Progress in the management of venous disease during our five decades as surgeons
Bo EKLÖF, MD, PhD2
1Trimiklini, Cyprus;
2Råå, Sweden
Abstract
The authors’ experience spans 5 decades of development in the management of venous disease. They describe their journey through the open surgery era; the transforming introduction of duplex ultrasound scanning; the establishment of consensus-driven classification, terminology, and investigatory planning, hugely important for research and patient care; and the emergence of minimally invasive endovascular procedures during the 21st century with greater acceptability by patients, minimal morbidity/ mortality, and wider application than open surgery. The management of patients with venous disease has progressed immensely since the 1960s.
Introduction
At the 12th annual meeting of the American Venous Forum in Phoenix, Arizona, year 2000, Professor Norman Browse gave the keynote lecture, in his provocative way questioning if anything was new in phlebology since the time of Hippocrates. His presentation was delivered right at the tipping point of the advancement of research and treatment of venous disease. Today in 2022, we can clearly state that much is new since ancient times. The authors’ decades-long journey in venous disease management and their contributions are described here. It is the personal experience of two vascular surgeons with a life-long interest in the diagnosis and treatment of venous disease, who also for several years were fortunate and privileged to closely collaborate with two pioneers in venous disease, Bob Kistner (with Bo Eklöf) and Seshadri Raju (with Peter Neglén). This presentation should not be viewed as a well-referenced comprehensive review and does not lay claim to be an accurate or objective account of the evolution of modern phlebology. It is a subjective record of the events that affected us.
1960-1990: era of open surgery
In the 1960s and 1970s, early in our careers, the prevalent misconceptions of phlebology were that venous disease equated to varicose veins, that it involved the science of reflux alone, and the belief that surgery cannot be performed in the deep system. Only a few general and vascular surgeons were interested in venous disease worldwide, especially in the United States.
Contrarily, the Scandinavians performed basic research in its diagnosis and management at that time.1 The percutaneous ultrasound was not available, and investigations were made by venography and invasive flow measurements. It was in that inherited tradition that our interest in venous disease was nurtured. At that time, invasive surgical treatment of varicose veins was predominant. The technique was refined over time. The radical operations with large incisions under general anesthesia and several days in hospital evolved to minimally invasive techniques (great saphenous vein [GSV] stripping with local avulsions) as an outpatient procedure performed under regional or local anesthesia. Present-day surgery is further advanced with ultrasound guidance and minute stab avulsions. Liquid sclerotherapy was used alone or in combination with high ligation of the GSV with variable results, but it was still inferior to the minimally invasive open surgery.2
Already in 1968, Bob Kistner described the first successful repair of a deep venous valve, an internal valvuloplasty, and the results in 17 patients were later presented in 1975.3,4 He proceeded to perform transposition (1975) and external valvuloplasty (1991) as an alternative to treat deep venous reflux. Although the internal valvuloplasty was not initially recognized as a breakthrough, it was an extraordinary feat. Generally, it showed that surgery of the deep venous system did not invariably lead to thrombosis, as was the predominant belief, and, specifically, that it was possible to repair the delicate venous valve leaflets. It rekindled interest in deep venous disease, especially deep venous reflux, and the treatment of extensive deep venous thrombosis (DVT).
