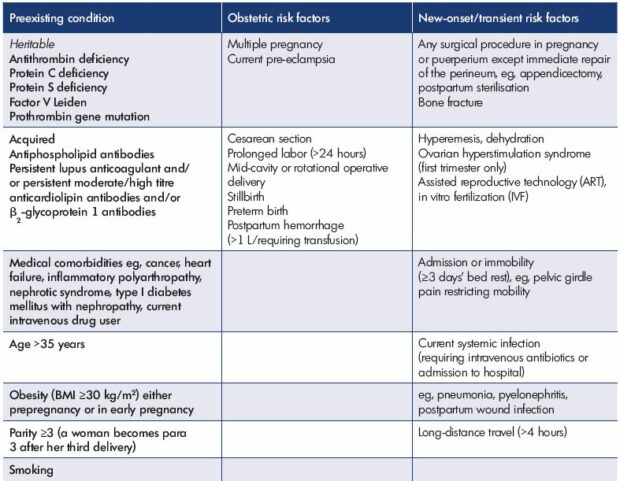
Table I. Risk factors for venous thromboembolism.
From reference 30: Royal College of Obstetricians and Gynaecologists.
https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf.
Starting thromboprophylaxis
In women who have received VTE thromboprophylaxis previously, prophylaxis should be started as early as possible during pregnancy, while women without a previous VTE, but with 3 other risk factors, antenatal prophylaxis can begin at 28 weeks of gestation (grade B, grade C).
Interrupting thromboprophylaxis
When evaluating thromboprophylaxis interruption, women receiving antenatal low-molecular-weight heparin who are having an elective cesarean section should receive a thromboprophylactic dose of low-molecular-weight heparin 1 day before delivery, and, on the day of delivery, any morning dose should be omitted and the operation performed that morning. After delivery, if there is no postpartum hemorrhage and regional analgesia has not been used, the first thromboprophylactic dose of low molecular- weight heparin should be administered as soon as possible. If spinal anesthesia was used, low-molecular weight heparin should be given after 4 hours or after the epidural catheter has been removed; the catheter should not be removed within 12 hours of the most recent injection. If a hemorrhagic problem develops while taking low molecular- weight heparin, the treatment should be stopped and a hematology specialist should be consulted. In women with a VTE after a cesarean section, others risk factors may also be identified, ie, severe pre-eclampsia, reoperation, twin pregnancy, obesity, immobilization, and placenta praevia.34 The risk of a VTE after a cesarean section is at least 2 times higher than after a vaginal delivery.25 In addition, the risk of a VTE is after an emergency cesarean section is 2 times higher than after an elective cesarean section, which is 4 times than the risk after a vaginal delivery.35
According to the official guidelines, all women who have had emergency cesarean sections should be considered for thromboprophylaxis with low-molecular-weight heparin for 10 days after delivery, apart from those having an elective cesarean section who should only be considered for thromboprophylaxis with low-molecular-weight heparin for 10 days after delivery if they have any additional risk factors (Table I, grade C). Low-molecular-weight heparin thromboprophylaxis should be continued for 6 weeks in high-risk women and for 10 days in intermediate-risk women (grade C). The dose of low-molecular-weight heparin is determined based on the patient’s weight (Table II). The use of low-molecular-weight heparin should be reduced in women with renal impairment. Low molecular- weight heparin is safe while breastfeeding. This treatment should be avoided, discontinued, or postponed in women at risk of bleeding after carefully considering the balance between the risks of bleeding and thrombosis (grade D), while women with allergic reactions to low molecular- weight heparin should be offered an alternative preparation or an alternative form of prophylaxis (grade D). The contraindications for low-molecular-weight heparin are shown in the Table III.
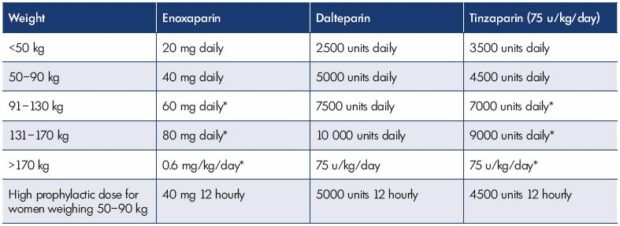
Table II. Low-molecular-weight heparin dosage.
From reference 30: Royal College of Obstetricians and Gynaecologists.
https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf.
