Role of duplex ultrasound investigation in the management of postthrombotic syndrome
investigation in the management of
postthrombotic syndrome
Caroline MENEZ, MD
CHU de Grenoble, France
Abstract
Duplex ultrasound is a method for analyzing the anatomy and hemodynamic profile of lower-limb veins; it can also be used for pelvic and abdominal examinations. For postthrombotic syndrome, duplex ultrasound can recognize specific anatomical abnormalities in the venous lumen, wall, and valves. Reflux can be easily diagnosed with duplex ultrasound, although some controversy is present concerning the extent of the reflux detected compared with descending venography. Venous obstruction is more challenging to quantify; nevertheless, simple indirect signs, such as phasic-flow disappearance and low–flow velocity in the common femoral vein, suggest suprainguinal obstruction. Before operative recanalization, duplex ultrasound can be used to determine the procedure, feasibility, expected permeability, and safest venous access site; evaluate suprainguinal venous segments and infrainguinal vessels to determine the landing zone; distinguish between postthrombotic syndrome, primary and congenital incompetence, or compression. Duplex ultrasound is currently used during postoperative follow-up after repermeation and stenting to determine the permeability of the stented veins and recognize complications, such as thrombosis, residual stenosis, and intrastent intimal hyperplasia. Currently, duplex ultrasound is the first-line examination for postthrombotic syndrome diagnosis, preoperative investigation, and postoperative follow-up because it provides relevant information for the operative management of obstruction and reflux, even if the preoperative assessment must be completed by venography and other instrumental investigations.
Introduction
Postthrombotic syndrome includes all of the venous signs and symptoms occurring after a deep venous thrombosis in the lower limb. Major clinical features include dilated veins, edema, leg pain, and cutaneous changes. Obstruction of the deep venous system may lead to venous claudication. Diagnostic and quantification of postthrombotic syndrome are based on clinical criteria, which are described in the Villalta scale.1
Often underestimated, postthrombotic syndrome is responsible for important disabilities in daily life. The development of new endovascular interventional techniques offers appealing treatment possibilities, even for patients without tissue damage, that are complementary to conservative treatments. Deep endovenous stenting is safe, resulting in low morbidity and mortality, and effective, with a high rate of technical success, patency, ulcer healing, and clinical improvement.2 The main objectives are to improve the patients’ quality of life and possibly reduce the risk of recurrence by removing the obstruction.
In addition to a physical examination, duplex ultrasound is a mandatory and complementary assessment for patients presenting with chronic venous disease. Current guidelines strongly recommend using duplex ultrasound as the primary diagnostic test for superficial venous insufficiency, suspected abdominal or pelvic venous pathology, postthrombotic syndrome, or clinical suspicion of other forms of iliac or inferior vena cava obstruction.3 Examining deep veins is more challenging than superficial veins, but duplex ultrasound may provide very useful information during all stages in the management of postthrombotic syndrome.
Duplex ultrasound techniques
Duplex ultrasound is a method for analyzing the anatomy and hemodynamic profile of lower-limb veins; it can also be used for pelvic and abdominal examinations. Duplex ultrasound techniques have been extensively described in consensus documents.4,5
For superficial veins, it is recommended to use a highresolution linear ultrasound transducer (12-18 MHz) and to have the patient in a standing position. Basic duplex ultrasound examination of superficial veins includes assessing perforating veins and all of the saphenous vein junctions, trunks, and tributaries. The hemodynamic analysis is used to diagnose reflux elicited by the calf compressionrelease maneuver and/or the Valsalva maneuver. Cut-off duration for reflux is 0.5 seconds for superficial veins and 0.35 seconds for perforating veins. The anatomical analysis measures the diameter of the refluxing saphenous trunks, which is measured ≈15 cm away from the saphenofemoral junction for the greater saphenous vein, at mid-calf for the small saphenous vein, and at the fascia for perforating veins. In all cases, the sources and extension of the reflux must be recognized. Results of duplex examination are commonly reported on cartography.