Several alternative approaches to direct valvuloplasty have since been described. Alternative external methods to control deep reflux were already introduced in 1972, so called “cuffing” by Hallberg. In the absence of valve leaflets, such as in postthrombotic disease, a transplantation of a valve-carrying axillary vein segment to the femoral or popliteal veins was described in 1982 by Taheri. Angioscopy-assisted valvuloplasty was used in 1991. As late as 2007, Maleti developed a unique “neovalve” reconstruction by skillful dissection of the vein wall to build at least 1 valve leaflet. Valvuloplasty in patients with primary vein reflux has been shown to have a sustained cumulative clinical improvement and valve competence long term (around 75%), whereas competency in postthrombotic patients steadily deteriorates over the years to a low rate of 25% after 8 years. Under the best circumstances, these patients can be offered an ulcer-recurrence–free interval of 60% for 6 years.5,6 The valve repair in the most common postthrombotic group is nowadays therefore considered a “last ditch” attempt in patients with severe chronic venous insufficiency (CVI). Open deep repair was never popularized; Drs Kistner, Raju, and Sotturai were most active in the United States, and Dr Perrin had the largest experience in Europe.7 The necessary skill set, the careful selection of patients, and the variable long-term durability have today limited its use to a few interested venous surgeons. However, there is no doubt that relief of an axial deep reflux leads to dramatic clinical improvement with 95% of primary healing of venous ulcers. One-third of patients have complete relief of symptoms with no compressive stockings for more than 10 years.5
In Europe and Scandinavia, a few enthusiastic surgeons started early to explore flow-directed or systemic deep thrombolysis with streptokinase to treat acute DVT. It never caught on because the complications frequently were severe. Open femoro-ilio-caval thrombectomy was popularized in some centers in Europe. During the 1970s and 1980s, the technique was advanced using contrast-dye–filled venous Fogarty balloons, more efficient distal clearance, temporary arteriovenous fistulae, percutaneous closure of the arteriovenous fistulae, and percutaneous balloon dilation of any residual stenosis.8 We also started to better understand the hematological contributing factors to acute DVT as multiple types of thrombophilia were discovered. Our collected experience for 3 decades working in Sweden, Kuwait, and Hawaii includes over 200 treated patients. The long-term outcome has been favorable in pooled large series (465 patients) with a 73% patency rate, femoro-popliteal patency/competence in 44%, and symptom-free lower limb in 63% of operated patients. Despite positive results, no properly powered randomized study has ever been performed.
The basic experiences gathered from this “open surgery era” thrombectomy were important for facilitating the later introduction of percutaneous removal of thrombus. Although clinical and physiological improvements were shown with open thrombectomy, the Achilles’ heel was the inability to treat the frequently revealed underlying venous stenosis and to achieve a satisfying clearance of the femoral-popliteal segment. It was not until percutaneous catheter-directed urokinase infusion and venous stenting were introduced in the 1990s9,10 that the treatment paradigm of iliofemoral DVT shifted and early thrombus removal was popularized.
Chronic venous obstruction was, during the “open surgery era,” treated like arterial obstruction with bypass surgery. The Palma procedure (suprapubic transposition of the GSV; a “cross-over by-pass”) was already introduced in 1960.11 When a suitable GSV was unavailable, a 10-12-mm ringed polytetrafluoroethylene (PTFE) graft was later used, but with a much inferior patency rate. Using artificial grafts for venous bypass surgery was frowned upon in the 1980s due to the high failure rate. Short and long iliac, ilio-caval, and femoroilio- caval occlusions were, however, treated by in-line ringed PTFE grafts. Open surgical venous reconstructions were and are still challenging and demand life-long anticoagulation, and patency is affected by the type of conduit, graft material, low venous pressure, and the presence of thrombophilia. Open venous bypass surgery was only considered in select patients, those fit for surgery and with severely symptomatic, preferably short chronic venous obstruction. With correct selection of patients, Palma vein and iliofemoral/ilio-caval PTFE bypasses have been shown to have excellent cumulative secondary patency rates (70%-83% patency at 3-6 years and 85% at 10 years; respectively) with good symptomatic relief.12,13 With later introduction of percutaneous venous stenting this treatment paradigm changed.
1990: era of diagnosis and classification
The authors worked together from 1971 to 1990. In the early 1990s, we were privileged to start working with pioneers in venous surgery both in research and practical management. Bo Eklöf joined Bob Kistner in Hawaii in 1991, and Peter Neglén moved to Mississippi to share practice with Seshadri Raju in 1997 after a stint as Visiting Professor at the University of Mississippi in 1990-1991 (Figure 1).