Using warfarin during pregnancy
Warfarin has been described to cross the placenta, which increases the risk of congenital abnormalities in approximately 5% of fetuses exposed between 6 and 12 weeks of gestation.36-40 According to the official guidelines, using warfarin during pregnancy is restricted to the few situations where heparin is considered unsuitable, eg, women with mechanical heart valves (grade B). Women receiving long-term anticoagulation with warfarin can be converted from low-molecular-weight heparin to warfarin postpartum when the risk of hemorrhage is reduced, usually 5 to 7 days after delivery. Warfarin is safe while breast feeding.
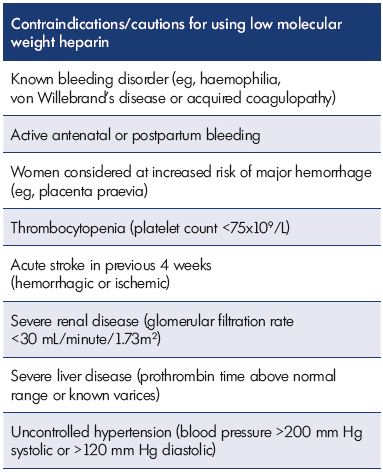
Table III. Contraindications/caution for using low-molecularweight
heparin.
From reference 30: Royal College of Obstetricians and
Gynaecologists.
https://www.rcog.org.uk/globalassets/documents/guidelines/
gtg-37a.pdf.
Non–vitamin K antagonist oral anticoagulants and pregnancy
Although treatment with warfarin is effective, it has numerous limitations, such as a slow onset and offset of action, dosage variations based on differences in dietary vitamin K intake, and multiple drug interactions. To overcome these limitations, new non–vitamin K antagonist oral anticoagulants (NOACs) have been developed to replace warfarin in many indications,41-44 including three inhibitors of factor Xa–rivaroxaban, apixaban, and edoxaban– and one inhibitor of thrombin–dabigatran. In contrast to warfarin, NOACs have a rapid onset and offset of action, a more predictable anticoagulant response with no impact from dietary vitamin K intake, and few drug interactions.
NOACs are indicated, among other indications, for preventing VTE and venous thrombosis. Several large trials compared conventional treatments for VTE with dabigatran, rivaroxaban, apixaban, and edoxaban.45-50 In a recent meta-analysis that compared NOACs with warfarin, 24 455 patients with acute VTE were analyzed for recurrent VTE, fatal pulmonary embolism, and all-cause mortality.51 While the final outcomes were not significantly different (ie, lower) in patients treated with NOACs, both the rate of major bleeding was significantly lower in patients receiving NOACs vs those receiving warfarin (risk reduction [RR], 0.60; 95% CI, 0.41-0.88), as was the rate of fatal bleeding (RR, 0.36; 95% CI, 0.15-0.87).51 Overall, for VTE treatment, NOACs are noninferior to conventional therapies; however, they are associated with a lower rate of bleeding.
There is limited data published concerning the use of NOACs during pregnancy. According to the official guidelines,52 oral direct thrombin inhibitors (dabigatran) and antifactor-Xa inhibitors (rivaroxaban, apixaban) are not recommended during pregnancy (Grade 1C). A recent Canadian study that examined the rate and extent of rivaroxaban transfer across the human placenta53 found that rivaroxaban transfer across the term human placenta occurs rapidly from both the mother-to-fetus and fetus-to- mother directions. Still, bearing in mind that rivaroxaban is highly bound to plasma proteins, the authors suggest that the amount of unbound drug that might reach the fetus is probably much lower.53 In a study by Hoeltzenbein et al,54 63 exposed pregnancies where identified among 94 requests concerning rivaroxaban use during childbearing age. Indications for rivaroxaban were VTE, knee surgery, and atrial fibrillation. After surveillance of 37 pregnancies, there were 6 spontaneous abortions, 8 elective terminations of pregnancy, and 23 live births.54 After recognition of pregnancy, most women discontinued the use of rivaroxaban, mostly in the first trimester, except for one woman that continued until gestational week 26. There was only one case of bleeding in the whole series. There is an obvious risk of taking dabigatran, rivaroxaban, or apixaban during pregnancy and breastfeeding; therefore, larger prospective studies concerning this issue are expected.
Compression stockings and venoactive agents
Therapy for venous insufficiency during pregnancy is mainly directed toward compression stockings and venoactive agents. Some studies showed that compression stockings improved venous blood flow of the lower extremities with a simultaneous decrease in the lumen diameter of the superficial femoral and common femoral veins in patients during late pregnancy and early postpartum.55-57 In patients with known chronic venous insufficiency, compression stockings should be initiated at the start of pregnancy and after the appearance of the first venous disorder in patients with no previous venous abnormalities.58 According to the official guidelines,30 compression therapy of appropriate size and graduated compression pressure of 14 to 15 mm Hg at the calf is recommended during pregnancy and the postpartum period for women who are hospitalized and have a contraindication to low-molecular-weight heparin (grade D), which refers to women who are hospitalized after a cesarean section (combined with low-molecular weight heparin), women considered to be at a particularly high risk of a VTE, and women travelling long distances, ie, >4 hours (grade D).