Examination of deep veins requires different probes that are convex and/or microconvex and have a lower frequency (3-8 MHz). For postthrombotic syndrome, duplex ultrasound checks for deep venous reflux at the femoral and popliteal veins in patients who are standing and it uses a cut-off value of 1 second for reflux duration. Obstruction is measured using an augmentation maneuver in patients in a supine position.6
Postthrombotic syndrome diagnosis
Deep veins abnormalities
Anatomical abnormalities
At the acute stage of an obstructive deep venous thrombosis, the occluded vein appears as a dilated and noncompressible vein with a clot filling the lumen of the vein that is more or less echolucent according to the age of the thrombus. For a nonocclusive thrombosis, the thrombus is usually floating in the lumen of a nondilated vein.
During follow-up, different evolutions of the thrombus can be observed with the entire spectrum from a complete resolution with recanalization without any residual abnormality to a persistent occlusion with vein shrinkage.7 Consequently, deep venous abnormalities can be very obvious, but they can also be very limited or absent, and, in such a case, distinguishing between postthrombotic and primary deep venous insufficiency can be challenging. On the other hand, according to the depth of the veins, duplex ultrasound is usually more precise for infrainguinal vein examination than for the inferior vena cava and iliac veins that require using a low frequency transducer for better penetration, even though this results in a lower resolution.
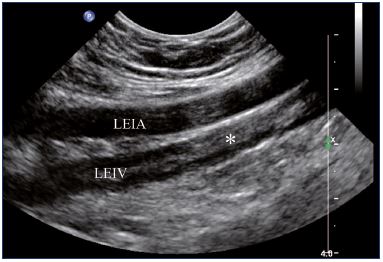
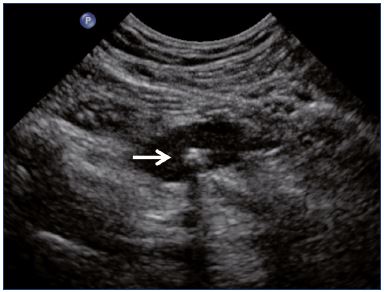
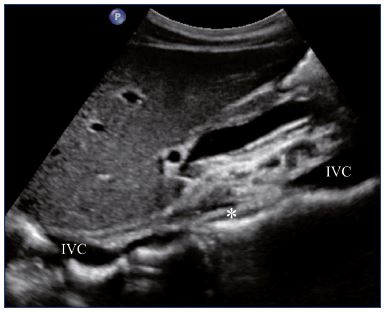
Figure 1. Residual fibrotic thrombus* in the left external iliac vein
(sagittal view).
Abbreviations: LEIA, left external iliac artery; LEIV, left external iliac vein.
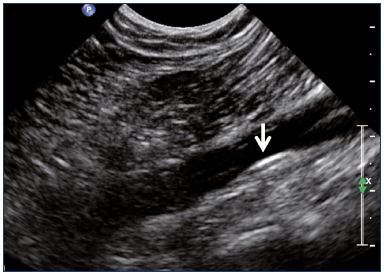
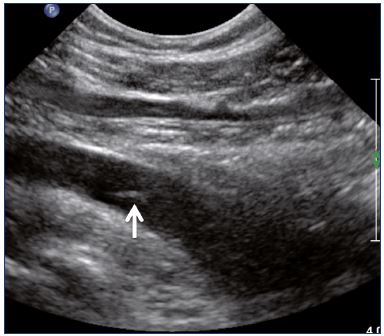
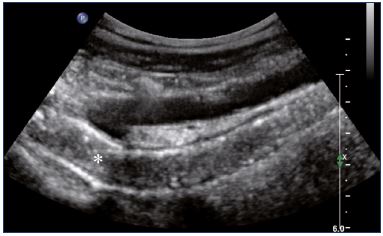
Postthrombotic changes may involve the venous lumen, wall, and valves. According to the extent of the lysis, the following can be observed in the venous lumen: (i) persistence of a thrombus that usually decreases in size and becomes more echogenic, and, in such cases, the vein is not totally compressible (Figure 1); (ii) partial recanalization of the vein with residual intraluminal fibrotic material presenting as more or less extended webs or synechia that lead to compartmentalization of the lumen (Figure 2); (iii) localized intraluminal calcifications—phleboliths (Figure 3); and (iv) complete recanalization of the vein without any abnormality. The following can be observed in the vein wall: (i) more or less shrunken, with possible complete fibrosis and disappearance from ultrasound detection (Figure 4); and (ii) isolated venous wall thickening and/or rigidity (Figure 5).8 Finally, venous valves are usually thin and mobile in the lumen of the vein and they can also present with the following abnormalities: (i) thickening and abnormally very echogenic (Figure 6); and (ii) rigid without spontaneous or induced valve movement. Nevertheless, in some cases, no anatomical abnormality can be observed with duplex ultrasound in patients with a confirmed history of deep vein thrombosis.