Figure 1. The 3 venous musketeers and D’Artagnan (from left to right, Bob Kistner, Peter Neglén, Seshadri Raju, and Bo Eklöf). Photo provided courtesy of Peter Neglén.
During the late 1980s, real-time and duplex ultrasound scanning (DUS) arrived. It revolutionized venous investigations as it opened the venous system for noninvasive studies in acute and chronic disease. It was now possible to separate venous segments in the deep and superficial systems. The definition of valve function was changed as the duration of retrograde flow could be quantified (1989).14 Multi-segment reflux scores were shown to correlate to clinical severity. Morphological segmental venous obstruction could be visualized confidently. Several studies showed in 1992-1993 that DUS was superior to ascending and descending venography as a diagnostic tool and replaced it as the main morphological investigation. Thus, the availability of DUS changed the whole paradigm of diagnosis. The ongoing controversy whether venous ulcer would only develop in the presence of deep venous reflux was quickly laid to rest. It was shown that superficial reflux alone can result in ulcer formation. The dispute whether descending venography was to be performed in supine or semi-erect position was buried. DUS quickly made the continuous wave Doppler (CWD) obsolete in most countries, and venography became largely a preoperative investigation. Air plethysmography was first presented in 1987.15 It was rapidly accepted as a good research and teaching tool on global venous hemodynamics. It was possible to differentiate between reflux (venous filling index was validated to reflect global reflux) and calf muscle pump function (ejection fraction). Initially, it was thought that residual volume fraction correlated linearly to ambulatory venous pressure, but several reports subsequently showed this not to be true.
An advanced vascular laboratory using noninvasive investigations including DUS and invasive pressure measurements was established in Mississippi in the early 1990s. It was the foundation for basic venous physiological research and assessment of results of surgery, resulting in numerous publications.16 The factors involved in the development of CVI proved to be multiple, involving parallel systems with reflux and/or obstruction and microvascular events (Figure 2).
Despite comprehensive investigations, the results of preoperative investigation did not always correctly reflect the severity of disease in individual patients, and clinical improvement was not necessarily resulting in physiological improvement post intervention. Although correlations were found in groups of patients, the tests were unable to place a patient in a specific class. This was very disappointing. The challenge was and still is to be able to identify and quantify the presence of reflux and obstruction in each vein segment and to assess the contribution of each individual or groups of segments to the global hemodynamics. If so, we would be able to direct the treatment to the dominant contributor in a complex multisystem disease. It would be immensely helpful because it has been clearly shown that the clinical condition is improved by partial correction of the pathology. The difficulty to assess physiological outcome led to the introduction and popularization of clinical severity scores and quality-of-life (QOL) assessments.
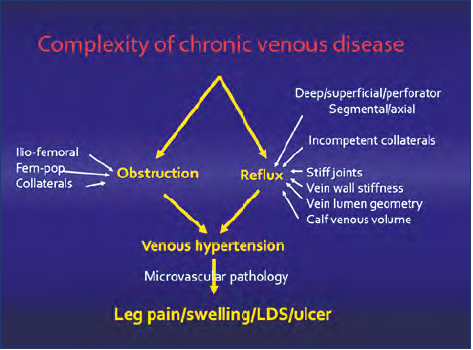
Figure 2. The complexity of the pathology of chronic venous disease is schematically outlined. Axial or segmental reflux may occur in 3 parallel axial systems: the profunda, femoropopliteal, and saphenous systems. The contribution of obstruction depends on which segment(s) is involved in the popliteo-femoro-ilio-caval outflow. The calf muscle pump may compensate for reflux or malfunction, increasing the venous pressure. The microvascular pathology induced by hypertension finally results in venous signs and symptoms. Abbreviations: Fem-pop, femoro-popliteal; LDS, lipodermatosclerosis.
