Specific criteria of the transcutaneous Doppler ultrasound in unusual causes of lower limb varicose veins
Milka GREINER2
2American Hospital of Paris, OPD 10, 63
Bd Victor Hugo, 92200 Neuilly/Seine,
France
Abstract
Transcutaneous Doppler ultrasound is the technique of choice to diagnose lower limb varicose veins; it is often the only technique needed. The relative contributions of the great saphenous vein, small saphenous vein, and incompetent perforating veins in the development of superficial venous insufficiency of the lower limbs are well recognized; however, information on unusual causes are lacking. Yet, there are Doppler ultrasound pathognomonic signs that can help diagnosis some of the unusual cases. The aim of this paper is to itemize these Doppler ultrasound signs and to study their embryology and anatomical forms; three entities will be detailed. The first entity is intraosseous perforating vein (also called bone perforators) incompetence as related to abnormal intraosseous venous drainage. While their pathophysiology remains to be clarified, Doppler ultrasound makes their diagnosis easy and accurate. The second entity concerns sciatic nerve varicose veins, which may be truncular or plexiform at the femoral level, and they are explained by venous embryology. The presence of a tubular venous trunk, which is parallel to, but situated outside of the small saphenous vein, indicates that a varicose vein developed in the territory of the superficial fibular nerve and its fibular communicating branch. It is pathognomonic of sciatic nerve varicose veins. The third entity relates to when an inguinal leak point has a normal anatomical drainage path, but with a flow that is reversed secondary to intrapelvic venous hyperpressure, allowing pelvic varicose veins to drain to the lower limb. The presence of a dystrophic venous network, which is dilated, tortuous, and incontinent, located above the inguinal ligament is pathognomonic of the presence of this inguinal venous reflux of pelvic origin.
Introduction
Beside the classic causes of superficial varicose veins in the lower limbs (ie, insufficiency of the great or small saphenous veins or incompetence of standard perforating veins), some specific anatomic sources must be known. They are detected by Doppler ultrasound (DUS) pathognomonic criteria. The DUS diagnosis with these criteria does not require specific equipment. All current devices have sufficient ultrasound imaging quality and Doppler sensitivity. The anatomical structures studied in this article are all superficial. They should favor the high frequencies and wideband probes (eg, 8 to 15 Mhz) to obtain the best image resolution. Flow imaging is based on the Doppler effect, color mode, and wideband power mode. With the exception of the inguinal leak point, which is identified when a patient is lying down, all exams are done with the patient in a standing position. This paper will focus on three entities: incompetent intraosseous perforating veins, sciatic nerve varicose veins, and inguinal leak points.
Incompetent intraosseous
perforating veins
For the first time, in 1962, Schobinger and Weinstein1 described paratibial leg varicose veins in connection with intraosseous venous dilation. Around 20 years ago, the term ”abnormal intraosseous venous drainage” appeared, but it was replaced recently with the term ”bone perforator.”9 However, the term intraosseous perforating veins or transosseous veins seems more suitable for these veins that perforate the bone cortex and that are physiological and numerous. They allow bone venous drainage toward the great circulation. They can become pathological, dilated, incompetent, and feed leg varicose veins. The term “incompetent intraosseous perforator vein” or incompetent transosseous veins” is more accurate. The clinical expression of these varicose veins is stereotyped: these are paratibial varicose veins that have developed on the anteromedial side of the middle third of the leg (Figure 1).
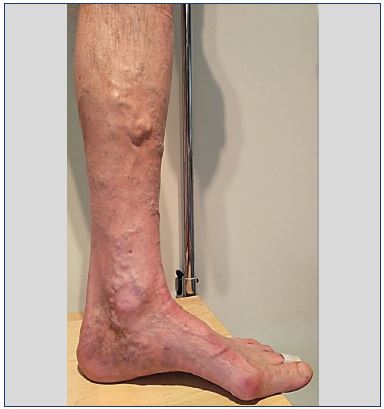
Figure 1. Typical clinical appearance of a varicose vein fed by
a bone perforator: anterolateral side of the middle third of the
leg.
