Recent Guidelines in Chronic Venous Disease: the place of MPFF at a dose of 500 mg
Françoise PITSCH
Suresnes, France
This article addresses some of the newer guidelines, the purpose of which is to help clinicians manage patients with chronic venous disease (CVD) of the lower extremities.
What is chronic venous disease? CVD covers a full spectrum of venous conditions ranging from telangiectasias to the ultimate complications (venous ulcers). Symptoms are commonly associated with signs of CVD.
Venous symptoms are defined as tingling, aching, burning, pain, muscle cramps, swelling, sensations of throbbing or heaviness, itching skin, restless legs, leg tiredness and/or fatigue, which may be exacerbated during the course of the day or by heat, but relieved with leg rest or elevation or both.1 Venous signs are visible manifestations of CVD, which include dilated veins (telangiectasias, reticular veins, varicose veins), leg edema, skin changes, and ulcers, as described in the clinical, etiology, anatomy, pathophysiology (CEAP) classification.2
A common language is needed before building guidelines in CVD: It should first be stressed that no consensus on guidelines is possible without the use of a common language. A leap forward was made recently thanks to a common terminology on venous anatomy,3 the CEAP classification proposed by the ad hoc committee of the AVF in 1994 and subsequently adopted worldwide as a basis for improved patient description2,4 a consensus on terminology related to CVD to avoid misunderstanding and lack of precision in publications. The VEIN TERM consensus document provides the definition of 33 widely used clinical venous terms and was published in J Vasc Surg 2009 under the aegis of the main American and European Scientific Societies (AVF, ACP, EVF, UIP, IUA, SVS).1
The CEAP classification includes a clinical assessment (C), an etiologic assessment of the patient’s disease (E), an anatomic assessment of location of the pathology (A), and the pathophysiologic basis for the underlying disease (P). It provides a broad-based, objective, anatomic, and physiologic basis for classification of venous disease. This is why CEAP has improved standardization, communication, decision making, and reporting of venous disease.
Understanding the pathophysiology of a disease state is essential for effective treatment. Results from studies that demonstrate treatment efficacy lead to guideline recommendations.
Ambulatory venous hypertension is the hemodynamic disease which is related to all symptoms and signs of CVD, the underlying components of venous hypertension being failure of the calf muscle pump, venous valvular incompetence, and luminal obstruction.5
After prolonged standing, venous pressure in the foot is approximately 90 mm Hg in both a patient with incompetent venous valves and a person with a normal leg. In CVD patients, ambulatory venous pressures remain high in the lower limbs during walking (more than 40 mm Hg in the present example), when normally these pressures should fall (to 30 mm Hg). Due to valve incompetence, venous refill time at APG is shorter in CVD patients than in healthy individuals.5 When venous pressures in the leg reach higher-than-normal levels and remain elevated for prolonged periods, a progressive increase in skin damage occurs. Nicolaides reported that nearly all patients with exercising venous pressures of >90 mm Hg experienced venous ulceration.6
The apparently simple concept of venous hypertension being responsible for CVD lies in the complex cellular and molecular processes set in motion by abnormal venous hemodynamics.
What initiates the inflammatory events in venous valves and walls is not yet clear. It is likely that venous hypertension and subsequent stasis lead to vein distension which in turn allows venous flow reversal and areas of low shear stress. Even in the absence of reflux, endothelial cells which are exposed to flow reversal become activated, together with leukocytes which are activated by low shear stress. The leukocyte-endothelial interaction initiates and maintains inflammation.5
When leukocytes are activated as a result of venous hypertension, they produce adhesion molecules which bind to intracellular adhesion molecules at the endothelial surface. This permits endothelial cell adhesion of leukocytes and initiates their migration through the vessel wall into the extravascular tissues, leading to degranulation and release of proteolytic enzymes [such as matrix metalloproteinases (MMPs), tissue inhibitors of MMPs (TIMPs), and transforming growth factor beta (TGF-β1)]7,8
THE CONSEQUENCES OF INFLAMMATION IN CHRONIC VENOUS DISEASE
Morphologic changes in venous valves occur. With prolonged pressure-induced inflammation, valve remodeling and damage occur as a result of leukocyte infiltration into valve leaflets, and reflux appears. The production of MMPs and in greater proportion of TIMPs leads to the accumulation of extracellular matrix material. In addition, increased production of TGF-β1 stimulates collagen synthesis and further increases production of TIMPs. The sum of these events results in the structural and hypertrophic changes in venous wall that typify patients with varicose veins.9
An early event in CVD is the elevation of endothelial permeability with opening of leakage sites between endothelial cells. As a result of such microvascular permeability, extravasation of water and water soluble nutrients leads to edema.10 In a further step, the extravasation of red cells leads to the hyperpigmentation of skin in lipodermatosclerosis.