Several organizations with specific interest in venous disease were established. In 1989, the American Venous Forum was founded by 20 members of the Society for Vascular Surgery. The first president was John J. Bergan. Initially, its membership was select and academic, but later it has substantially widened. Now, 33 years later, it is the leading phlebological society in the world with 800 members and a strong influence on the management of venous and lymphatic disease through its publication, Journal of Vascular Surgery: Venous and Lymphatic Disorders, and important consensus work. In the United States, the American College of Phlebology (now renamed American Vein & Lymphatic Society) had a profound impact, especially on the treatment of varicose veins and other superficial venous disorders. Although the European national phlebological societies had been active since the 1970s, the increased interest for phlebology resulted in the founding of the European Venous Forum in 2000. During the same period, the Asian Venous Forum, the Latin American Venous Forum, and the Australian and New Zealand Society of Phlebology were established.
Importantly, all this interest and societal activity saw the beginning of consensus work on classification, terminology, and investigatory planning, which continues today. An accurate classification system in venous disease is fundamental to understanding the clinical disease processes and to facilitate communication. In 1994, the American Venous Forum convened a consensus committee that created the CEAP (Clinical, Etiology, Anatomy, Pathology) classification (revised in 2004 and 2020),17 which provided a snapshot description of each individual patient. The classification made an appropriate comparison of patient cohorts possible, hugely important for venous research. “Basic” CEAP is also very useful to guide diagnosis and workup in daily practice. It is an instrument to make a correct diagnosis and to guide appropriate treatment. As CEAP was not intended to be used for linear follow-up, the American Venous Forum created the VCSS (Venous Clinical Severity Score) for this assessment. The difficulty to assess physiological outcomes led to the popularization of QOL assessments, both generic and venous disease–specific tools.
During the 1990s, open surgery for valve repair continued and was continuously refined as described above. A game changer would have been an off-the-shelf durable artificial valve that could be placed percutaneously. During the last decades, we have seen many artificial valves pass our eyes. Millions of dollars have been invested by companies to develop this “Holy Grail.” Although several devices have had initial promising results in vitro or in animals, ultimately all of them have thrombosed or failed when placed in humans so far.
During the 1990s, the utilization of venous DUS increased exponentially. Multiple endovascular devices for percutaneous arterial interventions were developed. Simultaneously, interest in percutaneous procedures on the venous system emerged. When the first generation of radiofrequency and laser obliteration of saphenous veins was introduced in 1999-2000, interest in venous disease skyrocketed.
The 21st century: tipping point and era of endovenous interventions
The VEITH symposium continues to be the largest vascular meeting in the United States. In 2002, 7 papers were presented in a venous session on late Friday afternoon, when most delegates vanished to enjoy the Big Apple. Eleven years later, in 2013, 175 papers on venous disease were given during 17 sessions over 3 days, sometimes with simultaneous sessions. What had happened? Was it progress?
We believe that several factors converged during the first decade of the new century. A major reason was that endovascular procedures largely started to replace open surgery (except for valve repair) (Figure 3). After the initial introduction of saphenous vein closure, improvements and novel methods were approved, which used various laser frequencies, steam, glue, foam sclerosants, etc. Numerous prospective randomized studies have been performed comparing treatment modalities. All methods were shown to be efficacious and resulted in a similar improvement in VCSS and QOL.18 However, recanalization of the saphenous veins and repeated treatment were more frequent after foam sclerotherapy. The differences between modern open surgery and the endovenous procedures are insignificant in this aspect. No treatment modality can be recommended as superior to another. Modern open surgery is still the leading procedure in the world except for the United States and some European countries where endovenous procedures have taken over completely. This is probably due to the relatively high device costs or reimbursement issues.