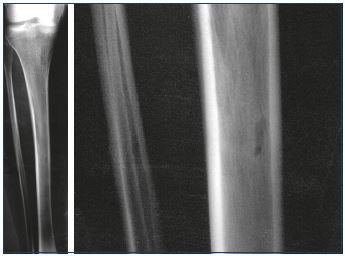
While a limited number of reports have been published,1-10 this type of varicose vein does not seem so uncommon. In 2016, Ramelet et al11 proposed a multicenter study on this specific type of varicose vein. Diaz-Candamio et al3 describes intrafibular varicose veins, an incidental finding in context of pain, swelling, and deep vein thrombosis. Imaging findings are similar to those of intratibial varix. Ultrasound investigations revealed a reflux from the intraosseous veins, which feeds subcutaneous venous dilations via an orifice in the bone cortex (Figure 2). The reflux is spontaneous or induced by the compression of muscles or varicose veins located below this cortical orifice. The cortical orifice is well visualized with DUS and it is always located at the anteromedial side of the middle third of the leg. It is characterized by a lack of continuity with the cortical bone. This bone defect is also clearly visible on a conventional radiography (Figure 3) and CT scan. It is sometimes possible to visualize intraosseous venous dilations under the cortical bone ultrasonically (Figure 4).
Within certain limits, ultrasound can analyze formations inside bone structures. The ultrasound image is based on the detection of waves reflected by the different acoustic interfaces encountered by the incident wave. An acoustic interface is the boundary between two tissues of different acoustic impedance. Acoustic impedance is a physical quantity characterized by a number Z and expressed as kg×m2/s. The greater the difference between two acoustic impedances, the higher the reflection coefficient (R) and the lower the transmission coefficient (T=1-R) per relation. Depending on the thickness and density of the bone, the transmission coefficient can exceed 60%. The transmitted waves, which are secondarily reflected, thus make it possible to analyze the structures behind the bone barrier. Conversely, the difference between the acoustic impedance of soft tissue and air is so great that less than 1% of the energy will be transmitted. The air is a true acoustic mirror and represents a much more impenetrable barrier to ultrasound than bone.
Although the clinical and ultrasonographic expressions of this specific type of varicose vein are well known, its pathophysiology remains to be clarified. The recall of bone vascularization enables a better understanding of this venous pathology. The arterial vascularization of the tibia is ensured by three networks (Figure 5). First, the main food for the bone is provided by the intramedullary artery or feeder of the tibia branch of the posterior tibial artery. It enters the bone through the main feeder hole, located on the posterior side of the middle third of the tibia. In the bone marrow, it is divided into two branches, upper and lower, which will develop into an intramedullary mesh arterial network. Second, metaphyseal arteries vascularize the metaphysis and epiphysis. They enter the bone through specific secondary feeding holes and communicate with the intramedullary network. Third, periosteal arteries, located along the entire length of the tibia, are derived, depending on the segment considered, from the lower genicular arteries, posterior tibial arteries, anterior tibial arteries, and fibular arteries. They communicate with the intramedullary mesh network through multiple transcortical feeder orifices.
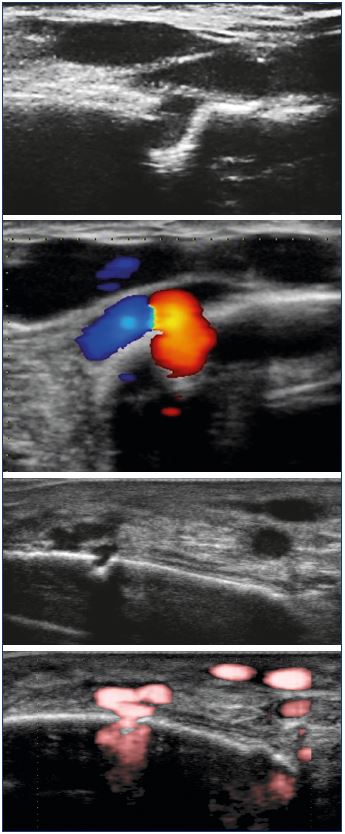
Figure 2. Ultrasonographic aspect of the incontinent bone
perforator: reflux of the intraosseous circulation toward the
great circulation, via a cortical defect.
