Randomized control trials on sclerotherapy for varicose veins
Vasculaire Jean Kulin, Lyon, France
ABSTRACT
Ultrasound-guided foam sclerotherapy (UGFS) is presently challenging other operative varices treatment. Randomized controlled trials are considered to be solid, evidence-based studies. In varices management, many randomized trials have been published in order to try to determine what the best treatment option is, and they are discussed in this review. Other randomized controlled trials dealing with varices sclerotherapy are analyzed including: (i) sclerosing agent versus placebo; (ii) liquid versus foam; (iii) the type, dose, and concentration of sclerosing agent; (iv) sclerotherapy in patients with or without thrombophilia; and (v) postsclerotherapy compression. The conclusions are that UGFS is both efficient and safe. Ideal sclerosing agent and dose as well as postsclerotherapy compression are still under discussion. Compared with other treatments, ultrasound-guided foam sclerotherapy is credited with more frequent recurrences, but they are easy to treat by redoing the injection with good success. Presently, in terms of cost effectiveness, UGFS is in a perfect position.
ABBREVIATIONS
VV = varicose veins
SFJ = saphenofemoral junction
UGFS = ultrasound-guided foam sclerotherapy
RCT = randomized controled trial
GSV = great saphenous vein
HL = high ligation
AVP = ambulatory venous pressure
VFS = visual foam sclerotherapy
RT = refill time
VCSS = venous clinical severity score
VSDS = venous segmental disease score
AVVQ = Aberdeen Varicose Vein Questionnaire
PE = pulmonary embolism
INTRODUCTION
In the last decade operative treatments for varices have been enriched with new methods. Presently, procedures for varicose vein (VV) treatment can be classified into 2 groups:1 (i) open surgery; and (ii) endoluminal procedures.
1. Open surgery with techniques to distinguish between sparing the saphenous trunk or not
The first subgroup encompasses incompetent tributary phlebectomy, (French acronym ASVAL), ambulatory Conservative HemodynamIc management of Varicose Veins, (French acronym CHIVA), saphenofemoral junction (SFJ) valvuloplasty, ligation, or cuffing + incompetent tributary phlebectomy.
The second one groups classical open surgery together with SFJ preservation or not, +/- perforator ligation, +/- incompetent tributary phlebectomy.
2. Endoluminal procedures including thermal ablation (laser, radiofrequency, steam, and cryostripping) and chemical ablation (glue and sclerotherapy)
The latter, although used for several centuries, has been drastically improved by ultrasound guidance and by delivering the sclerosing agent combined with gas to form foam. (Figures 1-6). Ultrasound-guided foam sclerotherapy (UGFS) is gaining favor among phlebologists. Many articles on UGFS including consensus analyses, meta-analyses, observational studies, and randomized controlled trials (RCT’s) have been published. The American College of Chest Physicians has established recommendation grades that are adopted and used worldwide (Table I).2-5
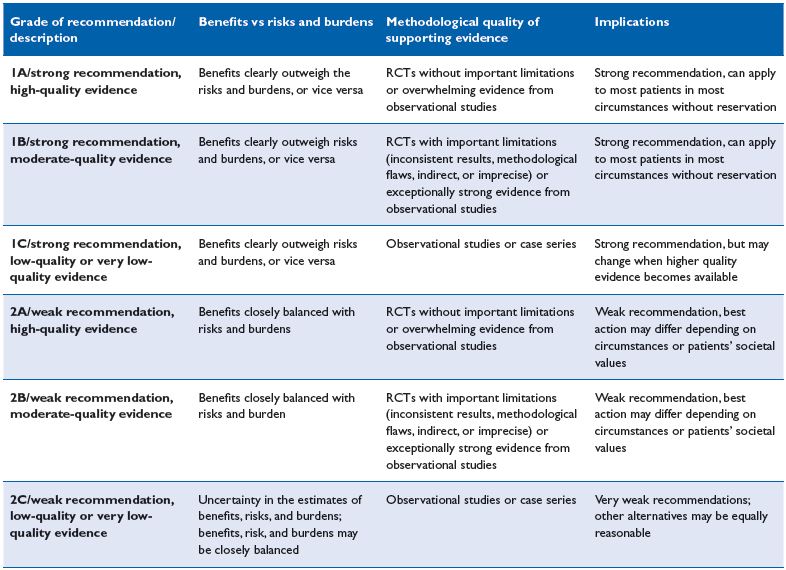
Table I. Grading recommendations (Chest, 2006;129:174-181)
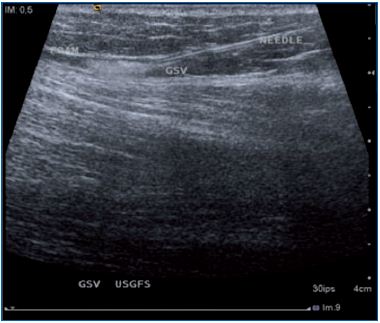
Figure 1. The needle and the foam injected in the great
saphenous vein are clearly identified.