Fluid transport through the lymphatic vasculature completes the body’s circulatory loop. The lymphatic vessels maintain tissue homeostasis and compensate for capillary leakage by absorption of extravasated interstitial fluid.11 In the case of intense blood capillary leakage, the lymphatic capacity of drainage is insufficient to absorb excess fluid and macromolecules. This adds to the formation of venous edema, which spares the toes, unlike lymphatic edema.
PHARMACOLOGICAL TREATMENT OF CHRONIC VENOUS DISEASE
A number of venoactive drugs (VADs) of plant or synthetic origin are available for the treatment of the symptoms of CVD, as illustrated in Table I.12
What are the mechanisms at work in pharmacological treatment of CVD by VADs?13
Most VADs increase venous tone, and thereby reduce venous distensibility and stasis.
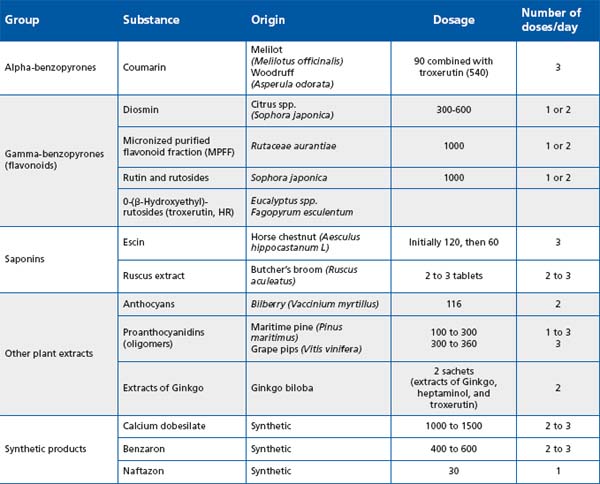
Table I: Classification of the main venoactive drugs. Adapted from Ramelet et al, 200812
Beneficial effects on capillary abnormal permeability have been demonstrated for almost all VADs. Only 3 VADs have been shown to improve lymphatic drainage.
Only one available VAD has documented evidence of its ability to attenuate the effects of various mediators of the inflammatory cascade, particularly leukocyte-endothelial interactions, which are important in many aspects of the disease (Figure 1).
The mechanism of the beneficial effects of VADs on venous tone have been studied and published for micronized purified flavonoid fraction (MPFF)14,15 and ruscus extracts.16 These 2 VADs appear to increase venous tone by prolonging the vasoconstrictor effect of noradrenaline on the vein wall. This increases the venous return and reduces venous pressure in patients suffering from CVD.
The improvement of abnormal permeability has been described for VADs, while evidence of increased lymphatic flow is available for only 3.17-19
Among available drugs able to block leukocyte adhesion to the venous valves and wall and thereby stop venous inflammation very early in the disease process, only MPFF so far has been studied in detail. In an animal model of venous hypertension, MPFF delayed the development of reflux and suppressed damage to the valve structures by decreasing the interaction between leukocytes and endothelial cells.20
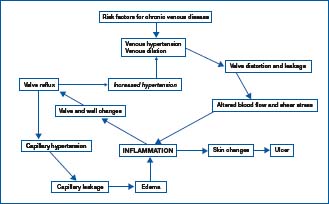
Figure 1. The vicious circle of venous hypertension/venous
inflammation. Adapted from Bergan et al, 20065
A NEW GRADING SYSTEM FOR CHRONIC VENOUS DISEASE GUIDELINES
Many grading systems are available in guidelines (Figure 2).
Cochrane reviews:
In a recent Cochrane review on VADs, randomized, double-blind, placebo-controlled trials (RCTs) were classified as level A (low risk of bias), level B (moderate risk of bias), or level C (high risk of bias). Special emphasis was placed on the method of randomization, the conditions of blinding, and the inclusion of an intention-to-treat analysis. The effect of treatment with VADs was estimated using relative risk (RR), with its corresponding 95% confidence interval (CI), by applying a random effects statistical model used in meta-analyses. The presence of statistically significant heterogeneity was also established. In the presence of heterogeneity, the results had to be interpreted carefully.
A total of 10 Cochrane reviews of CVD have been published since 2000;21-30 2 are on VADs.21,22
Significant and homogeneous results were found for most VADs in terms of edema reduction, decrease in restless legs syndrome, and improvement in trophic disorders.21 Some VADS performed better than others in improving venous disorders, while the review on HCSE did not use the same methodology and consequently cannot be compared with the review on other VADs.21
European guidelines
In European guidelines, studies are usually classified as: grade A (at least two RCTs with large sample sizes, metaanalyses combining homogeneous results), grade B (RCTs with small sample size, single RCT), or grade C (other controlled trials, nonrandomized controlled trials).13

Figure 2. European and US guidelines that have considered the
drug treatment of chronic venous disease in recent years.
Two of these guidelines deal with VADs. The article of Ramelet et al in Clinical Hemorrheology and Microcirculation represents the proceedings of the International Medical Consensus Meeting held in Siena on “Veno-active drugs in the management of symptoms of chronic venous disease”.31 Eighty-three publications on the effects of VADS on venous symptoms were analyzed.
International guidelines on the management of CVD, published in International Angiology,13 used the same grading system as that of the Siena experts except for meta-analyses which were grade B. Outcomes included not only symptoms but also edema and venous ulcer healing. In all, 128 publications on VADs were analyzed in this document.
According to these two recent guidelines on VADs, and because of the consistency of the evidence,13,31 a grade A was assigned to 2 VADs: MPFF (MPFF at a dose of 500 mg), and HRoxerutins for their effects on symptoms, edema, and skin changes (summarized in Table I).
American guidelines
The method of determining the strength and quality of the recommendations in American guidelines deserves mention. Recommendations are generally accompanied by a number, which refers to the strength of the recommendation, and a letter, which refers to the quality of the evidence supporting the recommendation. Recent guidelines for venous disease have used two levels for the strength of their recommendations depending mainly on the benefits/risks ratio: Grade 1 for strong and Grade 2 for weak. They further indicate that statements accompanied by a Grade 1 level are “recommendations” and statements accompanied by a Grade 2 level are “suggestions.”32
The quality of evidence upon which the strength of the recommendation is based ranges from “A” for high quality, which is consistent evidence from randomized trials, to “B” for moderate quality, which is evidence from nonrandomized trials or inconsistent evidence from randomized trials. Level “C” is low quality, which is suggestive evidence from nonrandomized trials, observational reports, or expert opinion. Writing committees are increasingly aware of the cost of care and patient values and preferences, as are physicians. These are also considered in the strength of recommendation.