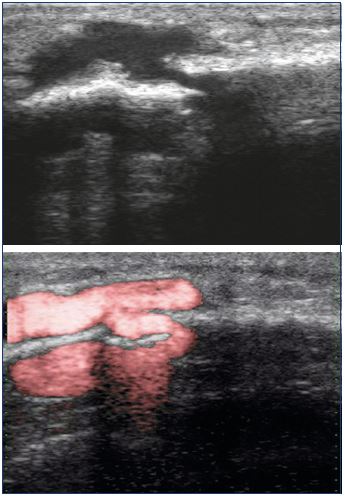
Figure 4. Visualization of subcortical venous dilations, which
communicate via a cortical orifice, with extraosseous venous
dilations.
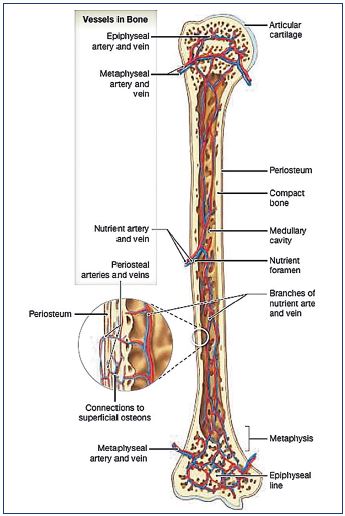
Figure 5. Schematic depiction of the arterial and venous
vasculature of a long bone.
© 2015, Pearson Education, Inc.
Several pathophysiological hypotheses can be proposed, including:
(i) Malformations during embryogenesis with either an absence of the posterior main feeder hole or hypo or agenesis of the tibia feeder vein;
(ii) Traumatic complications with either localized trauma with closure of the posterior main feeder or transverse diaphyseal fracture with interruption of venous continuity. However, in the multicenter study by Ramelet et al,11 significant limb trauma was found in only 6 out of 34 cases (12%), but it should be noted that minor trauma was not reported in this study; and
(iii) Hyperpressure in the tibia feeder vein secondary to primary or postthrombotic reflux in the posterior tibial vein is possibly associated with proximal venous reflux. In this case, the posterior feeder orifice, which is physiologically the main drainage pathway for the intraosseous venous system, becomes a point of reflux, meaning that the intraosseous vein drainage is forced to use other secondary cortical orifices, which may progressively widen by erosion due to vascular hyperflow.
Some authors2,4,8 describe, on CT scan and MRI, a dilatation of the tibia feeder vein in cases of an incontinent bone perforator, in favor of hyperpressure and/or venous stasis. However, Ramelet et al reported the presence of reflux of the posterior tibial vein in only 3 cases out of 35.11
In all of these hypotheses, an incontinent bone perforator is the expression of abnormal intraosseous venous drainage, but this raises three unresolved questions: (i) why is this pathology only located in the tibia and at the same level?; (ii) why is there a lack of a satellite artery?; and (iii) why does chemical ablation or phlebectomy of this vein have no detrimental effect,11 if it ensures intraosseous venous drainage. Concerning the first question, only one case was described at the fibula, none at either the femur or the lateral or posterior side of the tibia. Hydrostatic pressure (making intraosseous venous drainage toward the great circulation more difficult) or mechanical stress do not explain this anatomical fixity. Regarding the second question, in the studies and in our experience, the presence of a satellite artery has not been recorded. If the pathological transosseous vein matches the dilation of a physiological communication between the centromedullary circulation and the periosteal circulation, it should be accompanied by an artery; however, this small caliber artery could be difficult to identify by DUS. Lastly, the last question leads to the proposal of a fourth pathogenic hypothesis–a pathological transosseous vein could be a persistent embryonic vein, and the other bone drainage pathways are normal. This fourth hypothesis would help answer the three previous questions.
Key points
• Transosseous vein or intraosseous perforating vein are better term than bone perforator.
• These veins are numerous and physiological.
• The main transosseous vein is the drainage pathway for the feeder vein from the tibia to the posterior tibial vein. The other drainage pathways mainly correspond to the connections between the feeder vein of the tibia and the periosteal veins.
• Transosseous veins can become pathological after intraosseous abnormal drainage of congenital or acquired origin.
• These veins could also be persistent embryonic veins, with no abnormal intraosseous venous drainage.
• In front of isolated varicose veins located on the anteromedial side of the leg, incompetent tibial perforators have to be searched for using DUS.