Conclusion
Venoactive agents are very useful for treating the symptoms of venous insufficiency.59 So far, flavonoids have not shown teratogenic effects, and updated guidelines on the management of chronic venous disorders recommend the use of flavonoids because the benefits clearly outweigh the risks and there is moderate-quality evidence (grade 1B).59 Flavonoids can be used to treat pregnant women with a gestational age greater than 12 weeks; however, the use of flavonoids is not recommended while breastfeeding.

REFERENCES
1. Evans CJ, Fowkes FGR, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53(3):149-153.
2. Fowkes FG, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology. 2001;52(suppl 1):S5-S15.
3. Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27(1):1-59.
4. Callam MJ. Epidemiology of varicose veins. Br J Surg. 1994;81(2):167-173.
5. Rabe E, Pannier F. Clinical, aetiological, anatomical and pathological classification (CEAP): gold standard and limits. Phlebology. 2012;27(suppl 1):114- 118.
6. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248- 1252.
7. Stansby G. Women, pregnancy, and varicose veins. Lancet. 2000;355(9210):1117-1118.
8. Laurikka JO, Sisto T, Tarkka MR, Auvinen O, Hakama M. Risk indicators for varicose veins in forty- to sixty-year-olds in the Tampere varicose vein study. World J Surg. 2002;26(6):648-651.
9. Cordts PR, Gawley TS. Anatomic and physiologic changes in lower extremity venous hemodynamics associated with pregnancy. J Vasc Surg. 1996;24(5):763- 767.
10. Calderwood CJ, Jamieson R, Greer IA. Gestational related changes in the deep venous system of the lower limb on light reflection rheography in pregnancy and the puerperium. Clin Radiol. 2007;62(12):1174-1179.
11. Pemble L. Reversibility of pregnancyinduced changes in the superficial veins of the lower extremities. Phlebology. 2007;22(2):60-64.
12. Ciardullo AV, Panico S, Bellati C, et al. High endogenous estradiol is associated with increased venous distensibility and clinical evidence of varicose veins in menopausal women. J Vasc Surg. 2000;32(3):544-549.
13. Rabe E, Breu FX, Cavezzi A, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(6):338-354.
14. Sultan AA, West J, Tata LJ, Fleming KM, Nelson-Piercy C, Grainge MJ. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156(3):366- 373.
15. Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6(4):632-637.
16. Jackson E, Curtis KM, Gaffield ME. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol. 2011;117(3):691-703.
17. Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost. 2008;6(6):905-912.
18. James AH. Prevention and management of venous thromboembolism in pregnancy. Am J Med. 2007;120(10 suppl 2):S26-S34.
19. Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999;94(4):595-599.
20. Liu S, Rouleau J, Joseph KS, et al; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Epidemiology of pregnancy-associated venous thromboembolism: a populationbased study in Canada. J Obstet Gynaecol Can. 2009;31(7):611-620.
21. Virkus RA, Løkkegaard EC, Bergholt T, Mogensen U, Langhoff-Roos J, Lidegaard Ø. Venous thromboembolism in pregnant and puerperal women in Denmark 1995-2005. A national cohort study. Thromb Haemost. 2011;106:304-309.
22. Gherman RB, Goodwin TM, Leung B, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94(5 pt 1):730- 734.
23. James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol. 2005;193(1):216-219.
24. Blanco-Molina A, Trujillo-Santos J, Criado J, et al; RIETE Investigators. Venous thromboembolism during pregnancy or postpartum: findings from the RIETE Registry. Thromb Haemost. 2007;97(2):186-190.
25. Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176(4):455-460.
26. Sultan AA, Tata LJ, West J, et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood. 2013;121(19):3953-3961.
27. Virkus RA, Jørgensen M, Broholm R, Bergholt T. Successful treatment of massive deep vein thrombosis using catheter-directed thrombolysis and inferior vena cava filter in a puerperal woman. Acta Obstet Gynecol Scand. 2012;91(2):269-270.
28. Won HS, Kim DY, Yang MS, Lee SJ, Shin HH, Park JB. Pregnancy-induced hypertension, but not gestational diabetes mellitus, is a risk factor for venous thromboembolism in pregnancy. Korean Circ J. 2011;41(1):23-27.
29. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year populationbased study. Ann Intern Med. 2005;143(10):697-706.
30. Royal College of Obstetricians and Gynaecologists. Reducing the risk of thrombosis and embolism during pregnancy and the puerperium. Greentop Guideline No. 37a. https://www. rcog.org.uk/globalassets/documents/ guidelines/gtg-37a.pdf. Published April 2015. Accessed June 19, 2017.
31. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e691S-e736S.
32. Duhl AJ, Paidas MJ, Ural SH, et al; Pregnancy and Thrombosis Working Group. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromboembolism and adverse pregnancy outcomes. Am J Obstet Gynecol. 2007;197(5):457.e1- e21.
33. Samama CM, Albaladejo P, Benhamou D, et al; Committee for Good Practice Standards of the French Society for Anaesthesiology and Intensive Care (SFAR). Venous thromboembolism prevention in surgery and obstetrics: clinical practice guidelines. Eur J Anaesthesiol. 2006;23(2):95-116.
34. Jacobsen AF, Drolsum A, Klow NE, Dahl GF, Qvigstad E, Sandset PM. Deep vein thrombosis after elective cesarean section. Thromb Res. 2004;113(5):283- 288.
35. Macklon NS, Greer IA. Venous thromboembolic disease in obstetrics and gynaecology: the Scottish experience. Scott Med J. 1996;41(3):83- 86.
36. Holzgreve W, Carey JC, Hall BD. Warfarin-induced fetal abnormalities. Lancet. 1976;2(7991):914-915.
37. Born D, Martinez EE, Almeida PA, et al. Pregnancy in patients with prosthetic heart valves: the effects of anticoagulation on mother, fetus, and neonate. Am Heart J. 1992;124(2):413- 417.
38. Sareli P, England MJ, Berk MR, et al. Maternal and fetal sequelae of anticoagulation during pregnancy in patients with mechanical heart valve prostheses. Am J Cardiol. 1989;63(20):1462-1465.
39. Schaefer C, Hannemann D, Meister R, et al. Vitamin K antagonists and pregnancy outcome – a multi-centre prospective study. Thromb Haemost. 2006;95(6):949-957.
40. Vitale N, De Feo M, De Santo LS, Pollice A, Tedesco N, Cotrufo M. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol. 1999;33(6):1637-1641.
41. Hirschl M, Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism – a systematic review with indirect comparisons. Vasa. 2014;43(5):353-364.
42. Cohen AT, Hamilton M, Mitchell SA, et al. Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLoS One. 2015;10(12):e0144856.
43. Morimoto T, Crawford B, Wada K, Ueda S. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66(6):466-474.
44. Lip GY, Mitchell SA, Liu X, et al. Relative efficacy and safety of non-vitamin K oral anticoagulants for non-valvular atrial fibrillation: network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88-94.
45. Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352.
46. Schulman S, Kakkar AK, Goldhaber SZ, et al; RE-COVER II Trial Investigators. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764-772.
47. Bauersachs R, Berkowitz SD, Brenner B, et al; EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510.
48. Büller HR, Prins MH, Lensing AW, et al; EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287-1297.
49. Agnelli G, Buller HR, Cohen A, et al; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808.
50. Büller HR, Décousus H, Grosso MA, et al; Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369(15):1406-1415.
51. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-328.
52. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S-e226S.
53. Bapat P, Pinto LS, Lubetsky A, Berger H, Koren G. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol. 2015;213(5):710.e1-e6.
54. Hoeltzenbein M, Beck E, Meixner K, Schaefer C, Kreutz R. Pregnancy outcome after exposure to the novel oral anticoagulant rivaroxaban in women at suspected risk for thromboembolic events: a case series from the German Embryotox Pharmacovigilance Centre. Clin Res Cardiol. 2016;105(2):117-126.
55. Nilsson L, Austrell C, Norgren L. Venous function during late pregnancy, the effect of elastic compression hosiery. Vasa. 1992;21(2):203-205.
56. Büchtemann AS, Steins A, Volkert B, Hahn M, Klyscz T, Jünger M. The effect of compression therapy on venous haemodynamics in pregnant women. Br J Obstet Gynaecol. 1999;106(6):563- 569.
57. Jamieson R, Calderwood CJ, Greer IA. The effect of graduated compression stockings on blood velocity in the deep venous system of the lower limb in the postnatal period. BJOG. 2007;114(10):1292-1294.
58. Partsch H, Flour M, Smith PC; International Compression Club. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Int Angiol. 2008;27(3):193-219.
59. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs – guidelines according to scientific evidence. Int Angiol. 2014;33(2):87-208.