Two guidelines on venous diseases used this system:
• American College of Chest Physicians Evidence-Based Practice Guidelines (8th edition) published in 2008 in Chest, which included a section on the treatment of venous leg ulcers in patients with venous thromboembolic disease that reviews the evidence for therapies added to conventional compression.33
• the second is the latest edition (3rd) of the Handbook of Venous Disorders, Guidelines of the American Venous Forum.34
Acceleration of the healing of venous leg ulcers has been demonstrated by several double-blind studies using MPFF (MPFF at a dose of 500 mg) in combination with compression. This was confirmed in 2005 by a metaanalysis of 5 trials with MPFF as an adjunct to standard compression treatment in 723 patients in class C6 according to the CEAP classification.35
On the basis of this review, the author of the chapter devoted to “drug treatment of varicose veins, venous edema, and ulcers” of the latest edition (3rd edition) of the Handbook of Venous Disorders, Guidelines of the American Venous Forum, assigned VADs a grade 2B in the improvement of symptoms and edema associated with chronic venous disease. In the same chapter, only MPFF (MPFF at a dose of 500 mg) was quoted in the pharmacological treatment of venous ulcer. The use of MPFF in combination with compression in long-standing or large venous ulcers of primary etiology was recommended (Grade 1B).34
The recent ACCP guidelines stated that MPFF should be added to compression (Grade 2B) in patients with persistent venous ulcers of secondary etiology.33
In summary and based on the quality of evidence, it is possible to propose a strong recommendation, based on evidence of moderate quality, for the use of MPFF and rutosides in symptoms and edema.
HCSE and Ruscus extracts have also proven effective against CVD-related symptoms and lower limb edema, although the volume and quality of evidence is less than for the previous two drugs (2C). Calcium dobesilate has been associated with a potential safety concern relating to rare cases of agranulocytosis. Guidelines writers have considered it is only possible to give a weak recommendation for its use, given the uncertainty over the balance between benefits and harms (2B).
There is evidence from a meta-analysis of RCTs that MPFF is effective in the healing of venous ulcers. In the absence of important safety concerns, its use in this indication can be given a strong recommendation in primary ulcers (1B) and a weak one in secondary ulcers (2B).
UPDATING GUIDELINES IN CVD
An update of the “guidelines for testing drugs for CVD” is needed that will allow the pharmaceutical industry investing the necessary resources to perform large and definitive clinical trials that could improve the recommendations, which are useful for clinicians and organizations involved in decision making in this important field of CVD. Such guidelines could:
Reiterate the basic principles that should prevail when reporting from (and setting up) any RCT, using the Consolidated Standards of Reporting Trials (CONSORT) statement,36 as for meta-analyses with the QUORUM checklist.37
Describe comprehensively patients at selection in a study, using the advanced CEAP classification.4 Promote the use of validated tools to assess symptoms, edema, and venous leg ulcer, and have a consensus on end-points.38
Encourage the adoption of a simple and universally understood system of grading.34
REFERENCES
1. Eklof B, Perrin M, Delis K , Rutherford R. Updated terminology of chronic venous disorders: the Vein Term Transatlantic Interdisciplinary Consensus Document. J Vasc Surg. 2009;49:498-501.
2. Porter JM, Moneta GL. Reporting standards in venous disease: an update. J Vasc Surg. 1995;21:635-645.
3. Caggiati A, Bergan JJ, Gloviczki P et al. J Vasc Surg. 2002;36:416-422.
4. Eklöf B, Rutherford RB, Bergan JJ, et al: Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248- 1252.
5. Bergan JJ, Schmid-Schönbein G, Coleridge-Smith P, Nicolaides A, Boisseau M, Eklof B: Chronic venous disease. N Engl J Med. 2006;355:488- 498.
6. Nicolaides AN, Hussien MK, Szendro G, et al. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414-419.
7. Saharay M, Shields DA, Porter JB, Scurr JH, Coleridge Smith PD. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;26:265-273.
8. Takase S, Schmid-Schönbein G, Bergan JJ. Leukocyte activation in patients with venous insufficiency. J Vasc Surg. 1999;30:148-156.
9. Badier-Commander C, Couvelard A, Henin D, Verbeuren T, Michel JB, Jacob MP. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol. 2001;193:398-407.
10. Nicolaides AN. Investigation of chronic venous insufficiency; a consensus statement. Circulation 2000 Nov 14; 102: e126-63.
11. Priollet P. Venous edema of the lower limbs. Phlebolymphology. 2006;13:183- 187.
12. Ramelet AA, Perrin M, Kern P, Bounameaux H . Phlebology. 5th ed. Issy les Moulineaux, France: Elsevier Masson 2008.
13. Nicolaides A, Allegra C, Bergan J, et al.: Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Int Angiol. 2008;27:1-59.
14. Ibegbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G. Venous elasticity after treatment with MPFF at a dose of 500 mg. Angiology 1997;48:45-49.
15. Juteau N, Bakri F, Pomies JP, Foulon C, Rigaudy P, Pillion G, et al. The human saphenous vein in pharmacology: effect of a new micronized flavonoidic fraction (MPFF at a dose of 500 mg) on norepinephrine induced contraction. Int Angiol. 1995;14:8-13.
16. Bouskela E, Cyrino FZ, Marcelon G. Possible mechanisms for the venular constriction elicited by Ruscus extract on hamster cheek pouch. J Cardiovasc Pharmacol. 1994;24:165-170.
17. Labrid C. A lymphatic function of MPFF at a dose of 500 mg. Int Angiol. 1995;14:36- 38.
18. Borzeix MG, Angignard J, Dedieu F, Dupont JM, Miloradovich T, Leutenegger E. Effect of a combination of coumarin derivatives and rutoside on venous and lymphatic circulations during severe constriction of the caudal vena cava in rabbits. Arzneimittelforschung. 1995;45:262-266.
19. Piller NB. The lymphogogue action of calcium dobesilate on the flow of lymph from the thoracic duct of anesthetized and mobile guinea pigs. Lymphology 1988;21:124-127..
20. Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schonbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg 2004;28:484-493.
21. Martinez MJ, Bonfill X, Moreno RM, Vargas E, Capellà D. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003229.
22. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. 2006 Jan 25;(1):CD003230.
23. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD000265.
24. Bamigboye AA, Smyth R. Interventions for varicose veins and leg oedema in pregnancy.
Cochrane Database Syst Rev. 2007 Jan 24;(1):CD001066.
25. Tisi PV, Beverley C, Rees A. Injection sclerotherapy for varicose veins. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD001732.
26. Rigby KA, Palfreyman SJ, Beverley C, Michaels JA. Surgery versus sclerotherapy for the treatment of varicose veins. Cochrane Database Syst Rev. 2004 Oct 18;(4):CD004980.
27. Hardy SC, Riding G, Abidia A. Surgery for deep venous incompetence. Cochrane Database Syst Rev. 2004;(3):CD001097.
28. Kolbach DN, Sandbrink MW, Neumann HA, Prins MH. Compression therapy for treating stage I and II (Widmer) post-thrombotic syndrome. Cochrane Database Syst Rev. 2003;(4):CD004177.
29. Stones RW, Mountfield J. Interventions for treating chronic pelvic pain in women. Cochrane Database Syst Rev. 2000;(4):CD000387.
30. Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2000;(3):CD001484.
31. Ramelet AA, Boisseau MR, Allegra C, et al. Veno-active drugs in the management of chronic venous disease. An international consensus statement: current medical position, prospective views and final resolution. Clin Hemorheol Microcirc. 2005;33:309- 319.
32. Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Zelman Lewis S, Schünemann H. Grading Strength of Recommendations and Quality of Evidence in Clinical Guidelines: Report From an American College of Chest Physicians Task Force. Chest 2006;129;174-181.
33. Kearon C, Kahn S, Agnelli G, Comerota A, et al. American College of Chest Physicians Evidence-Based Practice Guidelines (8th edition). Chest. 2008;133:454S-545S.
34. Coleridge Smith PD. Drug treatment of varicose veins, venous oedema, and ulcers. In: Gloviczki P, ed. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 3rd ed. London, UK: Hodder Arnold; 2009: 359-365.
35. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30:198-208.
36. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 Explanation and Elaboration : updated guidelines for reporting parallel group randomised trials. BMJ 2010 (March 23);340:c869.
37. Clarke M. The QUORUM statement Lancet. 2000;355(9205):756-757.
38. Vasquez MA, Munschauer CE. Venous Clinical Severity Score and Quality-of- Life Assessment Tools: Application to Vein Practice. Phlebology. 2008;23(6):259-275.