• The ultrasound diagnosis is simple. We propose three diagnostic ultrasound criteria: (i) venous dilatations on the medial side of the tibia above (and/or below) the cortical bone; (ii) a cortical defect; and (iii) flux through this cortical hole from intraosseous to extraosseous circulation.
Sciatic nerve varicosis
Varicose veins around the sciatic nerve have been known for a long time. The first description was that of Verneuil in 1890.12 Since then, cases are episodically reported in the literature, with variable diagnostic circumstances: (i) in the context of Klippel Trenaunay syndrome13; (ii) during amputation of the thigh (discovery of a vascular pedicle, arterial and venous, around the sciatic nerve requiring a specific ligation)14; (iii) in front of recurrent sciatic pain in connection with the menstrual cycle14; or (iv) before recurrent varicose veins of the posterior side of the leg.15
In 2001, we proposed16 an ultrasound definition of this varicose vein, based on its embryological origins and nerve anatomy. In 2005, Ricci et al17,18 completed this description by recalling the ultrasound semiology of the sciatic nerve and its branches of the popliteal fossa. The best way to understand the pathophysiological aspects of this varicose vein is to summarize Gillot’s work on venous embryogenesis.19-22 In the embryo, the capillary networks precede the appearance of the nerves, but remain, at first, undifferentiated. It is only after the appearance of the nerves and at their contact that these embryonic venous plexuses, initially dispersed, will concentrate and differentiate. For the record, there are variations. Some veins have no nerve director (for example, at the level of the lower limb, the angio-directing element of the deep femoral and fibular veins is the femoral or fibular diaphysis, respectively). Three main angiogenic nerves intervene in the embryological development of the veins of the lower limbs. They are classified by their relation to the axis of the member: (i) the axial nervous plexus of the embryo will become, in an adult, the sciatic nerve in the thigh, the tibial nerve in the popliteal region, and the sural nerve in the leg; (ii) in front of this axis, the preaxial nervous plexus of the embryo will become the femoral nerve; and (iii) behind this axis, the postaxial nervous plexus will become the posterior femoral cutaneous nerve.
In contact with each nervous plexus, a satellite venous plexus grows (Figure 6). During embryologic development, these venous, axial, and pre- and postaxial plexuses expand or regress, leading to the final venous anatomical arrangement after the formation of interplexus anastomoses. From the evolution of the axial and preaxial venous plexuses, Gillot proposed three possible arrangements for the venous drainage of the thigh, defining three types. Evolution type 1 (Figure 7) is the most common where the axial system regresses. There are only a few discontinuous venous arches along the sciatic nerve that communicate on several levels with the deep femoral vein. The preaxial system normally evolves toward the femoral vein, which then becomes the main venous return pathway in the thigh. Evolution type 2 (Figure 8) occurs when the preaxial system can evolve normally toward the femoral vein, but the axial system does not regress and generates a tubular vein, named by Gillot as the axiofemoral trunk. It carries out a full-channel communication, without variation in size between the popliteal vein and most often the deep femoral vein, but sometimes the common femoral vein. According to the development of the femoral vein, the venous drainage of the thigh will then be ensured by two equivalent axes or preferably by this axiofemoral trunk (Figure 9). Evolution type 3 (Figure 10) is a rare evolution type where the preaxial system does not develop; instead, it regresses toward femoral vein atresia or hypoplasia. The axial system evolves toward a tubular, avalvular vein, the persistent sciatic vein, which is prolonged by the lower gluteal vein. It provides the main venous drainage pathway of the limb from the popliteal vein up to the internal iliac vein.
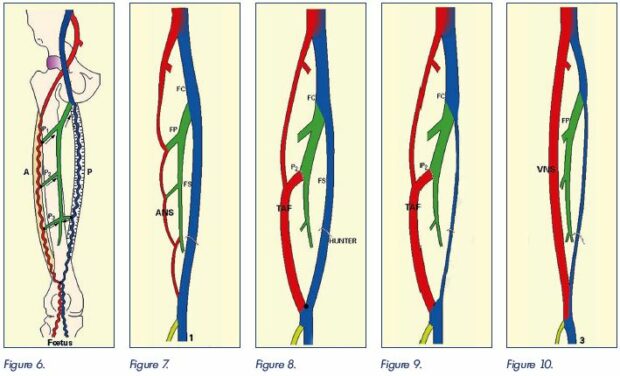
Figure 6. Schematic depiction of embryonic venous plexuses.