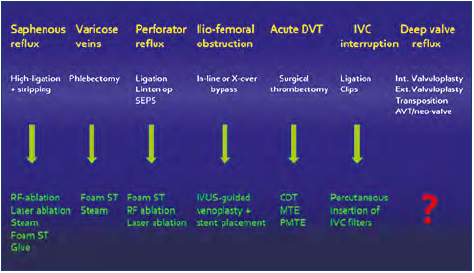
Figure 3. Open surgery is being replaced by minimally invasive endovenous procedures. Abbreviations: AVT, axillary vein transposition; CDT, catheterdirected thrombolysis; DVT, deep venous thrombosis; IVC, inferior vena cava; IVUS, intravascular ultrasound; MTE, mechanical thrombectomy; PMTE, pharmacomechanical thrombectomy; RF, radiofrequency; SEPS, subfascial endoscopic perforator surgery; ST, sclerotherapy.
Percutaneous placement of inferior vena cava (IVC) filters increased, and after a peak of generous usage, now have a defined, more restricted, temporary application. In 1995, the first cases of early clot removal for acute iliofemoral DVT were reported using catheter-directed urokinase and later recombinant tissue plasminogen activator (rt-PA) infusion, largely replacing the open thrombectomy.9 Mechanical devices with or without concomitant lysis were later developed. In the majority of cases, an underlying stenosis was revealed and treated in the same session with stent placement. The ultimate treatment would be to remove the blood clot, stent the causative stenosis, and have the patients return home within 24 hours. Much hope was tied to the ATTRACT study (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis) in 2017, a randomized study of anticoagulation and various clot removal methods, to once and for all ensure efficacy of early iliofemoral clot removal and stenting.19 Due to design and other issues, unfortunately, no dedicated conclusions could be made in this aspect.
Studies also emerged at that time showing that venous disease had a major impact on QOL and loss of work, although rarely being life or limb threatening. Long ignored, a large population had been underserved, and there was a present need of care. Campaigns by various organizations educated and made the population increasingly aware of the possible downsides of acute thromboembolism, postthrombotic disease, and varicose veins, and it wanted treatment. The novel minimally invasive procedures with low morbidity and rare mortality were certainly more readily accepted than the previous open surgery.
A major driver for the development was economy-based. As the procedures were attractively reimbursed (privately paid or by insurers), money could be made. With the evolution toward percutaneous therapy, not only surgeons but other specialties with catheter skills such as cardiologists and interventional radiologists were building venous therapy within their practices. Weekend-trained therapists with “hole-in-the wall” establishments jumped on the varicose vein treatment train. The medical device companies realized the emergence of a lucrative market in creating new technologies. Industry placed their commercial resources behind the surge, especially in the field of saphenous vein obliteration. Investors supported many start-ups with novel ideas.
Was the economy-based development unfavorable? A money driven system will always be flawed by a potential widening of indications or an unnecessary use of a treatment modality, especially if the therapists poorly understand the disease and are not properly certified. This abuse was and still is observed (Figure 4). On the other hand, the high frequency of treatment stimulated novel studies on multiple aspects of venous disease. The science and research then drove additional innovation and progress. There is no doubt that the endovenous treatments have substantially improved QOL for most patients, and without industry support, this would not have happened.

Figure 4. Professor Bo Eklöf lamenting the overuse of endovenous procedures in his signature way by singing a song (Blowing in the Wind) at an American Venous Forum gala: How many stents must a doctor insert? Before you call him a crook? Wrong indication, the patient is hurt The doctor should be on the hook! How many veins should be burned or be cooked when reasons are overlooked?The answer my friend is blowin’ in the wind. The answer is blowin’ in the wind. Photo provided courtesy of Peter Neglén.