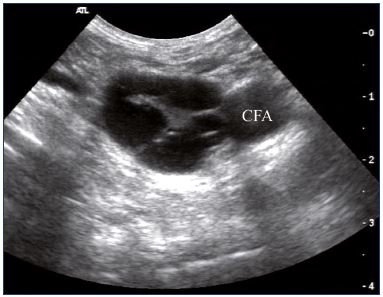
Figure 2. Intraluminal webs in the common femoral vein
(transverse view).
Abbreviations: CFA, common femoral artery.
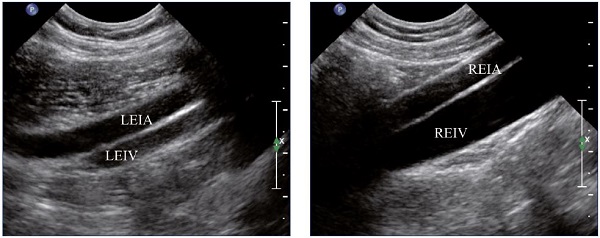
Figure 4. External iliac veins (longitudinal view).
Panel A shows shrinking of the LEIV. Panel B shows a healthy REIV.
Abbreviations: LEIA, left external iliac artery; LEIV, left external iliac vein; REIA, right external iliac artery; REIV, right external iliac vein.
Hemodynamic abnormalities
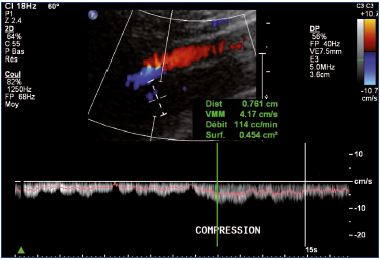
Postthrombotic changes may be responsible for reflux, residual complete obstruction, or limited lumen stenosis. Reflux can be observed mainly at the popliteal and femoral veins using a compression-release maneuver of the limb distal to the point of examination in a patient that is standing. A color Doppler ultrasound investigation is used for reflux screening. In a second step, a power Doppler ultrasound can measure reflux duration that must exceed the 1-second threshold to be considered significant (Figure 7). Duplex ultrasound is a very efficient technique, but there is some controversy concerning the evaluation of reflux extension compared with descending venography.9
Obstruction is more challenging to diagnose and therefore to quantify with duplex ultrasound, which can only measure velocities at different locations of the venous network, but cannot provide any relevant quantification of the global venous flow of the limb. In a normal patent vein, spontaneous blood velocities are low and they increase significantly with an augmentation maneuver. In this case, color Doppler shows a complete and homogenous filling of the lumen and pulsed Doppler shows an increase in the flow velocities with a steep slope of the curve. Furthermore, proximal veins, such as the iliac and common femoral veins, present a phasic flow with respiratory modulation.
In obstruction analysis, a duplex ultrasound can demonstrate the following for a remodeled vein:
• Absence of any flux in case of obstruction.
• Low velocities in case of partial recanalization.