Axial system (A); preaxial system (P); perforating veins (P1, P2, P3). For simplicity, the postaxial system is not shown.
Image courtesy of C. Gillot.
Figure 6. Figure 7. Figure 8. Figure 9. Figure 10.
Figure 7. Evolution type 1.
Evolution of the preaxial system to the femoral vein (FS). There is a regression of the postaxial system, which is reduced to simple
discontinuous arches (ANS) that communicate with the deep femoral vein (FP). The femoral vein is the main route for venous
drainage of the lower limb.
Image courtesy of C. Gillot.
Figure 8. Evolution type 2.
Normal evolution of the preaxial system to the femoral vein. Nonregression of the axial system, which results in a tubular vein, ie,
the axiofemoral trunk (TAF). There is direct communication between the popliteal vein and most often the deep femoral vein, but
sometimes the common femoral vein. Two equivalent lower limb venous drainage pathways.
Image courtesy of C. Gillot.
Figure 9. Evolution type 2 – other modality.
In this case, there is involution of the preaxial system with hypo or agenesis of the femoral vein. The axiofemoral trunk (TAF) and
the deep femoral vein are the main axis for the venous drainage of the lower limb.
Image courtesy of C. Gillot.
Figure 10. Evolution type 3.
Regression of the preaxial system with hypo or agenesis of the femoral vein. Nonregression of the axial system which results in a
tubular vein, avalvulated, which is prolonged by the inferior gluteal vein (persistent sciatic vein), which becomes the main axis for
drainage of the lower limbs.
Image courtesy of C. Gillot.
The ultrasound aspect follows directly from these embryological evolutions. In type 2, the popliteal vein is prolonged by two axes (Figure 11): the femoral vein, a more or less developed axis, at the medial side of the thigh is superficial and joins the common femoral vein and another deeper, median, sciatic nerve satellite, which usually drains to the upper third of the thigh into the deep femoral vein. When there are two popliteal roots, the most frequent case, we obtain an anatomical ”X” arrangement of the popliteal vein. In type 3, the ultrasound appearance is similar in the popliteal fossa, but the femoral vein is more often atretic (Figure 12) and the deep, medial trunk, a satellite of the sciatic nerve, does not curve inward to the upper third of the thigh, but extends to the medial part of the proximal part of the thigh to the gluteal fold in order to join the lower gluteal vein. Reflux of the axiofemoral trunk or the persistent sciatic vein is translated, on the femoral level, by a truncular varicose veins of the sciatic nerve (Figure 13). Type 1 corresponds to modal venous embryogenesis. The embryonic axial venous system has regressed, persisting only in the form of discontinuous venous arches along the sciatic nerve. The venous drainage of this nerve originates from venules located in the nerve trunk. They communicate with a plexiform network located around the perinerve, which joins, according to different patterns,23 the extraneuronal drainage veins.
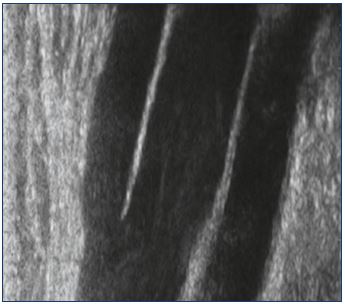
Figure 11. Ultrasound appearance of the extension of the
popliteal vein by two equivalent axes: the femoral vein and
the axiofemoral trunk.
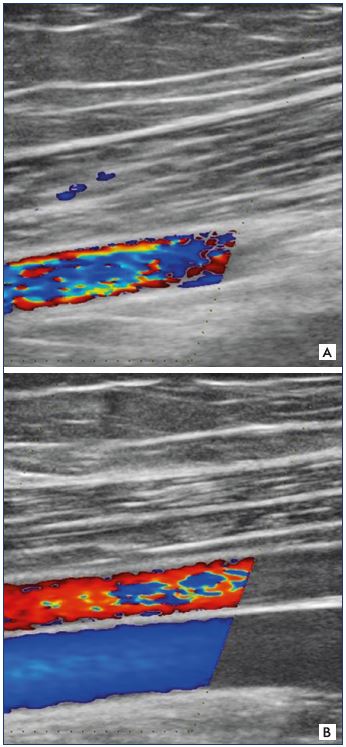
Figure 12. Femoral artery and vein.
