Proteolytic degradation and receptor cleavage in the microcirculation
Institute of Engineering in Medicine
University of California San Diego
La Jolla California, 92093-0412
ABSTRACT
We propose here a previously unrecognized pathogenic mechanism for hypertension and diabetes and the cluster of multifaceted cell dysfunctions characteristic of the metabolic syndrome. The evidence for this new hypothesis is derived from a genetic model with unchecked proteolytic activity, including that of matrix metalloproteinases, which causes cleavage of the extracellular domain of surface receptors and loss of their respective functions. For example, cleavage of the extracellular domain of the β2-adrenergic receptor promotes arteriolar vasoconstriction and elevates central blood pressure; cleavage of the insulin receptor reduces glucose transport and produces insulin resistance; cleavage of vascular endothelial growth factor receptor 2 induces endothelial apoptosis and loss of microvessels, ie, capillary rarefaction. Similarly, cleavage of leukocyte membrane adhesion molecules (CD18, ICAM-1) and the formyl-peptide receptor attenuates leukocyte-endothelial interactions and promotes immune suppression. Chronic blockade of unchecked proteinase activity attenuates cleavage of each of these receptor types and restores their respective cell functions. The effectiveness of chronic proteinase inhibition for patients with the metabolic syndrome remains to be explored.
INTRODUCTION
A characteristic feature of several vascular diseases is the clustering of multiple cell dysfunctions and organ complications. A classic example is the metabolic syndrome, in which multiple comorbidities are present at the same time.1 Besides obesity, patients with the metabolic syndrome have leptin resistance, elevated blood pressure, insulin resistance and hyperglycemia, reduced sleep quality and insomnia, dyslipidemia, capillary rarefaction, and immune suppression, to name a few. How is it possible that elevated arterial blood pressure and increased blood glucose levels with signs of insulin resistance2,3 cluster with microvascular dysfunctions?4 Furthermore, how can a single intervention against the metabolic syndrome, such as caloric restriction, interfere simultaneously with both hypertension and diabetes?5
The mechanism by which each condition arises individually is uncertain and there is as yet no conceptual framework that can explain such a clusteringof cell dysfunctions. Consequently, treatments are limited to symptomatic alleviation rather than targeting the underlying fundamental complication. Thus, the development of an overarching concept is potentially of major importance for the effective treatment of patients with multiple symptoms. Different cell dysfunctions may appear in the same individual to varying degrees and involve different receptors and molecular pathways. The condition as a whole predisposes toward enhanced cardiovascular complications, from atherosclerosis to heart and brain infarct, venous disease, and many other ailments.
In this article, we outline a new way of thinking about the multiple complications that may cause cell dysfunction in the metabolic syndrome. Most of the discussion is limited to an animal model, the spontaneously hypertensive rat (SHR). It is the only metabolic syndrome model in which we currently have a body of evidence in favor of this new hypothesis.
A NEW MECHANISM FOR INFLAMMATION: PROTEINASE ACTIVITY AND RECEPTOR CLEAVAGE
The SHR model was originally created as a high blood pressure phenotype.6 However, besides an elevated blood pressure, this strain exhibits other cell dysfunctions, which include insulin resistance with elevated glucose levels, capillary rarefaction, immune suppression, abnormal red blood cell aggregation, a defective fluid shear stress response, and reduced sleep quality, to name a few.7 Recent analysis of this model has brought to light a mechanism made possible by two key observations:
(i) a previously unrecognized mechanism for inflammation due to chronically unchecked proteinase activity, and
(ii) a set of new substrate targets for these proteinases.
UNCHECKED PROTEINASE ACTIVITY
In the SHR, in vivo microzymographic measurements and gel zymography—designed to detect proteinase activity in vivo in the microcirculation and in living tissue—reveal the presence of active proteinases, at increased levels compared with the control strains, the Wistar Kyoto rat strain and the Wistar strain. Techniques using a combination of fluorescently quenched proteinase substrates that are specific for individual proteinases, as well as antibody labeling, show enhanced matrix metalloproteinases (MMP) activity in the SHR in vivo. Specific MMPs found in the plasma include gelatinases (MMP-2 and MMP-9) and matrilysin (MMP- 7). In addition, MMP-1, MMP-1/-9, MMP-7, and MMP-8 activity can be detected on the endothelium along the mesenteric microvessels whereas less MMP-2 and MMP-3 activity is detected (Figure 1).8 The activity of these proteinases is elevated to a mild but significant degree and is not blocked by tissue inhibitors of metalloproteinases (TIMPs), as in control animals.
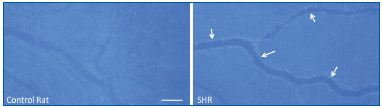
hypertensive rat (SHR) mesenteric microvessels.
Proteinase activity was detected by fluorescent intensity generated
after proteolytic cleavage of a quenched substrate cleaved by both
MMP-1 and MMP-9. The proteinase activity is significantly
enhanced on the endothelium (arrows). Length bar is 100 μm.
Adapted from reference 8.
What are the mechanisms that can generate such unchecked proteinase activity in the SHR? One clue comes from the observation that the SHR has chronically elevated levels of the nuclear transcription factor NF-κB,9 one of the transcription factors with multiple binding sites in the promoter region of most members of the MMP family of proteinases.10 Chronic pharmacological blockade of NF-κB reduces MMP levels in the SHR. The signaling pathway leading to such chronic overexpression of a proinflammatory transcription factor in this genetic form of the metabolic syndrome remains to be clarified.
MMPs are recognized for their ability to restructure the extracellular matrix and any tissue composed of extracellular matrix proteins. In hypertension, arterial hypertrophy and its transition to heart or renal failure, arterial aneurysm, or venous varicosities, are associated with enhanced activity of the members of the MMP family.11-13 This involvement in diseases has led to a number of clinical trials with new molecules that target the MMPs with differing levels of specificities. All these clinical trials are faced with the challenge that MMPs are a required component of wound healing. Therefore, chronic and specific inhibition of MMPs interferes with the resolution of inflammation and a more nuanced approach to pharmacological MMP inhibition needs to be developed. There is a need to identify specific MMPs that are associated with tissue damage. Then, ideally, only those particular proteinases should be targeted using graded levels of inhibitors.
RECEPTOR CLEAVAGE
In the SHR, the endothelium and the membranes of the circulating white and red blood cells show multiple signs of receptor cleavage due to unchecked MMP activity. They are diverse and affect a range of cell functions that are often found to be defective in the metabolic syndrome.
Using selected antibodies against the extracellular domain of the β2-adrenergic receptor, and antibodies against its intracellular domain as control, it is possible to show in vivo that, in the SHR, this particular receptor is cleaved at the extracellular domain.14 (Figure 2) The β2- adrenergic receptor is usually responsible for the vasodilation of the arterioles; thus, proteolytic cleavage of its extracellular domain undermines its ability to dilate the arterioles, a process that makes a major contribution to the elevated arterial blood pressure of the SHR strain. The extracellular domain of the receptor can be cleaved by several MMPs, and plasma of the SHR has even been shown to cleave the extracellular domain of the receptor in naive donor cells. In acute experiments, receptor cleavage can be blocked by several MMP inhibitors. Chronic blockade of the MMPs in the SHR with a mild but broad-acting MMP inhibitor serves to restore the density of the extracellular domain of the receptor, which in turn lowers systemic blood pressure in the SHR.14
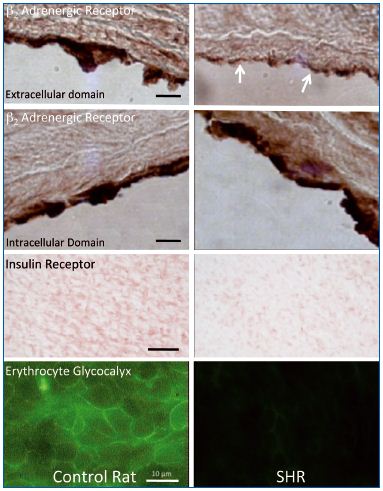
in control and spontaneously hypertensive rat (SHR) aorta.
β2-adrenergic receptor label density was detected by antibodies
against the extracellular (1st row) and intracellular domains (2nd
row) in control and SHR rat aorta. The SHR exhibits a
significantly lower expression of extracellular domains of β2-
adrenergic receptor (arrows) indicating extracellular receptor
cleavage. The extracellular domain label density for the insulin
receptor (3rd row) is also reduced in the SHR, and so is the
glycocalyx membrane density as detected with a lectin label on
fresh tissues (4th row). Length bars are 10 μm, except for the 4th
row in which the length bar is 100 μm.
Adapted from references 14, 16, and 30.
The SHR exhibits characteristic signs of insulin resistance similar to those observed in human hypertensive patients.15 We found evidence that insulin receptor α is associated with defective glucose transport due to proteolytic cleavage of its extracellular domain (Figure 2).sup>16 In several cell types of the SHR, the immunolabel density detected on the extracellular domain is lower than in controls. Extracellular domain density is restored by chronic MMP inhibition, a process that normalizes blood glucose values. Proteolytic cleavage of the extracellular domain of the insulin receptor was also observed in other experimental forms of insulin resistance,17 and was recently documented for the first time in a diabetic patient pilot study.18 Plasma of the SHR has the ability to cleave insulin receptor α in naive control cells and reduce glucose transport into the cell, a process that can also be blocked by several MMP inhibitors.
Another major defect in the SHR is extensive endothelial apoptosis associated, in the microcirculation of many organs, with a reduction of arteriolar and venular branching patterns and a loss of capillary networks, ie, capillary rarefaction.19 The extracellular domain density of VEGFR2, but not its intracellular domain density, is reduced in the SHR.8 Plasma of the SHR, as well as purified MMP-7 and MMP-9, cleaves the extracellular domain of VEGFR2, which then induces apoptosis. Chronic MMP blockade restores both receptor density and normal growth of the capillary networks.20
The SHR exhibits chronically elevated leukocyte counts in the circulation. This is associated with a general “immune suppression” and with reduced numbers of neutrophils and monocytes adhering to the postcapillary venules and migrating into the adjacent tissue.21 Measurement of the extracellular domain density of the CD18 integrin binding sites of leukocytes shows evidence of reduced density. Again, for this receptor, plasma of the SHR is able to cleave the extracellular integrin binding domain in the leukocyte membrane, a process that can also be inhibited by chronic MMP inhibition.16 Chronic blockade of the MMP activity in the SHR reduces the circulating leukocyte counts to control values.
ICAM-1, the counter receptor for CD18 on the endothelium, exhibits a mixed picture of receptor cleavage and overexpression that is organ-dependent.22 This reflects the fact that receptor density on a cell may be compensated by the overexpression of receptors after cleavage. Extracellular fragments of ICAM-1 accumulate in renal glomeruli, indicating that ultrafiltration in this organ may be associated with the accumulation of cleaved protein fragments, itself a potentially proinflammatory process.
The formyl-peptide receptor is involved in the control of pseudopod projection by cytoplasmic actin polymerization as a requirement for the amoeboid migration of leukocytes across the endothelium and into the tissue. The FPR is also required for mechanosensing exposure of leukocytes to fluid shear stress in the circulation.23,24 The fluid shear stress on the plasma membrane of leukocytes reduces pseudopod formation, a requirement for the normal passage of leukocytes through capillary networks and the stereotypic attachment to the postcapillary endothelium as a part of immune surveillance. While not completely abolished, the ability of SHR neutrophils to retract their pseudopods in response to fluid shear stress is significantly attenuated. This compromised ability to retract pseudopods leads to an increased number of leukocytes with pseudopods in the circulation and, consequently, an increase in capillary hemodynamic resistance.25 The reduced response of leukocytes to fluid shear stress is accompanied by proteolytic cleavage of the FPR in the plasma membrane, a process that is corrected with chronic blockade of MMPs in the circulation.26
The consequences of unchecked MMP activity in the circulation of the SHR may also include degradation of the tight junctions (occludin and claudin-5) and elevation of microvascular permeability. This was demonstrated in the SHR blood-brain barrier.27 Elevated permeability is accompanied by the appearance of lower molecular weight fragments of tight junction proteins in the surrounding astrocytes.
Just like many patients with the metabolic syndrome, SHRs have reduced sleep quality with more frequent disruption of their quiet sleep periods.28 We obtained initial evidence that the antibody label density against the extracellular domain of the 5HT-1A receptor is lower in the hypothalamic region of SHRs compared with the normotensive control rats.29 Once again, chronic inhibition of MMP activity may serve to reduce cleavage of the receptor and restore normal receptor density in the sleep centers of the brain.
The proteolytic cleavage of membrane receptors also affects the red blood cells. SHR plasma proteinases cleave the inner core of the red blood cell membrane glycoproteins facilitating dextran- but not fibrinogenmediated red blood cell aggregation.30 (em>Figure 2) The cleavage causes swelling of the red blood cells and impaired adhesion to the scavenger receptors of macrophages, and thus may reduce the rate of removal of the old red blood cells in organs like the spleen. In contrast to the cleavage of the fibrinogen binding sites by MMPs, amylases only cleave the carbohydrate portions of the red blood cell glycocalyx, reducing the surface charge on the cell surface and consequently facilitating fibrinogen-mediated red blood cell aggregation.
In summary, the current evidence in the SHR, a metabolic syndrome experimental model with characteristic hypertension, insulin resistance, etc, is consistent with the hypothesis of uncontrolled proteolytic damage to membrane protein structures and functions (Figure 3). Cells respond to the loss of membrane proteins by synthesizing new proteins, which can lead to a notable enhancement of receptor biosynthesis and cell turnover by enhanced mitosis and apoptosis. It is characteristic of the SHR,31 but at this time largely unexplored in patients.
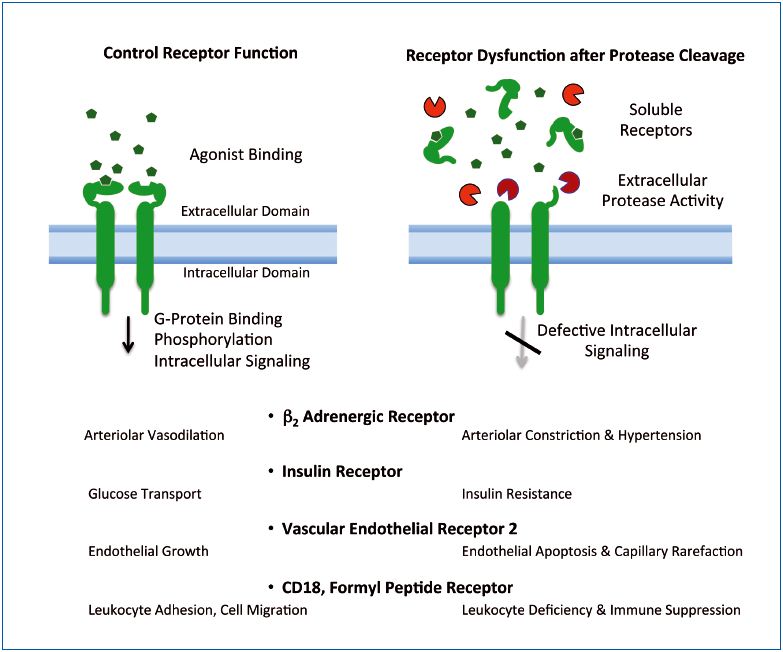
extracellular receptor domains.
EVIDENCE FOR MEMBRANE RECEPTOR CLEAVAGE IN HUMANS
Initial evidence that receptor cleavage may also be present in humans has been derived from biomarker studies in diabetes and essential hypertension patients indicating the presence of “soluble receptors.” These soluble receptors include the insulin receptor, VEGFR2, the IL-6 receptor, the angiopoietin receptor tie-2, the receptor for advanced glycation end products (RAGE), selectins, and other receptors. In general, soluble receptors are present in low concentrations in the plasma and typically constitute extracellular domain fragments of intact receptors.32 Sensitive techniques such as ELISA or mass spectrometry are required for their detection.
As yet, no essential physiological function for normal glucose metabolism or other metabolic needs have been identified for the receptor fragments present in the circulation. Rather, the presence of receptor fragments in plasma may have to be regarded as a consequence of uncontrolled proteolytic receptor cleavage. The fact that the concentration of insulin receptor fragments positively correlates with the glucose levels of diabetic patients32 supports the receptor cleavage hypothesis, ie, the more cleaved receptors are present in solution, the greater the accumulation of extracellular glucose due to defective insulin receptor function after cleavage.
RECEPTOR CLEAVAGE IN CHRONIC VENOUS DISEASE
Can the concept of proteinase activity and receptor cleavage be applied to chronic venous disease?33 Already well-established in chronic venous pressure elevation,34 increased MMP activity (eg, MMP-1, -8, and -9 and tissue inhibitors of metalloproteinases, TIMP-1 and -2) in endothelial cells and in leukocytes adhering to the postcapillary venules is also observed after short-term venous pressure elevations (associated in vivo with a shift in fluid shear stress).33 This has a direct effect on VEGFR2 expression and may contribute to the loss of endothelial cell response.
Taking all this evidence into account, the question arises of whether receptor cleavage may be one of the early trigger mechanisms for the inflammatory cascade that accompanies chronic venous disease. My hope is that we will be able to clarify this question in the short-term. MMP activation in venules, which may be associated with mechanical distension of veins, may benefit from a combination of approaches: on the one hand by a more effective traditional mechanical counteraction against venous vessel distension and, on the other hand, by a partial blockade of activated MMPs.
In either the metabolic disease or chronic venous disease, a pharmacological approach against MMP activity will require a nuanced approach since MMPs are an integral part of tissue repair in the inflammatory process.
CONFLICT OF INTEREST
Dr. Schmid-Schönbein is a scientific advisor to Leading BioScience, INC.

REFERENCES
1. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-1607.
2. Rizzo M, Rizvi AA, Rini GB, et al. The therapeutic modulation of atherogenic dyslipidemia and inflammatory markers in the metabolic syndrome: what is the clinical relevance? Acta Diabetol. 2009;46(1):1-11.
3. Mule G, Cottone S, Nardi E, et al. Metabolic syndrome in subjects with essential hypertension: relationships with subclinical cardiovascular and renal damage. Minerva Cardioangiol. 2006;54(2):173-194.
4. Serne EH, de Jongh RT, Eringa EC, et al. Microvascular dysfunction: causative role in the association between hypertension, insulin resistance and the metabolic syndrome? Essays Biochem. 2006;42:163-176.
5. Fontana L. Calorie restriction and cardiometabolic health. Eur J Cardiovasc Prev Rehabil. 2008;15(1):3-9.
6. Okamoto K. Spontaneous hypertension in rats. Int. Rev. Exp. Pathol. 1969;7:227- 270.
7. Suzuki H, Zweifach BW, Schmid- Schönbein GW. The multifaceted contribution of microvascular abnormalities to the pathophysiology of the hypertensive syndrome. In: Zanchetti A, Mancia G, eds. Handbook of Hypertension, Vol. 17, Pathopysiology of Hypertension. Amsterdam: Elsevier Science B.V.; 1997:482-523.
8. Tran ED, DeLano FA, Schmid- Schönbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47(5):423-431.
9. Wu K-IS, Schmid-Schönbein GW. NF kappaB and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension. 2011;57:261-268.
10. Clark IM, Swingler TE, Sampieri CL, et al. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40(6- 7):1362-1378.
11. Odenbach J, Wang X, Cooper S, et al. MMP-2 mediates angiotensin IIinduced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2010;57(1):123- 130.
12. Lehoux S, Lemarie CA, Esposito B, et al. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109(8):1041-1047.
13. Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23(2):85-98.
14. Rodrigues SF, Tran ED, Fortes ZB, et al. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299(1):H25-H35.
15. Mondon CE, Reaven GM. Evidence of abnormalities in insulin metabolism in rats with spontaneous hypertension. Metabolism. 1988;32:303-305.
16. DeLano FA, Schmid-Schönbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52(2):415-423.
17. DeLano FA, Zhang H, Tran EE, et al. New hypothesis for insulin resistance in hypertension due to receptor cleavage. Expert Review of Endocrinology and Metabolism. 2010;5(1):149-158.
18. Chen AY, Bonner M, Lefkowitz RB, et al. Insulin receptor alpha levels are lower and matrix metalloproteinase (MMP) levels are higher in obese humans with type 2 diabetes (DM2). Obesity 2011. 29th Annual Scientific Meeting. 2011;19 (Abstract suppl 1):524-P.
19. Prewitt RL, Chen IIH, Dowell RF. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am. J. Physiol. 1982;243:H243-H251.
20. Murfee WL, Schmid-Schönbein GW. Chapter 12. Structure of microvascular networks in genetic hypertension. Methods Enzymol. 2008;444:271-284.
21. Suematsu M, Suzuki H, Tamatani T, et al. Impairment of selectin-mediated leukocyte adhesion to venular endothelium in spontaneously hypertensive rats. J Clin Invest. 1995;96(4):2009-2016.
22. Tong S, Neboori HJ, Tran ED, et al. Constitutive expression and enzymatic cleavage of ICAM-1 in the spontaneously hypertensive rat. J Vasc Res. 2011;48(5):386-96.
23. Prossnitz ER, Quehenberger O, Cochrane CG, et al. Signal transducing properties of the N-formyl peptide receptor expressed in undifferentiated HL60 cells. J Immunol. 1993;151(10):5704-5715.
24. Makino A, Prossnitz ER, Bünemann M, et al. G Protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290:C1633-C1639.
25. Fukuda S, Yasu T, Kobayashi N, et al. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ Res. 2004;95(1):100-108.
26. Chen AY, Delano FA, Valdez SR, et al. Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am J Physiol Cell Physiol. 2010;299(6):C1441- C1449.
27. Yang Y, Estrada EY, Thompson JF, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697-709.
28. Kuo TB, Shaw FZ, Lai CJ, et al. Changes in sleep patterns in spontaneously hypertensive rats. Sleep. 2004;27(3):406-412.
29. Valdez SR. Serotonin 5HT-1A Receptor Density in the Brain of the Spontaneously Hypertensive Rats [MS]. University of California San Diego; 2010.
30. Pot C, Chen AY, Ha JN, Schmid- Schönbein GW. Proteolytic cleavage of the red blood cell glycocalyx in a genetic form of hypertension. Cell Mol Bioeng. 2011;4(4):678-692.
31. Hamet P, Thorin-Trescases N, Moreau P, et al. Workshop: excess growth and apoptosis: is hypertension a case of accelerated aging of cardiovascular cells? Hypertension. 2001;37(2 Part 2):760-766.
32. Group TSRS. Soluble insulin receptor ectodomain is elevated in the plasma of patients with diabetes. Diabetes. 2007;56(8):2028-2035.
33. Alsaigh T, Pocock ES, Bergan JJ, et al. Acute venous occlusion enhances matrix metalloprotease activity: Implications on endothelial dysfunction. Microvasc Res. 2011;81(1):108-116.
34. Raffetto JD, Qiao X, Koledova VV, et al. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48(2):447-456.