• Increase in velocity in case of venous stenosis with ratio over 2.5 (Figure 8).10
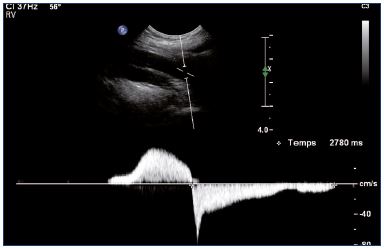
Usually low velocities are registered cranially to a segmental obstruction area or at a long remodeled segment of the vein. An increase in velocity is observed at a segmental stenosis. In the last two cases, color Doppler shows an irregular colorization of the vein lumen compared with a patent healthy vein (Figure 9). If the duplex analysis is performed proximally to an obstructive area, the duplex ultrasound will demonstrate a low increase in venous velocity during an augmentation maneuver with a flat slope of the waveform that is asymmetrical compared with a healthy limb (Figure 10).
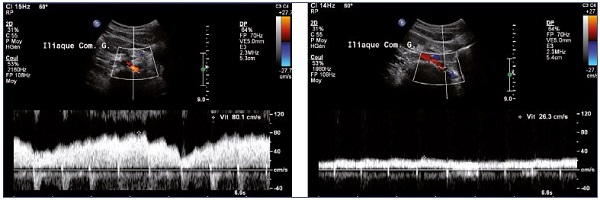
Figure 8. Increase in venous flux velocities at the termination of the left iliac vein (Panel A) compared with velocities measured distally
to the stenosis (Panel B) (spontaneous flow).
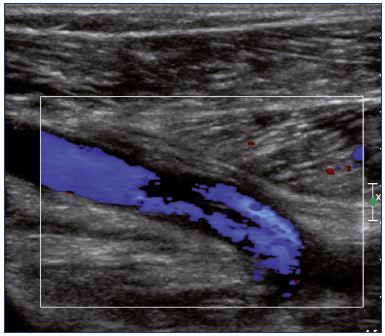
Figure 9. Color Doppler ultrasound of the popliteal vein
(longitudinal view).
There is a partial recanalization with irregular colorization of
the lumen vein.
For iliac vein obstruction, phasic flow measured in the common femoral vein usually disappears and a spontaneous low velocity flux can be observed (Figure 11).11 An analysis of the collateral veins is useful. As lower-limb veins are often duplicated or triplicated and connected with numerous collateral veins, a collateral pathway develops when deep vein thrombosis occurs and this network may or may not compensate for a residual occlusion of a deep vein. For example, for postthrombotic syndrome, spontaneous high-flow velocity in the great saphenous vein indicates that there is an obstruction of the infrainguinal deep vein. For suprainguinal postthrombotic syndrome, retrograde flow into the internal iliac vein indicates that there is an obstruction of the common iliac vein and other deep collateral veins can also be identified.
Superficial and perforating vein tests
Postthrombotic syndrome may mimic primary superficial venous insufficiency, which is why deep veins must also be investigated, especially in patients presenting with a history of thromboembolic disease and/or with an advanced clinical class (C) of the clinical, etiological, anatomical, and pathophysiological (CEAP) classification. Superficial venous insufficiency can be present in postthrombotic syndrome and can sometimes be worsened by deep venous reflux or obstruction. Duplex ultrasound examination of the superficial vein for postthrombotic syndrome is the same as for primary superficial venous insufficiency. Nevertheless, two points must be highlighted. For postthrombotic syndrome, superficial collateral veins can be dilated and tortuous and they can mimic varicose veins. In such cases, duplex ultrasound shows a spontaneous and continuous antegrade flow and no reflux during a compression-release maneuver. These features are commonly observed at the thigh and abdomen. If the varicose vein reflux originates from an incompetent perforating vein or if it is connected with a bidirectional perforating vein, duplex ultrasound must search for the source of the perforating venous reflux, which can be increased by reflux in an axial or a major deep vein.
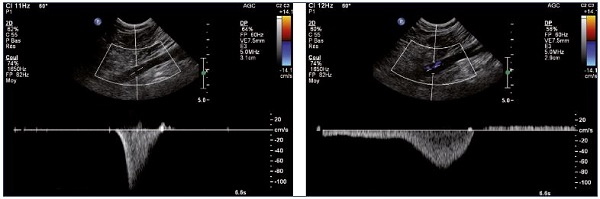
Figure 10. Left and right popliteal vein (longitudinal view).