Little attention was given to chronic pelvic outflow obstruction until the mid-1990s because of the limitations of invasive open surgery. DUS was rarely carried out above the groin. With the comprehensive workup performed in Mississippi, Peter Neglén and Seshadri Raju became interested in the frequently observed stenoses or occlusions in the outflow tract of the limb in patients with CVI. The prevailing thought was that stenting in the venous system would invariably result in thrombosis due to low phasic flow and pressure. They started iliofemoral venous stenting in earnest in 1997 (Figure 5). The favorable outcomes of the first 92 stented patients were reported in 2000.10,20 Already in 1999, clinical and investigatory follow up started to be prospectively entered into a time-stamped standardized electronic medical records program, allowing ideal retrospective analysis. In 2007, the Mississippi experience reported cumulative analysis at 6 years of stent-related outcome and clinical and hemodynamic results in 982 patients stented for chronic obstructive lesions of the femoro-ilio-caval vein under intravascular ultrasound (IVUS) guidance.21 It showed that venous stenting could be performed with low morbidity and mortality, a long-term high patency rate, and a low rate of in-stent restenosis. It resulted in major symptom relief in patients with chronic venous disease. However, this was not consistently reflected in any substantial hemodynamic improvement by conventional measurements. The beneficial clinical outcome occurred regardless of presence of remaining reflux, adjunct saphenous procedures, or etiology of obstruction. These results have been reproduced by numerous single cohort studies since then.
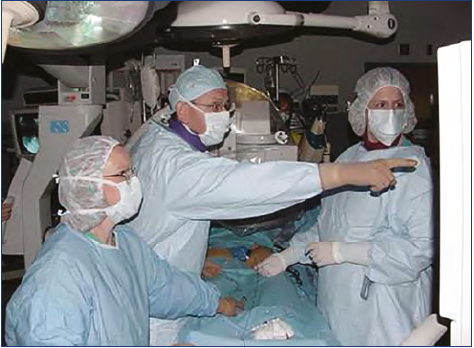
Figure 5. Early venous stenting in a dedicated interventional room in the operating theater of River Oaks Hospital, Mississippi, a rarity anywhere in 1997. Dr Neglén, seemingly excited, pointing at a venous outflow stenosis on the screen. Photo provided courtesy of Peter Neglén.
Nowadays, a thorough assessment of the ilio-caval venous outflow tract is mandatory in the workup of a patient with CVI. IVUS-guided stenting of the chronic venous femoro-iliocaval outflow has largely replaced open surgery and widened the strict indications for open surgery. Bypass surgery should nowadays not be performed unless a recanalization and stenting has been attempted or failed. In the 2010s, industry was convinced of the importance of venous stenting and the market opportunity. Several venous-dedicated stents have been developed and approved.
The unsatisfying aspect of diagnosis of obstruction is the lack of a validated hemodynamic test. The indication for placing a stent is a combination of symptoms and morphological measurement, arbitrarily shown to be more than 50% to 60% stenosis as detected by IVUS. As previously noted, it is not possible to detect the dominant pathology in a complex venous pathology. Therefore, our therapeutical approach is essentially blind. An initial treatment alternative is chosen because of its simplicity, minimal invasiveness, low mortality/ morbidity, and favorable clinical outcome in most patients. Therefore, venous stenting of the outflow of the limb and control of superficial reflux by minimally invasive methods are primary interventions in these patients. Compression therapy and local ulcer treatment is performed simultaneously (Figure 6). It is of paramount importance not to rely on these conservative measures alone, but to initially perform a proper investigation of the entire venous system of the lower limb and its outflow.

Figure 6. The champion of bandaging, Professor Hugo Partsch placing a compression bandage on the champion of deep valve repair, Dr Bob Kistner. This illustrates the new mantra that the cornerstone for management of chronic venous disease is not compression therapy, but an accurate diagnosis and classification of the underlying venous problem to direct appropriate treatment, be it conservative and/or interventional. Photo provided courtesy of Bo Eklöf.
To conclude, we can firmly state that the 21st century has seen a major emergence of successful treatment alternatives for venous disease. The DUS has been a pivotal tool for progress in understanding the venous circulation. Our main disappointment is that despite studies of the venous pathophysiology, we are still unable to build an adequate model of the venous circulation. In the future, we need to have a better understanding of venous physiology and develop accurate tests for obstruction and reflux to guide treatment. No doubt novel devices to treat venous disease minimally will be developed; perhaps the endovascular valve replacement will see daylight. New anticoagulants and agents targeting venous thrombus inflammation better preventing postthrombotic syndrome are on the horizon.