Panel A. Femoral artery is present, but it is missing the femoral
vein. Panel B. Femoral artery and veins are both present.
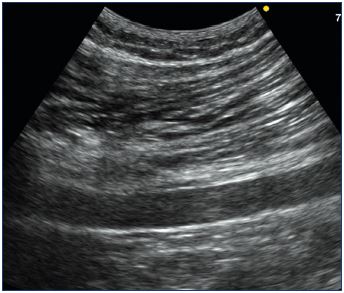
Figure 13. Truncular aspect of varicose veins of the sciatic
nerve (tubular venous trunk, posterior, media, deep, in contact
with the sciatic nerve).
It joins the deep femoral vein at the upper third of the thigh
(ie, the axiofemoral trunk). It extends to the buttock and drains
into the lower gluteal vein = persistent sciatic vein.
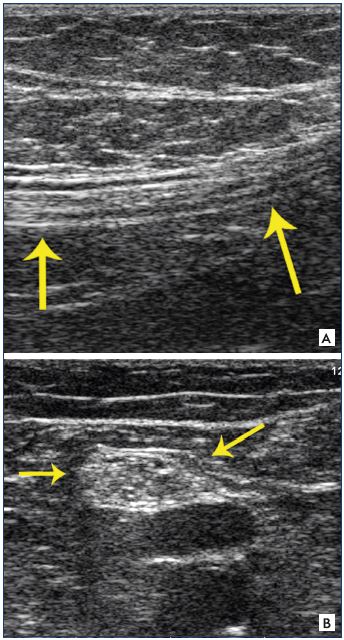
Figure 14. Sections of the sciatic nerve.
Panel A. Longitudinal section (fibrillary aspect). Panel B. Cross
section (honeycomb appearance).
The nerves are well visualized on ultrasound.17,24 In a longitudinal section, the nerves are visualized in the form of fibrillar structures, associating parallel striations, alternately hypo- and hyperechoic (Figure 14). The hypoechoic striations correspond to the nerve fibers and the endonerve, and the hyperechoic streaks to the epinerve and perinerve. A ”honeycomb” appearance is characteristic in cross sections. The main nerves involved in the expression of the sciatic nerve varicose veins are the tibial nerve, the common fibular nerve, the superficial fibular nerve, and its communicating branch. They are all very individualizable.
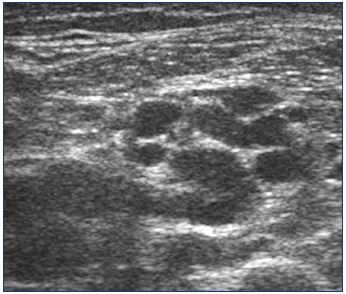
These veins have a small caliber; they are not spontaneously visible on ultrasound. They become so in case of reflux when they are dilated (Figure 15), especially the perinervous veins that dilate because the endovenous veins are contained by the nerve sheath. The expression of this varicose vein in the thigh is characterized by16,24,25: (i) the presence of a plexiform network that is located around the sciatic nerve (Figure 16) and is tortuous, more or less dilated, usually 3 to 5 mm, and refluxing; and (ii) veins located in the sheath of the nerve that are tubular, with a small caliber, and refluxing (Figure 17).
The sciatic nerve divides, usually at the top of the popliteal rhombus, into its two terminal branches, ie, the tibial and fibular nerves. Varicose veins may be of interest to these two nerves (Figure 18), but it preferentially follows the common fibular nerve. The ultrasound aspect is the same as the thigh. In the thigh and in the popliteal fossa, this varicose vein is located under the muscular fascia. In the leg, an echographic criterion is pathognomonic24,26: the presence of a tubular trunk, parallel to the small saphenous vein, located outside (average distance≈25 mm) (Figure 19), and incontinent, is, in our practice, always correlated with the presence of a varicose veins of the sciatic nerve. In fact, this vein, situated in a compartment analogous to that of the small saphenous vein, follows the course of the superficial fibular nerve. It feeds a subcutaneous varicose vein of the posterior face of the middle third of the calf, which can communicate with the small saphenous vein, when the varicose follows the fibular communicating branch (Figure 20).