Normal (Panel A) and pathological (Panel B) velocity profiles during an augmentation maneuver.
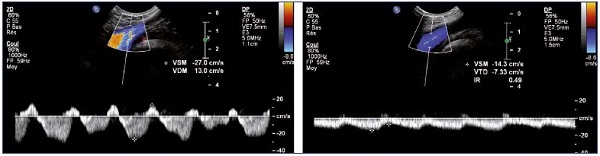
Figure 11. Left and right common femoral vein flux.
Right common femoral vein flux with a patent and healthy right iliac vein (Panel A) and left common femoral vein flux with left iliac
vein obstruction (Panel B).
Hemodynamic analysis for postthrombotic syndrome
Supra-inguinal obstruction
Iliac vein obstruction can be related to postthrombotic syndrome, but also to other conditions, such as the May-Thurner syndrome (left common iliac vein compression between the right iliac artery and the spine), other iliac vein compressions, or congenital deep venous anomalies, such as inferior vena cava atresia. Duplex ultrasound searches for indirect signs of iliac obstruction at the femoral vein, ie, phasic–flow disappearance and low-flow velocity. High venous pressure can be responsible for an increase in the femoral vein diameter compared with the contralateral side.
For postthrombotic syndrome, anatomical and hemodynamic direct abnormalities (as described above) can be observed at the common iliac vein and/or at an external vein, which sometimes extends to the femoral vein and/or the inferior vena cava, according to the location of the initial deep venous thrombosis. Some collateral veins can usually be recognized with duplex ultrasound, such as the superficial suprapubic vein (Palma collateral veins) or the lateral abdominal collateral vein (for inferior vena cava obstruction), but also deep veins involving the latero-uterine, ovarian, and lumbar veins can be identified. In some cases, collateral veins can mimic the course of the iliac vein.
For the May-Thurner syndrome, indirect hemodynamic anomalies of obstruction are present on the left side and deep collateral veins can be observed. Left internal iliac vein retrograde flux is frequent. The termination of the common iliac vein appears to be compressed by the right common iliac artery with a decrease in the vein diameter and an increase in the flux velocity. Proximally to the compression, the iliac vein diameter is larger with a decrease in venous flow velocities. For other compressions, duplex ultrasound can often identify the cause of the compression (tumor or retroperitoneal fibrosis). For inferior vena cava atresia, duplex ultrasound shows the absence of a normal inferior vena cava in the atretic area and usually obvious collateral veins (Figure 12).
Infrainguinal abnormalities
Combination of superficial and deep venous reflux
At the infrainguinal level, the combination of superficial and deep venous reflux is common, and normally, both can be easily evaluated. For combined reflux in the common femoral vein and the great saphenous vein or in the popliteal vein and the small saphenous vein above the saphenofemoral or the popliteal junction, respectively, deep venous reflux can be simply induced by the saphenous reflux. If the reflux occurs below the junction, it shows evidence of a true deep venous incompetence.
Combination of reflux and popliteal-femoral vein obstruction
As discussed previously, reflux in the deep veins is easier to demonstrate using duplex ultrasound than is obstruction. In current practice, duplex ultrasound is used to search for reflux at the popliteal and femoral veins, while obstruction is only searched for at the iliac vein. For postthrombotic syndrome, reflux and obstruction can coexist at the infrainguinal vein; therefore, these should both be analyzed.
For popliteal-femoral vein obstruction associated with great saphenous vein reflux, it can be challenging to evaluate the respective responsibility of deep venous obstruction and superficial venous reflux, insofar as refluxing superficial veins can also act as a collateral pathway. If the great saphenous vein appears to be refluxing in a standing position with a compression-release maneuver, it can also be efficient as a collateral pathway during exercise. In such a case, the decision to spare or ablate the refluxing great saphenous vein can be made by using the following tests: (i) a great saphenous vein duplex ultrasound can be performed during tiptoe-elevation movements to analyze the flux direction during exercise; and (ii) variations in the flow in the deep veins and other collateral veins of the thigh can be analyzed during compression of the great saphenous vein (Figure 13). If the great saphenous vein appears to be refluxing during exercise and/or if the deep venous flow increases during great saphenous vein compression, reflux is probably predominant compared with the collateral efficacy.