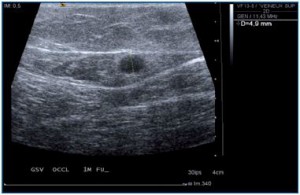
Figure 2. Spasm following foam injection into the great
saphenous vein.
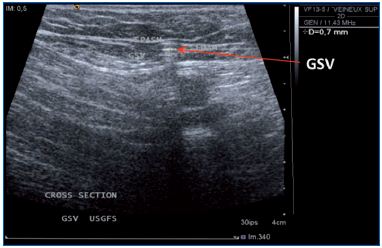
Figure 3. One month follow-up after UGFS. GSV, great
saphenous vein; EPIG; epigastric vein; FCV common
femoral vein.
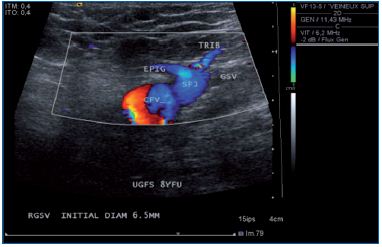
Figure 4. One month follow-up after UGFS. GSV, great
saphenous vein; EPIG; epigastric vein; FCV common
femoral vein.
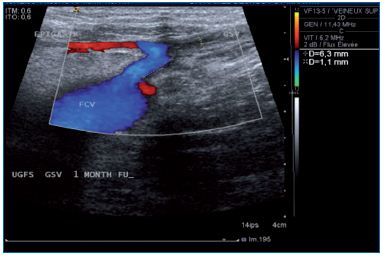
Figure 5. Eight year follow-up after ultrasound-guided
foam sclerotherapy. GSV, great saphenous vein; EPIG,
epigastric vein; TRIB, tributary; CFV, common femoral
vein; SFJ, saphenofemoral junction.
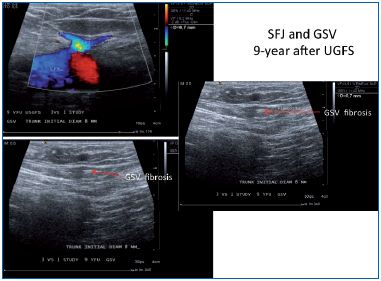
Figure 6. Nine years follow-up after ultrasound-guided
foam sclerotherapy. GSV, great saphenous vein; CFV,
common femoral vein; SFJ, saphenofemoral junction.
AIM
To report and review RCT’s on sclerotherapy for treating VV.
MATERIAL AND METHODS
A PubMed search was conducted in English and French for the years 2000 to 2012 with the keywords sclerotherapy, foam, ultrasound-guided foam sclerotherapy, and their counterparts in French. Only articles, but not meeting abstracts, dealing with lower limb VV were selected. RTC’s identified were classified into distinct groups: (i) sclerosing agent versus placebo; (ii) liquid versus foam; (iii) sclerosing agent usages (type, dose, and concentration); (iv) sclerotherapy in patients with thrombophilia; (v) postsclerotherapy compression; and (vi) outcome after sclerotherapy versus other treatment procedures.
RESULTS
1. RCTs of diverse sclerotherapy modalities Sclerosing agent vs normal saline injection6 (Table II)
Comment. The trial has demonstrated that polidocanol is efficient at closing the vein and modifying hemodynamics by measuring the venoarterial flow index by using ultrasound investigation.
Liquid versus foam7-11 (Table III)
Comment. German, Japanese, and French articles related to Varices (C2-C6) come to the same conclusions; foam is more efficient than liquid in terms of vein occlusion with polidocanol as the sclerosing agent by using the EASY-FOAM-kit with a 2-year follow-up or Tessari method after 1 year. Unfortunately, the 4 RCT’s did not provide clinical data except for a quality of life survey in the German article. The Catalan RCT is difficult to interpret, as the group is heterogeneous and the methodology questionable, but the outcome seems to be in favor of foam, although at 1 year there is no difference in terms of patient satisfaction. In conclusion, if sclerotherapy is considered for treating varices, the use of UGFS deserves a strong grade 1B recommendation.