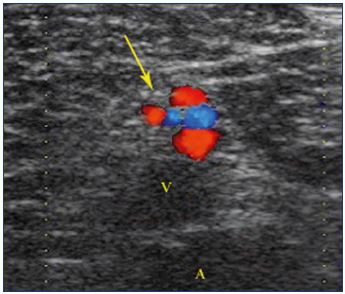
Figure 16. Cross section showing a venous, tortuous, and
incontinent network running inside and around the sciatic
nerve.
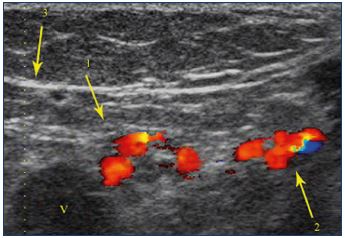
Figure 18. Cross section showing a venous, tortuous, and
incontinent network within and around the tibial and fibular
nerves.
Venous network within and around the tibial nerve (1). Venous
network within and around the common fibular nerve (2).
Popliteal vein (v).
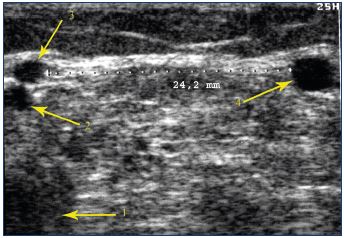
Figure 19. Cross section showing a tubular trunk that is parallel
to and located outside of the small saphenous vein.
Popliteal vein (1). Vein of the cutaneous medial sural nerve (2).
Small saphenous vein (3). Parallel vein in contact with the
superficial fibular nerve or its communicating branch (4).
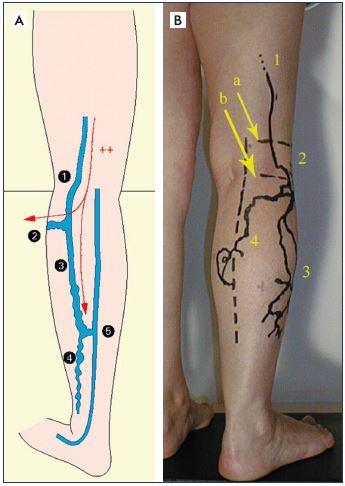
Figure 20. Calf varicose veins.
Panel A. Diagram of the appearance of calf varicose veins,
fed by sciatic nerve varicose veins. Varicose veins along
the common fibular nerve (1), which feeds a varicose vein
that bypasses the fibula head by following the deep fibular
nerve and an almost constant varicose vein that follows the
superficial fibular nerve and its communicating branch (3):
it corresponds to the pathognomonic ultrasound criterion. It
usually joins the small saphenous vein at the lower third of the
calf and feeds a posterolateral varicose veins of the lower half
of the leg.
Panel B. Skin mapping of a varicose vein of the calf, fed by a
sciatic nerve varicose vein. Varicose vein of the sciatic nerve (1),
located under the muscle fascia (a). Varicose vein of the
common fibular nerve, located between the muscle fascia and
the saphenous fascia (2). Varicose vein of the superficial fibular
nerve (3). Varicose vein of the communicating fibular branch
(4). Varicose veins 3 and 4 are located above the saphenous
fascia.
A postthrombotic origin has been evoked. In practice, postthrombotic sequelae of devalvation are very rarely associated with sciatic nerve varicose veins. Moreover, it is difficult to conceive that the venous network of the sciatic nerve, which, apart from the truncular forms in the context of a persistent embryonic vein, is plexiform with a small caliber, can become an effective supply pathway for the lower limb in case of obstructive syndrome. In its plexiform form, it does not fit into a venous malformation context either. The most likely hypothesis is that a primitive reflux disease is localized to the veins of the sciatic nerve or that it is part of a more general disease. One of the afferents of the inferior gluteal vein drains the satellite veins of the sciatic nerve. Thus, reflux of the inferior gluteal vein may cause sciatic nerve varicose veins, which then becomes an indirect criterion for pelvic varicose veins.
Key points
• The positive diagnosis of the sciatic nerve varices is made by DUS.
• Its morphological expression is a direct result of embryogenesis: it can be plexiform or truncal. The persistent sciatic vein is only a rare form of this pathology.
• The presence of an incontinent, interfascial, tubular trunk, located outside of and parallel to the small saphenous vein is pathognomonic of sciatic nerve varicose veins.