Interest in preoperative duplex ultrasound
Recanalization
Percutaneous transluminal angioplasty for iliocaval or iliofemoral obstruction can use a guidewire to go through the obstruction and then dilate and stent the obstructed vein segment. Preoperatively, duplex ultrasound provides relevant information about the extent of obstruction, the precise anatomy of affected segments to treat, the presence of collateral veins, and the association of deep and/or superficial venous reflux.
The entire deep venous system can be analyzed from the inferior vena cava up to the distal veins. Veins are described as normal, dilated, or shrunken, and the vein diameter is measured at each level. Postthrombotic abnormalities are classified as an absence of a patent vein lumen (residual obliteration), the presence of a patent central channel (with vein wall thickening), or compartmentalization of the vascular lumen by postthrombotic webs and/or synechia (Figure 1, 2, and 4). Moreover, duplex ultrasound identifies other anomalies, such as inferior vena cava atresia, May-Thurner syndrome, or vein compression.
Consequently, the duplex ultrasound examination focuses on selecting patients to be treated regarding feasibility; determining the expected mid- and long-term permeability; planning the procedure; choosing the most efficient site for safe venous access; selecting venous segments to be treated; evaluating infrainguinal vessels (ie, common femoral vein, deep femoral vein, femoral and popliteal veins, and saphenous veins) to determine the landing zone since proximal and distal termination of the stents have to be positioned in a normal healthy venous segment, even below the inguinal ligament. Infrainguinal dilatation and eventual stenting have not yet been validated, although they are considered interesting by some clinicians.12
Surgery for postthrombotic syndrome reflux
Duplex ultrasound is usually able to distinguish between postthrombotic, primary, and congenital incompetence. For primary incompetence, the refluxing vein appears as a normal vein, with a thin wall and no lumen abnormality. The vein can be compressed easily and completely. The only abnormality, except for an occasional, slightly enlarged vein, is valve incompetence (Figure 14). Sometimes the valve structure remains intact and is therefore suitable for external or internal valvuloplasty. For postthrombotic syndrome, different therapeutic options can be considered, including the replacement of the refluxing vein segment by transplantation of a vein segment containing a competent valve, transposition of a refluxing vein onto a competent one, or creation of a neovalve from the thickened vein wall.13
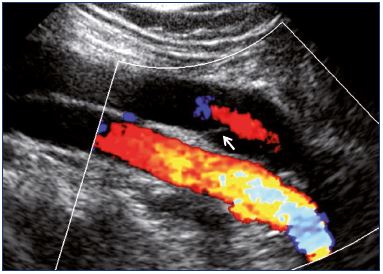
Figure 14. Popliteal vein as obtained from color Doppler
ultrasound showing central reflux through a nonthickened valve
(longitudinal view).
Even if the preoperative examination is based on phlebography, duplex ultrasound could be used to describe precisely the anatomical and morphological features of the vein to be transplanted or transposed and the features of the vein wall and lumen before neovalve creation.
Superficial and perforating vein ablation
If superficial vein ablation is planned, preoperative duplex ultrasound is mandatory. Duplex ultrasound examination will include an assessment of perforating veins and the saphenous vein junctions, trunks, and tributaries along their course with the results reported using cartography. Furthermore, duplex ultrasound will be used to guide endovenous treatment (thermal and chemical), which is largely used today, compared with conventional surgery.14
Intraoperative duplex ultrasound
Recanalization mainly uses fluoroscopic guidance. When available, intravascular ultrasound provides a precise evaluation of the venous stenosis and the result of the angioplasty. Nevertheless, transcutaneous duplex guidance as an adjunctive option should be considered, but it must first be evaluated. Duplex ultrasound can also be used for vein access guidance at the femoral and popliteal level. If the vein to be treated is clearly visualized with duplex ultrasound, echo guidance should be used for catheterization. Providing real time hemodynamic analysis, duplex ultrasound could also limit the use of contrast to manage and control venous recanalization.
Postoperative duplex ultrasound follow-up
Duplex ultrasound is the first-line imaging technique for the postoperative follow-up, regardless of the treatment modality–endovascular and open surgery (bypass or valvuloplasty). After recanalization, duplex ultrasound is used to check the patency of the treated vein and the collapse of collaterals. More precisely, duplex ultrasound measures flow into the stented veins. Complications are easily diagnosed with duplex ultrasound: (i) thrombosis usually occurs in the stented vein, which occurs more rarely in native veins (Figure 15); (ii) residual stenosis can be observed for inadequate angioplasty or stenting (Figure 16); (iii) can be observed during follow-up, intrastent intimal hyperplasia. For intrastent intimal hyperplasia, a power Doppler ultrasound can show a circumferential decrease in the diameter of the vein lumen with an echolucent area between the lumen and stented wall of the vein (Figure 17). Regarding surgical techniques, duplex ultrasound checks the bypass permeability and the disappearance of reflux after valvuloplasty.
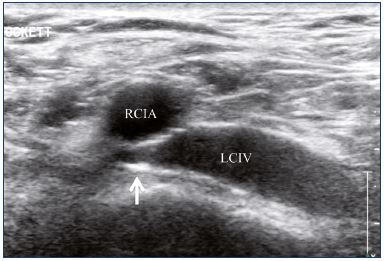
Figure 16. Inadequate angioplasty and stenting of the left
common iliac vein with residual stenosis.
Abbreviations: LCIV, left common iliac vein; RCIA, right common iliac artery.
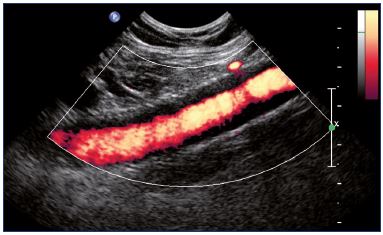
Figure 17. Power Doppler showing an intrastent intimal
hyperplasia of the external iliac vein (longitudinal view).
Role of duplex ultrasound in managing postthrombotic syndrome
Duplex ultrasound is recommended as the primary diagnostic tool for suspected abdominal or pelvic venous pathology to evaluate patients with postthrombotic syndrome or clinical suspicion of other forms of iliac or inferior vena cava obstruction or compression.3 Nevertheless, because the use of Duplex ultrasound for assessing the iliac veins and collateral veins can be limited, additional pelvic imaging studies, such as conventional descending venography, computed tomography, or magnetic resonance imaging, are usually performed to assess the extent of the disease in the iliocaval segment and to exclude extravascular disease causing obstruction, such as tumors or retroperitoneal fibrosis.15 Thus, duplex ultrasound is not systematically used as a preoperative examination before recanalization. Among 16 papers selected for a recent review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction, duplex ultrasound was only used in 30% of the reported studies as a preoperative examination, and it was always associated with other diagnostic techniques, including ascending and descending venography, computed tomography and magnetic resonance venography, venous pressure measurement, and plethysmography.16-20 However, in more recent publications on endovascular intervention, duplex ultrasound is always performed during follow-up to assess patency.21-24
Conclusion
Duplex ultrasound is a first-line examination for postthrombotic syndrome diagnosis and postoperative follow-up. It can provide relevant information for the operative management of obstruction and reflux, even if the preoperative assessment is based on computed tomography venography or magnetic resonance venography. Intraoperative ultrasound is not yet used, except for venous access.
REFERENCES
1. Villalta S, Bagatella P, Piccioli A, Lensing A, Prins M, Prandoni P. Assessment and validity and reproducibility of a clinical scale for the post-thrombotic syndrome [abstract]. Haemostasis. 1994;24(suppl 1):158a.
2. Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57:1163-1169.
3. Wittens C, Davies AH, Baekgaard N, et al. Management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2015;49:678-737.
4. Coleridge-Smith P, Labropoulos N, Partsch H, Myers K, Nicolaides A, Cavezzi A. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs–UIP consensus document. Part I. Basic principles. Eur J Vasc Endovasc Surg. 2006;31:83-92.