Our hope is that interest in venous disease will be less driven by monetary gain and more a requirement for proper education and certification of venous therapists. This would no doubt improve outcome and decrease any abuse. Teaching is provided by many societies and organizations such as the American Venous Forum with its fellows and attending courses and the European Venous Forum (EVF) with its EVF Hands-on Workshop (EVF HOW) and EVF HOW Plus courses (Figure 7). Certification is given by the American Vein & Lymphatic Society (AVLS) in the United States and a recently established European Board of Phlebology.

Figure 7. The authors (Peter Neglén left) visiting the operating theaters in Ankara during a meeting arranged by the Turkish Vascular Society in 2006. Photo provided courtesy of Peter Neglén.

REFERENCES
1. Eklöf B, Gjöres JE, Thulesius O, Bergqvist, eds. Controversies in the Management of Venous Disorders: Scandinavian Contributions on Venous Problems with Comments by International Authorities. D. Butterworths; 1989.
2. Einarsson E, Neglén P, Eklöf B. Sclerotherapy or surgery as treatment for varicose veins – a prospective randomized study. Phlebology. 1993;8:22-26.
3. Kistner RL. Surgical repair of a venous valve – case report. Straub Clin Proc. 1968;34:41-43.
4. Kistner RL. Surgical repair of the incompetent vein valve. Arch Surg. 1975;110:1336-1342.
5. Masuda EM, Kistner RL. Long-term results of venous valve reconstruction: a four-to twenty-one-year follow-up. J Vasc Surg. 1994;19:391-403.
6. Raju S, Fredericks RK, Neglén P, Bass JD. Durability of venous valve reconstruction techniques for “primary” and postthrombotic reflux. J Vasc Surg. 1996;23:357-67.
7. Perrin M. Reconstructive surgery for deep venous reflux: a report on 144 cases. Cardiovasc Surg. 2000;8:246-255.
8. Eklöf B. Surgical thrombectomy for iliofemoral venous thrombosis revisited. J Vasc Surg. 2011;54:897-900.
9. Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487-494.
10. Neglén P, Raju S. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7:79-91.
11. Palma EC, Esperon R. Vein transplants and grafts in the surgical treatment of the postphlebitic syndrome. J Cardiovasc Surg (Torino). 1960;1:94-107.
12. Halliday P, Harris J, May J. Femorofemoral crossover grafts (Palma operation): a longterm follow-up study. In: Bergan JJ, Yao JST, eds. Surgery of the Veins. Grune & Stratton, Inc; 1985:241-254.
13. Garg N, Gloviczki P, Karimi KM, et al. Factors affecting outcome of open and hybrid reconstructions for nonmalignant obstruction of iliofemoral veins and inferior vena cava. J Vasc Surg. 2011;53:383-393.
14. van Bemmelen PS, Bedford G, Beach K, Strandness DE. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425-431.
15. Christopoulos DG, Nicolaides AN, Szendro G, Irvine AT, Bull M, Eastcott HHG. Airplethysmography and the effect of elastic compression on venous hemodynamics of the leg. J Vasc Surg. 1987;5:148-159.
16. Neglén P, Raju S. A rational approach to detection of significant reflux with duplex Doppler scanning and air plethysmography. J Vasc Surg. 1993;17:590-595.
17. Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8:342- 352.
18. Rasmussen L, Lawaetz M, Serup J, Björn L, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for GSV varicose veins with 3 years follow-up. J Vasc Surg Venous Lymphat Disord. 2013;349-356.
19. S. Vedantham SZ, Goldhaber JA, Julian SR, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377:2240-2252.
20. Neglén P, Berry MA, and Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20:560-571.
21. Neglén P, Hollis KC, Olivier J, and Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979-990.
