Role of anticoagulation treatment in the prevention of post-thrombotic syndrome
PhD1;
Athanasios GIANNOUKAS,MD,
PhD2;
1Consultant Vascular Surgeon;
2Professor of Vascular Surgery and Head
of the Department; Department of Vascular
Surgery, University Hospital of Larissa,
Faculty of Medicine, School of Health
Sciences, University of Thessaly, Larissa,
Greece
Abstract
Post-thrombotic syndrome (PTS) develops after deep vein thrombosis (DVT) of the lower limbs and may affect up to 50% of patients after proximal DVT. Prevention is of paramount importance as there is no gold standard for treatment of established PTS. Pharmacological or mechanical thromboprophylaxis is recommended to prevent PTS. Effective DVT treatment with vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs), low-molecular-weight heparins (LMWHs), and fondaparinux are recommended as appropriate duration and intensity of anticoagulation therapy prevents DVT recurrence and PTS development. Rivaroxaban offers more favorable prognosis in terms of PTS development and severity than treatment with VKAs. LMWH have anti-inflammatory properties and may be superior to VKAs. Larger randomized controlled studies are needed to clarify optimal treatment for PTS prevention.
Introduction
Post-thrombotic syndrome (PTS) is a frequent complication of deep vein thrombosis (DVT) of the lower limbs.1 PTS refers to chronic clinical manifestations of venous valvular dysfunction following proximal DVT, including various symptoms affecting a patient’s quality of life (QOL).2 It is also associated with a reduced ability to work, with considerable consequences for both the patient and heath care systems.3 Almost half of the patients with DVT (20%-50%) will develop PTS within 2 years after the index event, despite conventional anticoagulation therapy.4 Others reported that the incidence of PTS continues to increase up to 10 years after the initial diagnosis of DVT.5,6
Venous hypertension is the main pathophysiological factor for PTS development.1 Outflow venous obstruction deriving from persistent residual vein thrombosis (RVT) and acute inflammatory response after venous thrombosis leads to valvular damage and reflux and occurrence of venous hypertension.1 Another important factor for PTS development is the chronic inflammation affecting the venous wall and the microcirculation. Excessive capillary leakage and impairment of skin nutrition leads to skin changes and ulceration in more severe forms of PTS.7 Inflammation also delays thrombus resolution and causes fibrosis in the vein wall.8,9
No standard criteria exist for diagnosis of PTS and thus its presence becomes evident when signs and symptoms of deep venous incompetence occurs after the acute episode of DVT has passed (after 6 months).10 The signs and symptoms of PTS include limb edema, various degrees of pain, heaviness, cramps, fatigue, itching, venous claudication, varicose veins due to venous stasis, and skin changes.11 Skin changes include hyperpigmentation, venous eczema, lipodermatosclerosis, and in more severe forms of PTS, venous ulceration (Figures 1, 2). Symptoms are provoked by standing position or walking and reduced by rest and elevation of the leg.
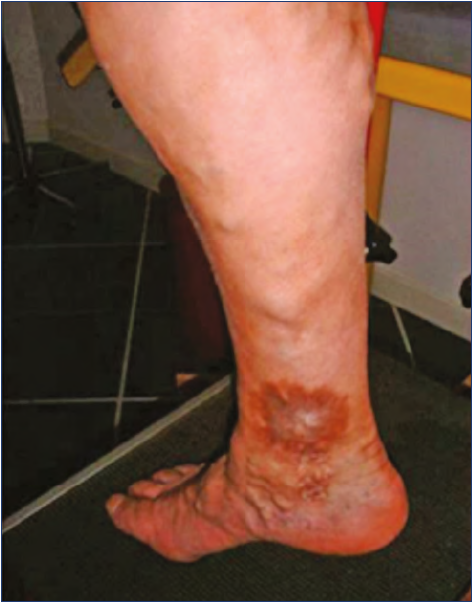
Figure 1. Post-thrombotic syndrome; healed ulcer with concomitant skin changes in the medial malleolus and varicose veins. Photo provided courtesy of Department of Vascular Surgery, University Hospital of Larissa, Larissa, Greece.
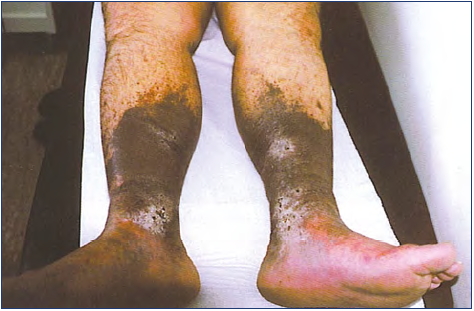
Figure 2. Post-thrombotic syndrome in both lower limbs with active ulcer and extensive concomitant skin changes in the gaiter area. Photo provided courtesy of Department of Vascular Surgery, University Hospital of Larissa, Larissa, Greece.
Pharmacological or mechanical thromboprophylaxis is recommended to prevent PTS.12 Appropriate duration and intensity of anticoagulation therapy prevents recurrent DVT and development of PTS.13 Vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs), low-molecular-weight heparins (LMWHs), and fondaparinux are recommended for treatment of DVT.14 The latest PTS guidelines from the American Heart Association do not recommend a specific anticoagulant over another, although the quality and type of anticoagulation could affect the risk of PTS.15 This review focuses on the role of conventional anticoagulation treatment in the prevention of PTS.
Vitamin K antagonists (VKAs)
For more than half a century, VKAs have been recommended as the standard treatment for DVT.13 VKAs, such as warfarin, acenocoumarol, and phenprocoumon, are administered orally and inhibit the γ-carboxylation of coagulation factors II, VII, IX, and X, and also the functional activity of the coagulation inhibitor proteins C and S.
Numerous studies have documented that the quality of VKA treatment plays an important role in PTS development.16,17 The Einstein DVT trial, a randomized controlled study (RCT) that compared the efficacy and safety of rivaroxaban with subcutaneous enoxaparin followed by a VKA in 3449 patients with acute DVT, demonstrated that 21% of patients treated with VKA were below the therapeutic international normalized ratio (INR) levels.18 A large Danish survey including 310 300 patients being treated with VKA reported that around 70% of patients were in therapeutic range, whereas another study from the United States (US) showed that only 54% of the patients had achieved continuing target levels of INR.19,20 A meta-analysis by Erkens et al21 reported that patients treated with VKAs are at the target level of anticoagulation (INR 2-3) for only about 50% of their treatment time, with a strong tendency toward subtherapeutic INR (42% in 0-1 month of treatment, 35% in 1-3 months, and 24.1% in 1-6 months).
Subtherapeutic anticoagulation with VKAs has been related to a 3-fold higher risk of PTS in patients who had an INR less than 2.0 for more than 50% of the treatment duration.16,17 About 30% of the patients, especially in the first weeks of treatment have subtherapeutic INR.16,17,21 In this period, delayed clot lysis and stimulated connective tissue growth due to thrombin generation may cause permanent fibrosis and venous damage.22 Therefore, it is crucial that patients treated with VKAs have adequate INR control during the first weeks of therapy for prevention of PTS.
Direct oral anticoagulants (DOACS)
DOACs have become over the last years the new standard of treatment for venous thromboembolism (VTE).14 DOACs can be given in fixed doses without routine monitoring. The following DOACs are licensed for VTE treatment: dabigatran, which inhibits thrombin, and rivaroxaban, edoxaban, and apixaban, which inhibits factor Xa (FXa). The latest guidelines by the European Society for Vascular Surgery (ESVS) recommend DOACs over VKAs for the treatment of VTE (grade 1A) in patients without cancer.14 Theoretically, DOACs may also reduce the incidence of PTS compared with VKA, due to their more reliable dosing and predictable pharmacodynamics. It remains unknown whether DOACs are equivalent to LMWH with respect to PTS prevention.