• When the popliteal vein is extended by a tubular, median, deep vein (axiofemoral trunk or persistent sciatic vein), the presence of a functional femoral vein must be verified because this vein occasionally provides the main venous drainage of the limb from the popliteal vein up to the internal iliac vein.
• Varicose veins of the sciatic nerve may be an expression of incompetence of the lower gluteal vein.
Pathognomonic expression of a pelvic venous leak: the inguinal point or I point
Pelvic varices are very common in multiparous women, but, in the vast majority of cases, they are asymptomatic. When they are symptomatic, the clinical signs can be located: (i) on the pelvic level, in the form of pelvic congestion syndrome27-29; and/or (ii) on the lower limbs in the form of varicose veins originating from the pelvis.24,30-34
Transmission of pelvic reflux to the lower limbs requires communication between these two floors. In an anatomical drainage pathway, an inverted flow (from the pelvis to the lower limbs) defines a leak point. These leak points may or may not be systematized. In 1989, in an anatomical study, Lefebvre et al30 had already described connections between extra and intra pelvic veins. In 2004, Franceschi and Bahnini described the DUS signs of 6 leak points originating from a hemipelvis (giving a total of 12 leak points).31,32 Among these systematized leak points, there is the inguinal point. The superficial veins of the Mount of Venus and some epigastric veins preferentially drain into the uterine vein via the vein of the round ligament, which runs through the inguinal canal, unusual causes of varicose veins in the lower extremities.
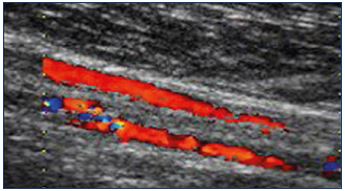
The ultrasound expression of the inguinal point is unequivocal.24,32,34 The reflux is externalized by the external orifice of the inguinal canal, situated above the inguinal ligament, outside the femoral vessels, by a trunk that travels from the abdominal-pelvic cavity toward the surface, with an internal concavity (Figure 21). The reflux may be spontaneous with breathing or be caused by abdominal hyperpressure maneuvers (manual anterior compression of the abdomen or Valsalva maneuver). The examination can be positive in the decubitus position, and it is often sensitized in orthostatism.
The saphenofemoral junction is an intervalvular segment,38 delimited by the preterminal valve and the terminal valve. This segment receives four major afférents39: the anterior saphenous vein, two abdominal afferents (the superficial epigastric vein and the superficial iliac circumflex vein), and a genital afferent (the lateral pudendal vein). Pieri et al39 demonstrated that a truncular reflux of the terminal part of the great saphenous vein could be present, despite the continence of the terminal valve, if the preterminal valve is incontinent. In this case, the truncular reflux is most often fed by the abdominal or genital afferents. It may or may not be caused by the Valsalva maneuver. The Valsalva maneuver generates hyperpressure in the abdominal-pelvic cavity. If this hyperpressure causes truncular saphenous reflux, it involves the presence of incontinent communication between the abdominal-pelvic cavity and the lower limb. In other words, in front of a preterminal reflux caused by a Valsalva maneuver, it is necessary to search for a leak point of pelvic origin, usually an inguinal point or a perineal point.
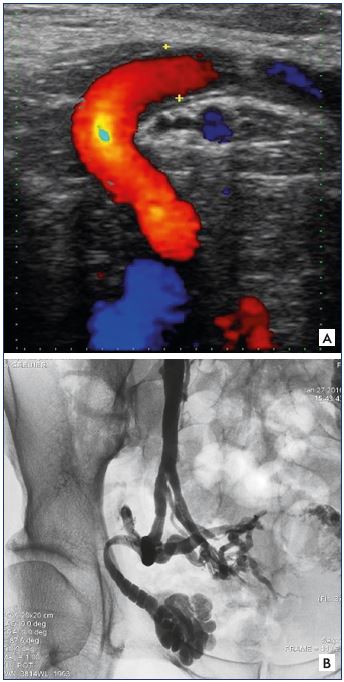
Figure 21. Iguinal point.
Panel A. Univocal ultrasound aspect of the inguinal point
(I point). Incontinent tubular trunk that is externalized through
the orifice of the inguinal canal, with a concave path inward.
Panel B. