Saphenopopliteal junctions are significantly lower when incompetent. Embryological hypothesis and surgical implications
SUMMARY
The aim of this study was to compare the levels of the small saphenous junction with deep veins in a control group versus a group of patients with insufficient short saphenous junctions. A comparative meta-analysis of the data from 15 series shows that the incompetent junctions were significantly lower in the control group. The comparison with anatomy and embryology shows that the functional and phylogenetic regression of the small saphenous vein might correspond to its embryologic regression. This embryologic regression is associated with a progressive descent of the anastomosis in the deep vein. This descent might also induce poorer competence of the small saphenous junction.
INTRODUCTION
Experience gained from surgical removal of the small saphenous vein (SSV), and repeated surgery following recurrence of a popliteal varicose vein after surgery of the SSV has shown us that the junction of the SSV was usually easily accessible via an incision in the popliteal fossa.1,2 In contrast, anatomical dissections of the SSV have revealed a high degree of variability in the level of anastomosis with the popliteal vein.3,4 This variability is not reflected in surgery for incompetent SSV. This discrepancy led us to speculate that the level of the junction could be linked with an incompetence of the SSV.
MATERIALS AND METHODS
In a meta-analysis of the various sets of anatomical data in the literature reporting the frequency of the different levels of the SSV junction, we have compared a control group with subjects presenting incompetent SSV. The classification used for this comparison was based on that of Kosinski (Figure 1).5 For our study (Figure 2) we defined type I anastomoses as located either at the popliteal bend level or between 0 and 7 cm above this bend, type II anastomoses as located more than 7 cm above the popliteal bend, and type III anastomoses as located below the popliteal bend. To compare the various classifications reported in the literature, the data for some groups were reassigned according to this classification. Apparently healthy saphenopopliteal junctions were studied in several sets of anatomical dissections5- 7 and on the basis of Doppler scans of healthy patients (Table I).8 Incompetent junctions were defined on the basis of the surgical findings or, in the preoperative phase, on the basis of the Doppler ultrasound scan or by venography (Table II).9-19 The distributions were compared by independent ÷2 tests, with á set to 5%. The degree of significance, P, for the ÷2 test and the value of the reduced deviation ì (square root of ÷2) are reported for each comparison.
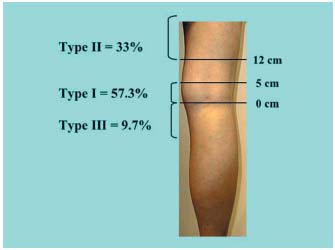 Figure 1. Classification used by Kosinski.5
Figure 1. Classification used by Kosinski.5 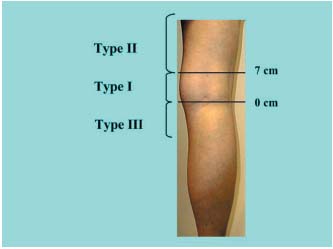 Figure 2. Classification used to compare the data in the various studies. Height in cm from the popliteal bend (0 located within the popliteal bend). (Type I, II, III).
Figure 2. Classification used to compare the data in the various studies. Height in cm from the popliteal bend (0 located within the popliteal bend). (Type I, II, III).
RESULTS
1/ Anatomy of the saphenopopliteal junction in the subjects in the control group presenting with an apparently healthy saphenopopliteal junction (Table I).5-8 This has been very well documented by Kosinski5 on the basis of 124 dissections. In this group (Figure 1), 57.3% of SSV anastomoses were located between 0 and 5 cm above the popliteal bend (less than half of them had a considerable postaxial extension which constituted a Giacomini vein (GSV) when it rejoined the great saphenous vein. Thirty-three percent of the cases were high anastomoses, located at least 12 cm above the popliteal bend. In two out of five cases, there was a single anastomosis with the femoral vein, in two out of five cases there was a single anastomosis with the GSV, and in one out of five cases there was a composite anastomosis. From the low junctions, ie, those located below the popliteal bend, 9.7% consisted mainly of direct or indirect junctions with the GSV below the knee. A junction with the gastrocnemius veins was seen only occasionally. Cibor7 found very similar percentages following cadaver dissections. On the basis of ultrasound scans of healthy subjects, Engel8 reported virtually the same percentages, with 52.4% of junctions located in the popliteal fossa, 46.6% high junctions, and 1% low junctions. According to Moosman,6 17.5% of SSVs dissected in cadavers had no connection with the popliteal vein.
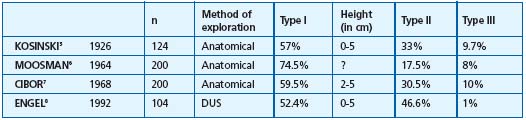
Table I. Type of saphenopopliteal junction in the control group subjects. These various groups correspond to SSVs investigated by anatomical dissection 5-7 or by Doppler ultrasound explorations (DUS) in groups of healthy subjects.8 Type I corresponds to an anastomosis located within the popliteal fossa (0 and 7 cm), type II to an anastomosis located more than 7 cm above this fossa, type III to an anastomosis located below the popliteal bend. ? = Values not reported by the author.
2/ Anatomy of the saphenopopliteal junction in subjects presenting with an incontinent saphenopopliteal junction (Table II).9-19 In patients with varicose veins, studies of the anatomy of the incompetent saphenopopliteal junction using Doppler ultrasound or venography, or observed intaoperatively, have shown that 80.1% of the popliteal junctions were located between the popliteal bend and a level 7 cm above it. High refluxes, more than 7 cm above the popliteal bend, were only found in 13.9% of cases.
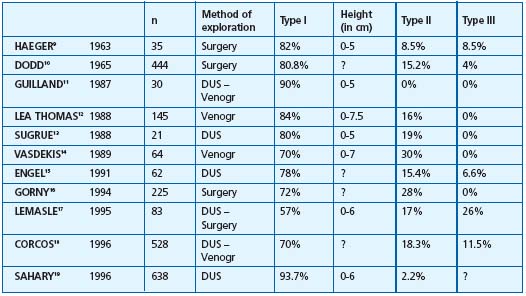
Table II. Type of saphenopopliteal junction in subjects presenting with incompetence of the SSV. The various groups correspond to incontinent short saphenous veins. Method of exploration: DUS = Doppler ultrasound, Surgery = peroperative assessment, Venogr = Venography. Type I corresponds to an anastomosis located within the popliteal fossa (between 0 and 7 cm), type II to an anastomosis located more than 7 cm above it, type III to an anastomosis located below the popliteal bend. ? = Values not reported by the author
3/ Statistical comparisons of the percentages in the varicose patient group and control subject group. These percentages are reported in Figure 3, which compares the junctions located within the popliteal fossa (type I), and in Figure 4, which compares the high junctions (type II) in these groups. The meta-analysis of these various pooled sets shows that the mean frequencies of the various locations were significantly different between the groups with and without junctional incompetence. The incompetent junctions were more often located between the popliteal bend and a level 7 cm above than the junctions in the control group (80.1% versus 62.6%, P<0.05). The junctions in the control group were more often located more than 7 cm above the popliteal bend than those in the incontinent junction group (29.5% versus 13.8%, P<0.05). This difference in frequency found by the meta-analysis of the type I and type II groups was significant (P<0.05) (Table III), ì (reduced deviation) = 1.978, P<0.05 for the comparison of the type I groups, and u = 1.997, P<0.05 for the comparison of the type II groups.
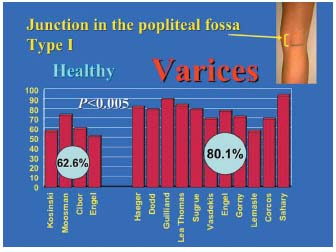
Figure 3. Comparison of type I junctions in the incompetent group and in the control group. Pairwise comparison of each of the 4 data sets comprising the control group of type I junctions (saphenopopliteal junction located within the popliteal fossa) with the 11 data sets from groups with type I saphenopopliteal junction incompetence (saphenopopliteal junction located in the popliteal fossa) shows that the junctions located within the popliteal fossa were usually incompetent (P<0.05).
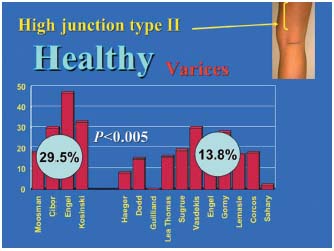
Figure 4. Comparison of type II junctions in the incompetent group and in the control group. The pairwise comparison of each of the 4 data sets for the type II control group (high saphenopopliteal junction) with the 11 data sets of the type II saphenopopliteal junction incompetence group shows that the high junctions were usually competent (P<0.05).
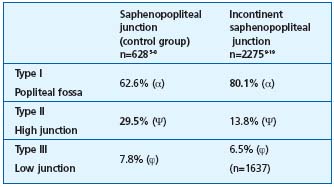
Table III. Comparison of the height of the junction in incompetent and competent SSV. The meta-analysis of the group (α) and (Ψ) has demonstrated a significant difference in the type-I and type II junctions (P<0.05). The difference was not significant for the comparison of groups (ϕ) with a type III junction. The type III, incompetent saphenopopliteal junction group included a different number of subjects, because one study19 was incomplete.
DISCUSSION
With regard to the junctions located in the popliteal fossa (Figure 3), only the percentages reported by Vasdekis14 and Lemasle17 and, to a lesser extent, those reported by Sugrue13 showed no highly significant difference from the control group (P>0.01, or not significant). With regard to the high junctions (Figure 4), only the percentages reported by Vasdekis14 and Sugrue13 showed no highly significant difference from the control group (P>0.01 or not significant). The data sets reported by Vasdekis14 and Sugrue13 in fact correspond to only 85 out of 2275 cases (3.7%), and their findings probably reflect recruitment bias. The data of Kosinski,5 Moosman,6 and Cibor,7 based on cadaver dissections, do not specify what percentage (if any) of the cadavers displayed incontinence of the SSV. In fact it is unlikely that the frequency of SSV incompetence (10% to 12% of the population with varicose veins) could affect the degree of significance, P, which is often less than 0.01 for pairwise comparisons of data sets. In contrast, the differences in the percentages reported for the two groups by Engel8,15 (normal SSV and incontinent SSV) and obtained using the same methodology are highly significant (P<0.01). A previous anatomical study of 41 incontinent saphenopopliteal junctions following surgery1 gave similar percentages. Although the determinations of the height above or below the popliteal fossa were not exactly the same, 78% of the junctions were located at the level of the popliteal fossa, versus only 12% located higher up. These figures match those of Van der Stricht, who found only 29 junctions at the level of the popliteal fossa out of 60 dissections of unselected cadavers.20
The notion that the lower the saphenopopliteal junction, the greater the risk of incompetence, could be explicable in terms of the embryology of the popliteal vein system.
1/ Embryology of the popliteal vein
In general, during the embryological development superficial veins appear prior to deep veins with a thicker histological texture. Similarly, the SSV appears before the great saphenous vein, also with a thicker histological texture. Initially, the venous return system is superficially located on the limb bud and only later is transferred to a deeper level. Finally, the residual superficial system is used for superficial cutaneous draining and thermoregulation.
In a 6-week-old embryo, the first collector networks can be clearly observed. The first venous network is superficial. It goes from the extremity to the root bud where the first anastomosis will be created with the deep venous pelvic system, and later the inferior vena cava. Later on, the preaxial superficial venous network will appear and form a secondary circulatory system.
In a 9-week-old embryo, deep veins appear in the form of an endothelialized vascular lacuna surrounding the arteries. These three networks– superficial primary postaxial, superficial secondary preaxial, and deep ones– prefigure globally and respectively the SSV, the long saphenous vein and femoral vein and the deep popliteal and femoral veins.
With time, anastomosis will occur between the superficial pre- and postaxial and the deep axial networks to facilitate blood draining at the limb root thanks to the deep network. Progressively, flow will be transferred from the limb extremity to its root, a transfer from the postaxial to the preaxial network that will finally ensure the main venous communication between the limb and the pelvis.21-26 In a 10-mm embryo, draining occurs via the postaxial system. The SSV is prolonged by the postaxial vein that drains into the pelvis through the ischiatic vein.27
With ingrowths of the femoral vein, the axial vein normally involutes. It corresponds to limb elongation and to medial rotation of the anterior side of the limb. The anterior preaxial system becomes medial on the lower part of the limb.
In a 15-mm embryo, a secondary path appears to the detriment of the preaxial system. The superficial and deep femoral veins meet the long saphenous vein and flow into the iliac vein.
In a 20-mm embryo, with pelvic rotation and lower limb elongation, the draining system moves forward, from the ischiatic postaxial system to the iliac vein preaxial system. In a 25-mm embryo, numerous anastomoses can be observed. Flow transfer is organized to the fore between the postaxial and axial systems and the axial and preaxial system.
In a 35-mm embryo, after the thigh has extended from the trunk, the SSV individualizes and loses its connections with the ischiatic system to flow into the great saphenous vein. The final stage will only be achieved in the 50-mm embryo. The SSV connection with the deep vein moves progressively downward during the embryologic and fetal period.
Three nerves control vessel development:
1- The axial nerve or sciatic nerve that gives the tibial and common fibular nerves at knee level. It is followed by the axial venous plexus.
2- The preaxial or femoral nerve that gives a sensory branch called the saphenous nerve. It is followed by the preaxial venous plexus.
3- The postaxial or small sciatic nerve that is more particularly represented by its sensory branch: the posterior cutaneous nerve. It comes down along the posterior aspect of the lower limb. The postaxial venous plexus follows the posterior cutaneous nerve down to the calf tip. Anastomosis between these different plexuses yields the final venous system.28
A/ The first anastomosis is ventral. It establishes a connection between the axial plexus below knee level with the preaxial plexus above knee level. This anastomosis generates the popliteal vein that drains all the deep leg axes to the thigh axial plexus.
B/ The second anastomosis is dorsal. It establishes a connection between the postaxial plexus and the axial plexus, at knee level. It is facilitated by the joining of the plexuses derived from knee bending. This anastomosis ensures all or part of the postaxial plexus superficial blood draining of the leg towards the thigh axial plexus, and becomes the saphenopopliteal junction.
1/ A complete anastomosis will give a SSV junction of terminal type that connects with the popliteal vein at the popliteal fossa level (50%). The upper postaxial extension, a satellite of the posterior femoral cutaneous nerve, will not grow. In this case, it is possible to say that the typical saphenopopliteal junction development as a cross is a “chance mishap” on the long postaxial axis of the embryo. The lack of conductor nerves along this cross may explain its extreme variability.22-24
2/ A partial anastomosis will give, on the one hand, after the branching of the short saphenous trunk, a deep anterior collateral which, with a normal cross, connects with the popliteal vein at the popliteal fossa level. On the other hand, it also gives a more superficial trunk that follows the posterior femoral cutaneous nerve on the fascia deep side. This postaxial extension is called the Hyrtl vein or Stolic vein. When it perforates the fascia at the upper level thigh part, crosses the medial thigh part to join the long saphenous vein at the femoral triangle level, it is called the Giacomini vein. It then creates an anastomosis between the postaxial leg plexus and the upper level thigh preaxial plexus. At the branching level, a deeper ascending collateral can appear; in this case the axial extension that follows the back of the sciatic nerve to join the axial trunk (sciatic nerve vein) constitutes another axiopostaxial anastomosis (Figure 5).29
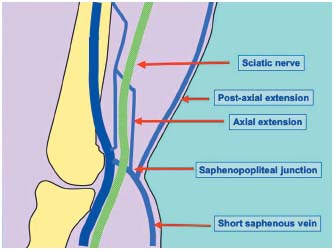
Figure 5. The SSV and its venous extensions.
3/ The lack of anastomosis entails a SSV without a junction. In this case, the trunk extends into a canal filled with the postaxial extension (Figure 6).30 Embryology can explain the great diversity of types and heights of the saphenopopliteal junction.31 The classically described modal junction only represents roughly 50% of cases. It then comes with a diameter of about 5 mm, is straight and 3 to 5 cm long, slightly bent forwards, ascending with a thick white multivalved wall (two to three valves). This SSV directly joins the popliteal vein some centimetres above the condyles, on the posterior side of the popliteal vein. The other endings reflect the major embryologic stages. The SSV often splits into three branches at the popliteal fossa: the postaxial extension upwards, subfascial, touching the posterior femoral cutaneous nerve; the axial extension at the rear of the tibial nerve and touching it; and the real saphenopopliteal junction that is a residue of the axiopostaxial anastomosis of the embryo. By this definition, postaxial extension characterized by the following features: a diameter exceeding 2 cm, a straight course, vertical, in the limb posterior axis, completely subfascial, accompanying the posterior femoral cutaneous nerve, in direct continuity with the SSV trunk and anatomically with valves oriented to allow centripetal reflux.
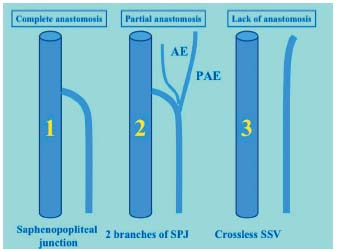
Figure 6. Different types of saphenopopliteal junction. 1: regular junction. 2: saphenopopliteal junction with axial and postaxial extensions. 3: small saphenous vein without a junction.
2/ Phylogenesis and ontogenesis
In phylogenesis and ontogenesis, superficial veins appear prior to the deep veins. Important differences can be observed between the evolution, the anatomy, the structure and the function of both superficial and deep veins and also between the great and short saphenous veins.32,33 In mammals, the latter develop differently and can vary within the same group. From an embryological point of view, the SSV, which is a primary vein, comes with a thicker and better-developed wall and is therefore better prepared for its superficial vein function than a secondary vein.34 SSV insufficiency only accounts for 15%.
3/ Comparative anatomy of the short saphenous vein
Embryologic downward regression of the SSV can also be found in mammalian phylogenetic evolution. Actually, in quadrupedal mammals, ungulate and plantigrade animals, the SSV is the most important hind vein whereas, in apes, it becomes much smaller and less functional. In big mammals, the short saphenous vein is generally the greater collecting vein toward the pelvis.24 It usually connects at a very high level and frequently in the middle of very large muscular masses. The lower junction of the short saphenous vein in the popliteal fossa (a specific adaptation in large bipedal mammals) may be an adaptation to lower-limb elongation. In big mammals, the great saphenous vein is regularly connected according to the same pattern, whereas the short saphenous vein connection can present several different patterns, each of them specific to a mammal species and corresponding to each embryologic stage.35 Therefore, in the different mammal species, normally and specifically, it is possible to observe the different kinds of connections in human beings.36,37
In quadrupedal mammals, the short saphenous vein is a very important vein with a usually double connection situated much higher than the knee-bending level, and is always embedded into large muscular masses. In pigs, a triple connection can be observed: in the circumflex femoral vein, in the gluteal veins and in the codalis femoralis vein (the equivalent of a deep femoral vein). In dogs, the short saphenous vein connects to the popliteal vein to form the femoral vein. In equids, it flows very high into the posterior thigh side or into the sciatic vein. In bovids, its two similar branches flow into the deep femoral vein and into the superficial femoral. Embryologic and phylogenetic regression of the small saphenous vein may induce its functional regression. Actually, short saphenous vein dysfunction or reflux is a frequent disorder in human beings but has never been observed in animals. The theory of small saphenous vein phylogenetic functional regression accompanying the progressive lowering of its connection into the axial plexus could explain the relation between the small saphenous vein caliber and the height of its connection in the popliteal vein. According to Gillot,23 the higher the small saphenous vein popliteal connection, the greater its caliber.
The SSV is a particular superficial vein.34 Unlike the great saphenous vein, the SSV is the oldest vein. It is a primary vein with a primary pattern (and has never been accompanied by an artery during embryologic development). It is the most important vein in mammals with a stronger and thicker wall than the GSV and is better prepared for its superficial vein function.
4/ Surgical implications
Incompetent saphenopopliteal junctions are in fact usually easily accessible during surgery. Out of 41 operated junctions, 78% were located within the popliteal fossa.1 It is unusual to have to operate on incompetent junctions located far above the popliteal fossa. When these high refluxes are associated with varices, they are often linked to pelvic vein insufficiency that follows the trajectory of the axial venous system. These are indeed high refluxes, but they are not directly transmitted by the short saphenous vein. They are not directly accessible for local surgery. When a very high junction does present with a reflux, this reflux is often centripetal and so it flows in the same direction during both compression and decompression movements of the calf muscles (Figure 7). This is in fact a venovenous shunt running alongside the path of the deep vein in the thigh. Varices of the popliteal fossa are usually collaterals of this second pathway. Surgery is not, of course, appropriate for a pathway displaying a systolic reflux with a very high junction.
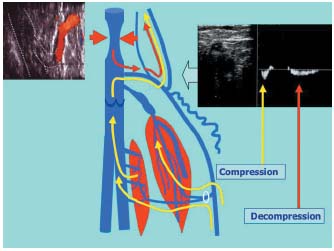
Figure 7. Systolic reflux at the saphenopopliteal junction. If the flow through the saphenopopliteal junction or through a proximal extension occurs in the same direction during both compression and decompression of the calf muscles, this indicates the presence of a physiological shunt. A very high junction of the small saphenous vein or of an axial or postaxial extension of this vein corresponds to the upper anastomosis of a deep vein-vein shunt.
With regard to popliteal recurrences after surgery of the short saphenous vein, the long stumps responsible for recurrences2 are also very often located within the popliteal fossa. Long stumps that have been inadequately resected because they were inaccessible will probably not give rise to a recurrence, when they were not incompetent at the time of the first operation.
ACKNOWLEDGEMENTS
Statistical analyses were carried out by F. Kohler, PR Spieao, Medical University of Nancy, France.