5. Auvert JF, Chleir F, Coppé G, Hamel- Desnos C, Moraglia L, Pichot O; French Society for Vascular Medicine. Quality standards for ultrasound assessment of the superficial venous system of the lower limbs [in French]. J Mal Vasc. 2014;39:26-46.
6. Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003;38:793-798.
7. Abai B, Labropoulos N. Duplex ultrasound scanning for chronic venous obstruction and valvular incompetence. In: Gloviczki P, ed. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 3rd ed. London, UK: Hodder Arnold; 2009:142-155.
8. Quarto G, Genovese G, Apperti M, Amato B, Benassai G, Furino E. Is the fibrotic parietal thickening a reliable parameter for diagnosing previous asymptomatic deep vein thrombosis? Ann Ital Chir. 2015;86:427-431.
9. Neglen P, Raju S. A comparison between descending phlebography and duplex Doppler investigation in the evaluation of reflux in chronic venous insufficiency: a challenge to phlebography as the “gold standard.” J Vasc Surg. 1992;16:687- 693.
10. Labropoulos N, Borge M, Pierce K, Pappas PJ. Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg. 2007;46:101- 107.
11. Zygmund J Jr, Pichot O, Dauplaise T. Practical phlebology venous ultrasound. In Kabnick LS, Sadick NS, eds. Venous ultrasound. 2nd ed. Taylor & Francis Group; 2013:67-71.
12. Spencer EB, Stratil P, Mizones H. Novel treatment techniques for recanalization of femoral-popliteal deep venous occlusion from chronic thrombosis. Tech Vasc Interv Radiol. 2014;17:114-120.
13. Maleti O, Perrin M. Reconstructive surgery for deep vein reflux in the lower limbs: techniques, results and indications. Eur J Vasc Endovasc Surg. 2011;41:837-848.
14. Pavlović MD, Schuller-Petrović S, Pichot O, et al. Guidelines of the First International Consensus Conference on Endovenous Thermal Ablation for Varicose Vein Disease—ETAV Consensus Meeting 2012. Phlebology. 2015;30:257-273.
15. Mahnken AH, Thomson K, de Haan M, O’Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37:889-897.
16. Seager MJ, Busuttil A, Dharmarajah B, Davies AH. A systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg. 2016;51:100-120.
17. Raju S, Neglén P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50:360- 368.
18. de Wolf MA, Arnoldussen CW, Grommes J, et al. Minimally invasive treatment of chronic iliofemoral venous occlusive disease. J Vasc Surg: Venous Lym Dis. 2013;1:146-153.
19. Alerany BM, Lamoca LM, Ortega MR, Rivas IL, Desboeufs RZ, Kiuri SS. Endovascular treatment of iliofemoral chronic post-thrombotic venous flow obstruction. J Vasc Surg: Venous Lym Dis. 2014;2:2-7.
20. Catarinella FS, Nieman FH, de Wolf MA, Toonder IM, de Graaf R, Wittens CH. Quality-of-life in interventionally treated patients with post-thrombotic syndrome. Phlebology. 2015;30(suppl 1):89-94.
21. de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Shortterm clinical experience with a dedicated venous nitinol stent: initial results with the sinus-venous stent. Eur J Vasc Endovasc Surg. 2015;50:518-526.
22. Yin M, Shi H, Ye K, et al. Clinical assessment of endovascular stenting compared with compression therapy alone in post-thrombotic patients with iliofemoral obstruction. Eur J Vasc Endovasc Surg. 2015;50:101-107.
23. Zhang Q, Huang Q, Shen B, Sun J, Wang X, Liu H. Efficacy and safety of endovascular intervention for the management of primary entire-inferior vena cava occlusion. Cardiovasc Intervent Radiol. 2015;38:665-671.
24. Meltzer AJ, Connolly PH, Kabutey NK, Jones DW, Schneider DB. Endovascular recanalization of iliocaval and inferior vena cava filter chronic total occlusions. J Vasc Surg: Venous Lym Dis. 2015;1:86- 89.