Sclerosing agent: type, dose, and concentration12-15 (Table IV).
Comment. Four articles (3 RCTs), without major bias, provided the same conclusion. Results obtained by using either 1% or 3% Polidocanol are equivalent, although the German study shows a trend that favors the 3% concentration in terms of cosmetic results at 1-year follow-up (P=0.569). Presently, there is no RCT comparing the most commonly used sclerosing agents, ie, polidocanol and tetradecyl sodium sulphate. In conclusion, if Polidocanol is used for treating varices by UGFS at a concentration of 1%, then it deserves a strong grade 1B recommendation.
Compression after sclerotherapy16-18 (Table V)
Comment. The 3 available RCTs assessed have different elements. The first trial perfectly documented recommending a short-term compression after classical surgery.16 Nevertheless, the type of compression prescribed in this trial is questionable: bandages followed by use of antithrombotic stockings. In conclusion, if compression is considered after UGFS treatment, short term compression is recommended, but related to above remarks, the grade of recommendation is weak, we suggest grade 2B.
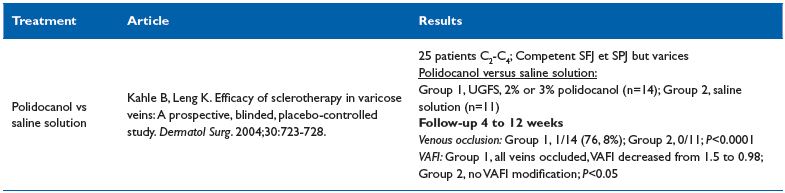
Table II. Sclerosing agent vs normal saline injection
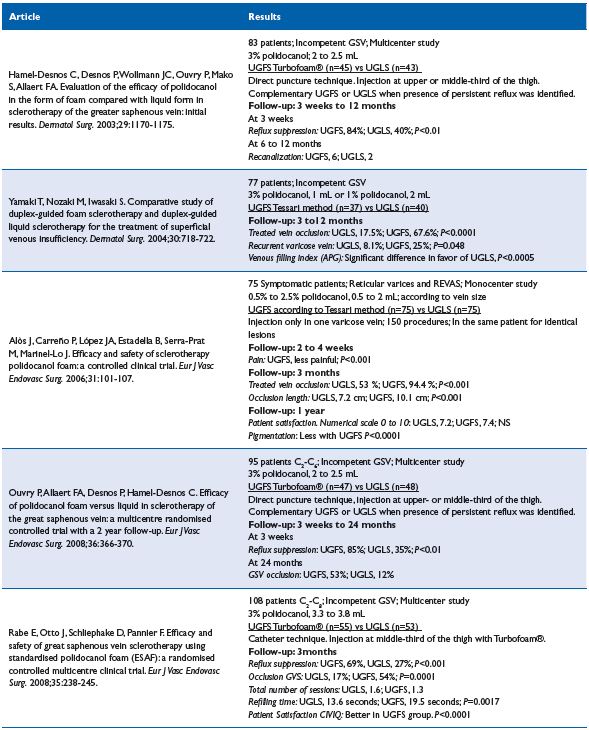
Table III. Liquid sclerotherapy vs foam sclerotherapy

Table IV. Sclerosing agent: dose and concentration
The second trial questions compression usefulness after UGFS.17 Although many parameters are assessed, the cohort studied is disparate both in terms of clinical class (C) as well as veins treated. The third trial compared biological changes in inflammation and coagulation between 2 post UGFS groups, one treated with compression and the other treated without compression therapy. No significant biological changes were observed between the 2 groups.18 Several observational studies argue for compression after sclerotherapy, but all of them used a liquid sclerosing agent.19-21 In conclusion, these trials received only a weak grade 2B recommendation, suggesting no compression is needed after UGFS.
isual foam sclerotherapy alone, or in combination with, UGFS22 (Table VI)
Comment. In two well-matched groups, 1% polidocanol was used for treating patients that were prospectively randomized to receive either visual foam sclerotherapy (VFS) alone or VFS combined with UGFS of the great saphenous vein (GSV). The results show that UGFS + VFS and VFS alone are equally effective for the treatment of GSV reflux, despite the lower volume of foam used for VFS alone. However, as the authors stated some parameters were not taken into account including optimal foam volume, injection techniques, vein diameter, and gas fabrication. In conclusion, no recommendation can be drawn from this trial.
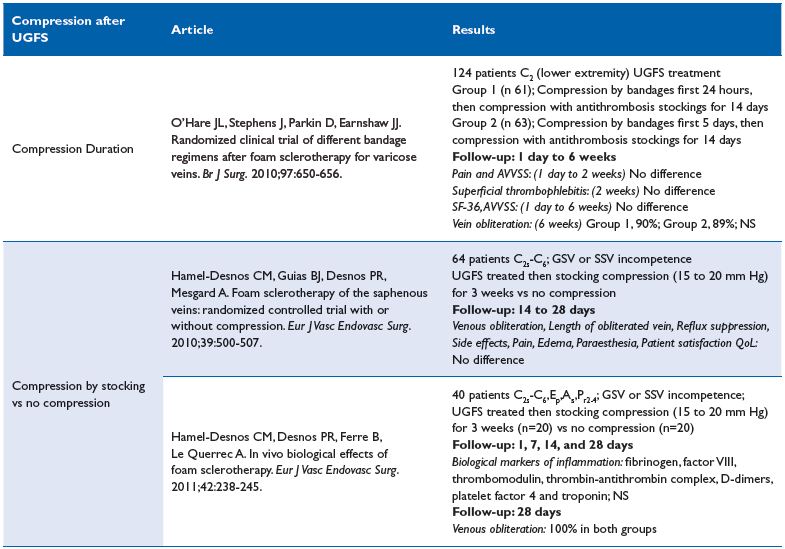
Table V. Compression after sclerotherapy
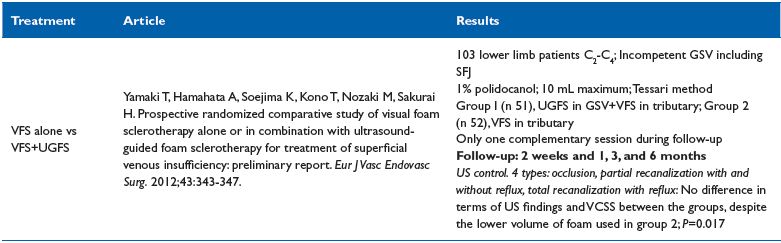
Table VI. Visual foam sclerotherapy alone or in combination with UGFS
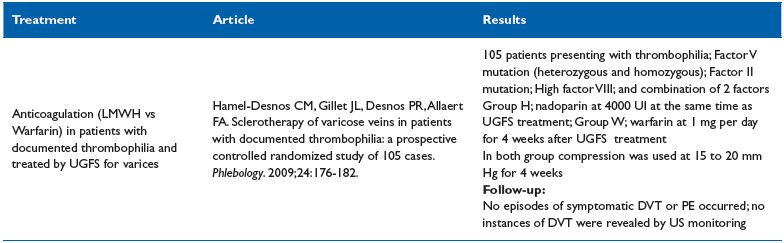
Table VII. Sclerotherapy of varicose veins in patients with documented thrombophilia
Sclerotherapy of varicose veins in patients with documented thrombophilia23
Comment. This study suggests that in the three most common forms of thrombophilia; sclerotherapy in combination with thromboprophylaxis, can be performed safely. Prophylaxis with low molecular weight heparin is easier to use than warfarin. In conclusion, this RCT can be graded 1B.
2. Sclerotherapy versus other operative treatments Sclerotherapy versus open surgery24-31 (em>Table VIII)
Comment. The main interest of Belcaro’s article is the 10-year follow-up duration, but its design is deceptive for various reasons. First, patients are distributed in 6 groups where procedures are either obsolete (in group 3, the technique used was isolated high ligation [HL]) or poorly described (in group 6, the sclerosing agent was tetradecyl sodium sulphate combined with the tensoactive agent J&J-93FA). In the follow-up, ultrasound examination and ambulatory venous pressure was routinely performed for measuring refill time, which is unusual and invasive; consequently, it is surprising that patients accepted this investigation. Furthermore, only noncomplicated varices (C2) were enrolled and we know that C2 refill time is usually not strongly modified. Additionally, the number of patients assessed at year 10 is not documented. To conclude, it looks difficult to draw valid conclusions from Belcaro’s article.24
The second article (Wright) compared VarisolveR with either open surgery or sclerotherapy.25 Unfortunately, open surgery involves various procedures including isolated HL, HL+stripping, and phlebectomy rendering the outcome difficult to assess. Nevertheless, the authors concluded that surgery was more efficacious. VarisolveR versus sclerotherapy was conclusive in favor of VarisolveR, but the sclerotherapy group was not homogenous as both liquid and handmade foam as well as polidocanol or sodium tetradecyl sulphate were used.25
Three other articles combining HL+UGFS and comparing the combination with conventional open surgery concluded more or less in favor of HL+UGFS, but followup was only a 6-month maximum duration.26-28
Conversely, Kalodiki’s article is a fully documented 5-year follow-up RCT, which concluded that treatment was equally effective between the surgical and foam groups, as demonstrated with the venous clinical severity score (VCSS), venous segmental disease score (VSDS), and the physical component of the SF-36 score improvements. The Aberdeen Varicose Vein Questionnaire (AVVQ) was significantly better in the surgery group, but the margins were small and this may not have any clinical significance.29 In addition, foam is less expensive; therefore, the cost effectiveness ratio is in favor of foam. It is important to keep in mind that in the above mentioned articles, UGFS was combined with HL.26-29
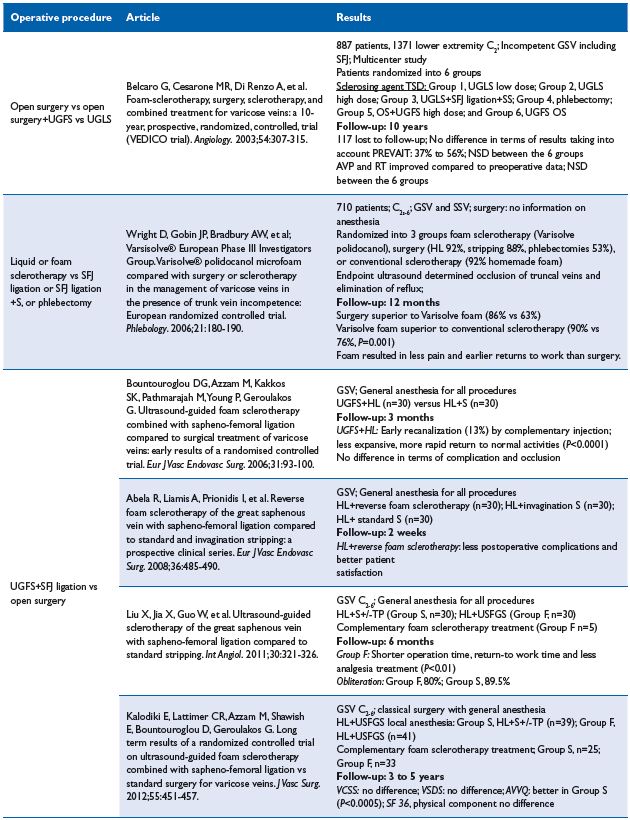
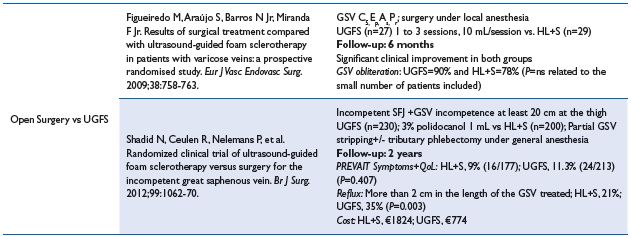
Table VIII. Sclerotherapy vs open surgery
stripping; SFJ, saphenofemoral junction; SSV, small saphenous vein; TP, tributary phlebectomy; TSD, tetradecyl sodium sulfate; UGFS, ultrasound-guided
foam sclerotherapy; UGLS, ultrasound-guided liquid sclerotherapy; VCSS, venous clinical severity score; VSDS, venous segmental disease score
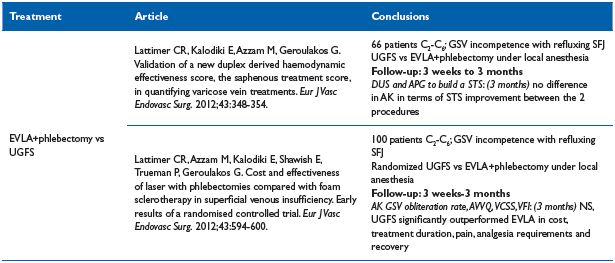
Table IX. Endovenous laser ablation vs UGFS
VCSS, venous clinical severity score; VFI, venous filling index
Does UGFS without HL provide the same results or, more importantly, are pulmonary embolisms (PE) more frequent. Two RCT’s without HL do not mention immediate postoperative PE.30-31 Shadid’s article provides a middle term outcome.31 There is no difference in terms of clinical results, but presence of reflux is significantly more frequent after UGFS, but conversely total cost is in favor of UGFS.
In conclusion, UGFS is credited as with having a better postoperative course, but recurrence of varices seems more frequent. The Society for Vascular Surgery and the American Venous Forum recommendations are not fully conclusive. They provided a grade 1B recommendation for UGFS in the treatment of varices, but there are no recommendations available to differentiate between open surgery and UGFS for the treatment of incompetent saphenous vein.32
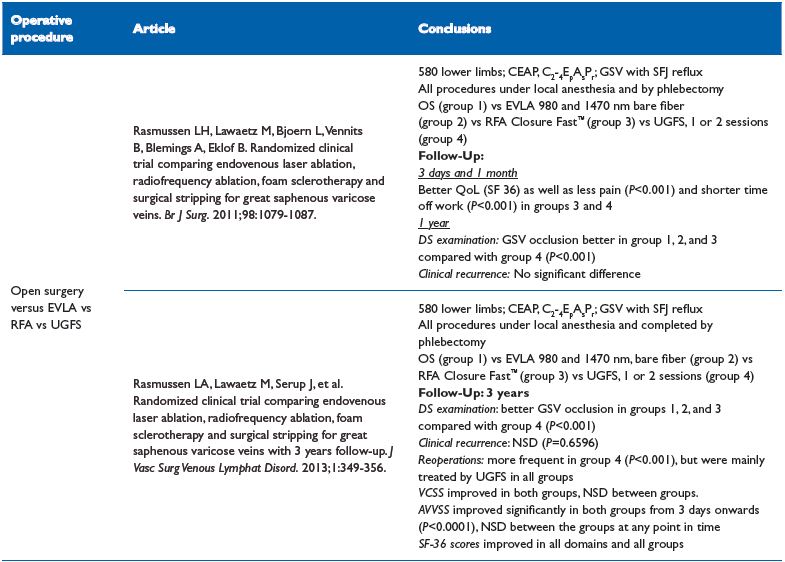
Table X. EVLA vs radiofrequency ablation vs UGFS vs surgical stripping
life; RFA, radiofrequency ablation; SFJ, saphenofemoral junction; UGFS, ultrasound-guided sclerotherapy; VCSS, venous clinical severity score
Endovenous laser ablation (EVLA) versus UGFS33,34 (Table IX)
Comment. This trial, based on 2 articles, favors UGFS, but the follow-up was short (3 months), besides the EVLA fiber was a bare one, and we know from various studies that jacket-tipped, radial fiber, or tulip–assisted EVLA improve the immediate postoperative course.35-38 Lattimer’s RCT conclusion contradicts the grade 1B recommendation from the Society for Vascular Surgery and the American Venous Forum. For treatment of the incompetent saphenous vein, they recommend endovenous thermal ablation over chemical ablation with foam.
EVLA vs radiofrequency ablation vs UGFS vs surgical stripping39,40
These two RCTs are for a five-year ongoing trial. Results after one year showed that all treatments are efficacious with a higher technical failure rate after foam sclerotherapy.39 Laser and foam sclerotherapy leads to faster recovery, less postoperative pain, and superior quality of life scores compared with laser and surgery.39
A 3-year follow-up duplex scan confirms that the GSV occlusion rate is lower in the UGFS group (P<0.001),40 but there was no difference in terms of clinical recurrence; VCSS, AAVVQ, and SF-36 scores. Reoperations were more frequent in UGFS group (P<0.001), but interestingly, recurrence were mainly treated by UGFS in all groups.40
Sclerotherapy versus other procedures for ablation of varices
There are no RCTs comparing sclerotherapy with open surgery preserving the saphenous trunk, other thermal ablation techniques (radiofrequency, foam), or a new technique such as ClariveinR41 or SapheonR42
DISCUSSION
Although RCTs remain the best tool for comparing clinical effectiveness, as mentioned in Grade system, nevertheless, RCTs must be evaluated carefully. Skepticism about conventional RCTs in nonpharmacological interventions (eg, sclerotherapy) remains, and expertise-based RCTs are suggested as an alternative where participants are randomized to clinicians with expertise in intervention A or clinicians with expertise in intervention B, and the clinicians perform only the procedure for which they are the expert.43
Accurate analysis of the presented RCTs is difficult as hidden bias can be hard to identify. For example, in some RCTs, surgery was performed either under local tumescent anesthesia or general anesthesia that may influence short-term evaluation.
CONCLUSION
Twenty-two RCTs (27 articles) and an analysis of observational studies were evaluated, which provided some conclusions regarding sclerotherapy. Sclerotherapy is a safe procedure with a low rate of adverse reactions. UGFS is more efficient in terms of obliteration than liquid sclerotherapy, where 1% and 3% polidocanol foam demonstrated equivalent efficacy in GSV treatment when the trunk was less than 8 mm in diameter. Benefits from optimal compression are still being debated. UGFS can be performed safely in combination with thromboprophylaxis in patients with thrombophilia. Compared with other operative treatments, UGFS is credited with more frequent recurrences, but they are easily treated by reinjection with satisfactory results. Presently, UGFS is cost effective when compared with other operative procedures for treating varices.

REFERENCES
1. Perrin M. Role of Surgery in the Treatment of Varicose Veins. In: Goldman MP, Guex J-J, Weiss RA, eds. Sclerotherapy: treatment of varicose and telangiectatic leg veins. 5th ed. Philadelphia, PA: Elsevier; 2011:282- 300.
2. Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines. Report from an American college of chest physicians task force. Chest. 2006;129:174-181.
3. Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924- 926.
4. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ; GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995-998.
5. Guyatt G H, Oxman A D, Kunz R, et al; GRADE Working Group. Going from evidence to recommendations. BMJ. 2008;336:1049-1051.
6. Kahle B, Leng K. Efficacy of sclerotherapy in varicose veins A prospective, blinded, placebocontrolled study. Dermatol Surg. 2004;30:723-728.
7. Hamel-Desnos C, Desnos P, Wollmann JC, Ouvry P, Mako S, Allaert FA. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg. 2003;29:1170-1175.
8. Yamaki T, Nozaki M, Iwasaki S. Comparative study of duplex-guided foam sclerotherapy and duplex-guided liquid sclerotherapy for the treatment of superficial venous insufficiency. Dermatol Surg. 2004;30:718-722.
9. Alos J, Carreno P, Lopez JA, Estadella B, Serra-Prat M, Marinel-Lo J. Efficacy and safety of sclerotherapy polidocanol foam: a controlled clinical trial. Eur J Vasc Endovasc Surg. 2006;31:101-107.
10. Ouvry P, Allaert FA, Desnos P, Hamel-Desnos C. Efficacy of polidocanol foam versus liquid in sclerotherapy of the great saphenous vein: a multicentre randomised controlled trial with a 2 year followup. Eur J Vasc Endovasc Surg. 2008;36:366-370.
11. Rabe E, Otto J, Schliephake D, Pannier F. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Vasc Endovasc Surg. 2008;35:238-245.
12. Hamel-Desnos C, Allaert FA, Benigni JP, et al. Etude 3/1. Mousse de polidocanol 3% versus 1% dans la grande veine saphene: premiers resultats. Phlébologie. 2005;58:165-173.
13. Ceulen RP, Bullens-Goessens YI, Pi-VAN DE Venne SJ, Nelemans PJ, Veraart JC, Sommer A. Outcomes and side effects of duplex-guided sclerotherapy in the treatment of great saphenous veins with 1% versus 3% polidocanol foam: results of a randomized controlled trial with1-Year follow-up. Dermatol Surg. 2007;33:276- 281.
14. Hamel-Desnos C, Ouvry P, Benigni JP, et al. Comparison of 1% and 3% polidocanol foam in ultrasound-guided sclerotherapy of the great saphenous vein: a randomized, double-blind trial with 2 year-follow-up. “The 3/1 study.” Eur J Vasc Endovasc Surg. 2007;34:723-729.
15. Blaise S, Bosson JL, Diamand JM. Ultrasound-guided sclerotherapy of the great saphenous vein with 1% vs. 3% polidocanol foam: a multicentre double-blind randomised trial with 3-year follow-up. Eur J Vasc Endovasc Surg. 2010;39:779-786.
16. O’Hare JL, Stephens J, Parkin D, Earnshaw JJ. Randomized clinical trial of different bandage regimens after foam sclerotherapy for varicose veins. Br J Surg. 2010;97:650-656.
17. Hamel-Desnos CM, Guias BJ, Desnos PR, Mesgard A. Foam sclerotherapy of the saphenous veins: randomized controlled trial with or without compression. Eur J Vasc Endovasc Surg. 2010;39:500-507.
18. Hamel-Desnos CM, Desnos PR, Ferre B, Le Querrec A. In vivo biological effects of foam sclerotherapy. Eur J Vasc Endovasc Surg. 2011;42:238-245.
19. Tazelaar DJ, Neumann HA, De Roos KP. Long cotton wool rolls as compression enhancers in macrosclerotherapy for varicose veins. Dermatol Surg. 1999;25:38-40.
20. Wenner L. Improvement of immediate and long-term results in sclerotherapy. Vasa. 1986;86:180-183.
21. Staubesand J, Seydewitz V. Etudes ultrastructurelles des varices sclerosees. Phlébologie. 1991;44:16-22.
22. Yamaki T, Hamahata A, Soejima K, Kono T, Nozaki M, Sakurai H. Prospective randomized comparative study of visual foam sclerotherapy alone or in combination with ultrasound-guided foam sclerotherapy for treatment of superficial venous insufficiency: preliminary report. Eur J Vasc Endovasc Surg. 2012;43:343-347.
23. Hamel-Desnos CM, Gillet JL, Desnos PR, Allaert FA. Sclerotherapy of varicose veins in patients with documented thrombophilia: a prospective controlled randomized study of 105 cases. Phlebology. 2009;24:176-182.
24. Belcaro G, Cesarone MR, Di Renzo A, et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology. 2003;54:307-315.
25. Wright D, Gobin JP, Bradbury AW, et al; VarsisolveR European Phase III Investigators Group. VarisolveR polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomized controlled trial. Phlebology. 2006;21:180-190.
26. Bountouroglou DG, Azzam M, Kakkos SK, Pathmarajah M, Young P, Geroulakos G. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: early results of a randomised controlled trial. Eur J Vasc Endovasc Surg. 2006;31:93-100.
27. Abela R, Liamis A, Prionidis I, et al. Reverse foam sclerotherapy of the great saphenous vein with saphenofemoral ligation compared to standard and invagination stripping: a prospective clinical series. Eur J Vasc Endovasc Surg. 2008;36:485-490.
28. Liu X, Jia X, Guo W, et al. Ultrasoundguided sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard stripping. Int Angiol. 2011;30:321-326.
29. Kalodiki E, Lattimer CR, Azzam M, Shawish E, Bountouroglou D, Geroulakos G. Long term results of a randomized controlled trial on ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation vs standard surgery for varicose veins. J Vasc Surg. 2012;55:451-457.
30. Figueiredo M, Araujo S, Barros N Jr, Miranda F Jr. Results of surgical treatment compared with ultrasoundguided foam sclerotherapy in patients with varicose veins: a prospective randomised study. Eur J Vasc Endovasc Surg. 2009;38:758-763.
31. Shadid N, Ceulen R, Nelemans P, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. Br J Surg. 2012;99:1062-70.
32. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases. Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53:2S-48S.
33. Lattimer CR, Kalodiki E, Azzam M, Geroulakos G. Validation of a new duplex derived haemodynamic effectiveness score, the saphenous treatment score, in quantifying varicose vein treatments. Eur J Vasc Endovasc Surg. 2012;43:348-354.
34. Lattimer CR, Azzam M, Kalodiki E, Shawish E, Trueman P, Geroulakos G. Cost and effectiveness of laser with phlebectomies compared with foam sclerotherapy in superficial venous insufficiency. Early results of a randomised controlled trial. Eur J Vasc Endovasc Surg. 2012;43:594-600.
35. Doganci S, Demirkilic U. Comparison of 980 nm Laser and bare-tip fibre with 1470 nm laser and radial fibre in the treatment of great saphenous vein varicosities: A prospective randomized controlled trial. Eur. J Vasc Endovasc Surg. 2010;40:254-259.
36. Pannier F, Rabe E, Maurins U. 1470 nm diode laser for endovenous ablation (EVLA) of incompetent saphenous veins – a prospective randomized pilot study comparing warm and cold tumescence anesthesia. Vasa. 2010;39:249-255.
37. Vuylsteke M, De Bo T, Dompe G, Di Crisci D, Abbad C, Mordon S. Endovenous laser treatment: is there a clinical difference between using a 1500 nm and a 980 nm diode laser. A multicenter randomised clinical trial. Int Angiol. 2011;30:327-334.
38. Vuylsteke ME, Thomis S, Mahieu P, Mordon S, Fourneau I. Endovenous laser ablation of the great saphenous vein using a bare fibre versus a tulip fibre: a randomised clinical trial. Eur J Vasc Endovasc Surg. 2012;44:587-592.
39. Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079-1087.
40. Rasmussen LA, Lawaetz M, Serup J, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins with 3 years follow-up. J Vasc Surg Venous Lymphat Disord. 2013;1:349-356.
41. Elias S, Raines JK. Mechanochemical tumescentless endovenous ablation: final results of the initial clinical trial. Phlebology. 2012;27:67-72.
42. Almeida J, Javier J, Mackay E, et al. One-year follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg. In press.
43. Devereaux PJ, Bhandari M, Clarke M, et al. Need for expertise based randomized controlled trials. BMJ. 2005;330:88-91.