Several studies have suggested that rivaroxaban, compared with warfarin, reduces the risk of PTS after DVT, although only a few of these studies were randomized and the incidence of PTS was the primary outcome.19,20,23-28 Two registries have also concluded a benefit of rivaroxaban, compared with warfarin, regarding the PTS prevalence.19,20 In the Danish registry, rivaroxaban was associated with decreased risk of PTS compared with warfarin within the 3-year follow-up (0.53 per 100 per year vs 0.55 per 100 per year, respectively).20 In a multicenter retrospective study from Norway, the risk of PTS development was lower after 2 years among patients treated with rivaroxaban than in warfarin-treated patients (odds ratio [OR], 0.5; 95% confidence interval [CI], 0.3 to 0.8; P=0.01).24 Another retrospective multicenter study by Ferreira and colleagues also reported a benefit of rivaroxaban over warfarin after 15 months among 127 patients with DVT (50.7% vs 69%; P=0.002).25 Prandoni and colleagues investigated the prevalence of PTS at 3 years in a prospective cohort study of 309 patients treated with rivaroxaban, apixaban, or dabigatran, compared with a historical cohort of 1036 patients treated with warfarin.26 Although the majority of the patients in the DOAC cohort were treated with rivaroxaban (84%), the risk of PTS in the DOAC-treated patients was reduced by 54% in comparison with patients treated with warfarin (OR, 0.46; 95% CI, 0.33 to 0.63).26 In a post hoc subgroup analysis of 336 patients previously included in the Einstein DVT trial, in which 48% were treated with rivaroxaban and 52% with warfarin, the prevalence of PTS 60 months after the acute DVT was numerically lower in the rivaroxaban group than in the warfarin group although statistically nonsignificant after adjustment for possible confounders (29% vs 40%; P=0.18).27 In a small RCT by de Athayde Soares and colleagues, rivaroxaban-treated patients had lower incidences of PTS and better total vein recanalization rates at 1-year follow-up than warfarin-treated patients.28 Finally, 2 recent meta-analyses found that the use of rivaroxaban for the treatment of DVT was associated with a roughly 50% reduction in PTS risk compared with warfarin.29,30
In a post hoc subgroup analysis of patients who participated in the RE-COVER studies, the long-term prevalence of PTS (mean time from the index event, 8.7 ± 1.4 years) was investigated.31 The RE-COVER studies randomized 5109 patients with DVT and/or pulmonary embolism (PE) to receive 6 months of treatment with either dabigatran (150 mg twice a day) or warfarin in double-blind design.32,33 After the completion of the studies, patient-reported Villalta and health-related QOL (HRQOL) questionnaires were sent by mail to the patients who agreed to participate. In total, 349 patients were included, of which 166 (48%) were treated with dabigatran and 183 (52%) with warfarin. PTS was diagnosed in 63% of patients with DVT and in 46% of patients with PE only and did not differ between the treatment groups; the crude odds ratio for PTS in patients treated with dabigatran compared with warfarin was 1.1 (95% CI, 0.6 to 1.8) after DVT and 1.2 (95% CI, 0.5 to 2.6) after PE only. It is possible that the higher quality of warfarin anticoagulation achieved in clinical trials than in clinical practice can partially explain why PTS prevalence did not differ between groups in the RE-COVER and Einstein DVT post hoc subgroup analyses.
In a recent published meta-analysis, 1894 patients were analyzed regarding the severity of PTS.30 Severe PTS was less common in patients treated with rivaroxaban, according to Villalta score, than in patients treated with warfarin (3.7% vs 6.4%) (OR, 0.55; 95% CI, 0.36 to 0.85; P=0.024).30 Furthermore, in another meta-analysis by Li and colleagues, rivaroxaban therapy was found to be associated with a reduced risk of mild PTS (OR, 0.64; 95% CI, 0.50 to 0.82; P=0.0005), moderate PTS (OR, 0.64; 95% CI, 0.45 to 0.91; P=0.01), and severe PTS (OR, 0.52; 95% CI, 0.33 to 0.82; P=0.005).29
DOACs may produce an earlier reduction in the RVT detected on duplex ultrasound and consequently an earlier vein recanalization. Prandoni and colleagues reported that the degree of RVT in the patients treated with rivaroxaban decreased from 50% to 40% at 3 months and continued to decrease even to 20% at 6 months after the episode of DVT.34 The study concluded that vein recanalization progressively increases over time in patients treated with DOACs in contrast with those treated with warfarin.34 Ferreira and colleagues25 demonstrated a significantly lower rate of RVT in patients treated with rivaroxaban (24.4%) than in patients treated with VKAs (64.6%). Others reported that the incidence of total recanalization 1 year after the episode was significantly higher in the rivaroxaban group than in the warfarin group, whereas partial or no venous recanalization was higher in the warfarin group.28
Low-molecular-weight heparins (LMWHs)
LMWHs are polysulfated glycosaminoglycans derived from unfractionated heparin by enzymatic or chemical depolymerization. The molecular weight is on average 4-5 kDa (range 2-9 kDa) and inhibits FXa over thrombin.35,36 LMWHs have anticoagulant and anti-inflammatory properties.37-39 Experimental studies have shown that LMWHs reduce venous wall inflammation,37 enhance endothelization,38 and reduce fibrosis,39 and it is probable that these anti-inflammatory properties could have a role in preventing PTS.
A systematic review by Hull and colleagues40 compared long-term LMWH (≥3 months) with VKAs for DVT treatment and showed a more favorable recanalization rate and lower incidence of venous ulceration with LMWHs. Another study that compared tinzaparin with VKAs showed a 23% reduction in signs and symptoms of PTS at 3 months with tinzaparin.41 In a subgroup analysis of 480 patients included in the home-LITE study, the prevalence of PTS was lower in the tinzaparin-treated patients than in warfarin-treated patients regardless of DVT location (iliac/noniliac).42 Patients with iliac DVT had an overall odds ratio of 0.53 (95% CI, 0.33 to 0.83; P=0.0079) for PTS, and patients with noniliac DVT had an odds ratio of 0.79 (95% CI, 0.67 to 0.93; P=0.0046), both in favor of tinzaparin. The study concluded that LMWH may be a suitable alternative for the prevention of PTS in patients with iliac DVT who are unlikely to undergo invasive thrombolysis.42
Conclusions
Anticoagulation remains the fundamental therapy for VTE, and in terms of PTS prevention, the quality of anticoagulation is particularly important. Rivaroxaban offers a more favorable prognosis in terms of PTS development and severity than treatment with VKAs. Careful INR monitoring remains critical for the outcome in case of VKA treatment. LMWH have anti-inflammatory properties in addition to anticoagulation and may provide a more favorable outcome. In future, more research is needed with large RCTs to better define the role of different DOACs compared with warfarin as well as LMWH and fondaparinux in the prevention of PTS.


REFERENCES
1. Kahn SR. How I treat postthrombotic syndrome. Blood. 2009;114:4624-4631.
2. Galanaud JP, Kahn SR. The postthrombotic syndrome: a 2012 therapeutic update. Curr Treat Options Cardiovasc Med. 2013;15:153-163.
3. O’Donnell TF Jr, Browse NL, Burnand KG, Thomas ML. The socioeconomic effects of an iliofemoral venous thrombosis. J Surg Res. 1977;22:483-488.
4. Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82:423-428.
5. Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4(4):734-742.
6. Haig Y, Enden T, Grøtta O, et al. Postthrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5 year follow-up results of an open label, randomized controlled trial. Lancet Haematol. 2016;3(2):64-71.
7. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-498.
8. Deatrick KB, Elfline M, Baker N, et al. Postthrombotic vein wall remodeling: preliminary observations. J Vasc Surg. 2011;53:139-146.
9. Henke PK, Varma MR, Moaveni DK, et al. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 2007;98:1045-1055.
10. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7(5):879-883.
11. Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164:17-26.
12. Kahn SR, Lim W, Dunn AS, et al; American College of Chest Physicians. Prevention of VTE in non surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e195Se226S.
13. Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e419S-e494S.
14. Kakkos SK et al. European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61(1):9-82.
15. Kahn SR, Comerota AJ, Cushman M, et al; on behalf of the American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. The Post-thrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies. A scientific statement from the American Heart Association. Circulation. 2014;130:1636-1661.
16. van Dongen CJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost. 2005;3:939-942.
17. Chitsike RS, Rodger MA, Kovacs MJ, et al. Risk of post thrombotic syndrome after subtherapeutic warfarin anticoagulation for a first unprovoked deep vein thrombosis: results from the REVERSE study. J Thromb Haemost. 2012;10(10):2039-2044.
18. Einstein Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510.
19. Coleman CI, Beyer-Westendorf J, Bunz TJ, Mahan CE, Spyropoulos AC. Postthrombotic syndrome in patients treated with rivaroxaban or warfarin for venous thromboembolism. Clin Appl Thromb Hemost. 2018;24(4):575-582.
20. Søgaard M, Nielsen BP, Skjøth F, Kjældgaard JN, Coleman CI, Larsen TB. Rivaroxaban vs warfarin and risk of postthrombotic syndrome among patients with venous thromboembolism. Am J Med. 2018;131(7):787-794.
21. Erkens PM, ten Cate H, Büller HR, Prins MH. Benchmark for time in therapeutic range in venous thromboembolism: a systematic review and meta-analysis. PLoS One. 2012;7(9):e42269.
22. Baglin T. Prevention of post-thrombotic syndrome: a case for new oral anticoagulant drugs or for heparins? J Thromb Haemost. 2012;10(8):1702-1703.
23. Jeraj L, Jezovnik MK, Poredos P. Rivaroxaban versus warfarin in the prevention of post-thrombotic syndrome. Thromb Res. 2017;157:46-48.
24. Utne KK, Dahm A, Wik HS, Jelsness- Jørgensen LP, Sandset PM, Ghanima W. Rivaroxaban versus warfarin for the prevention of post-thrombotic syndrome. Thromb Res. 2018;163:6-11.
25. Ferreira T, Huber SC, de Moraes Martinelli B, et al. Low prevalence of postthrombotic syndrome in patients treated with rivaroxaban. Vascul Pharmacol. 2020;124:106608.
26. Prandoni P, Ageno W, Ciammaichella M, et al. The risk of postthrombotic syndrome in patients with proximal deep vein thrombosis treated with the direct oral anticoagulants. Intern Emerg Med. 2020;15(3):447-452.
27. Cheung YW, Middeldorp S, Prins MH, et al. Post-thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/ vitamin K antagonists for acute deep-vein thrombosis. A post-hoc analysis. Thromb haemost. 2016;116(4):733-738.
28. de Athayde Soares R, Matielo MF, Brochado Neto FC, Nogueira MP, Almeida RD, Sacilotto R. Comparison of the recanalization rate and postthrombotic syndrome in patients with deep venous thrombosis treated with rivaroxaban or warfarin. Surgery. 2019;166(6):1076-1083.
29. Li R, Yuan M, Cheng J, et al. Risk of postthrombotic syndrome after deep vein thrombosis treated with rivaroxaban versus vitamin-K antagonists: a systematic review and meta-analysis. Thromb Res. 2020;196:340-348.
30. Karathanos C, Nana P, Spanos K, et al. Efficacy of rivaroxaban in prevention of post-thrombotic syndrome: a systematic review and meta-analysis. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1568- 1576.
31. Wik HS, Kahn SR, Eriksson H, et al. Postthrombotic syndrome in patients with venous thromboembolism treated with dabigatran or warfarin: a long-term cross-sectional follow-up of RE-COVER study patients. J Thromb Haemost. 2021;19(10):2495-2503.
32. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352.
33. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764-772.
34. Prandoni P, Ageno W, Mumoli M, et al. Recanalization rate in patients with proximal vein thrombosis treated with the direct oral anticoagulants. Thromb Res. 2017;153:97-100.
35. Hirsh J, Raschke R. Heparin and lowmolecular- weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:188S-203S.
36. Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e24S-e43S.
37. Downing LJ, Strieter RM, Kadell AM, Wilke CA, Greenfield LJ, Wakefield TW. Low-dose low-molecular-weight-heparin is antiinflammatory during venous thrombosis. J Vasc Surg. 1998;28(5):848-854.
38. Moaveni DK, Lynch EM, Luke C, et al. Vein wall re-endothelialization after deep vein thrombosis is improved with low molecular weight heparin. J Vasc Surg. 2008;47:616- 624.
39. Obi AT, Diaz JA, Ballard-Lipka NL, et al. Low-molecular-weight heparin modulates vein wall fibrotic response in a plasminogen activator inhibitor 1-dependent manner. J Vasc Surg Venous Lymphat Disord. 2014;2:441-450.
40. Hull RD, Liang J, Townshend G. Long-term low-molecular-weight heparin and the post-thrombotic syndrome: a systematic review. Am J Med. 2011;124(8):756-765.
41. Hull RD, Pineo GF, Brant R, et al; LITE Trial Investigators. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. Am J Med. 2009;122(8):762-769.
42. Hull RD, Liang J, Tazmin Merali T. Effect of long-term LMWH on post-thrombotic syndrome in patients with iliac/noniliac venous thrombosis: a subanalysis from the Home-LITE study. Clin Appl Thromb Hemost. 2013;19(5):476-481.
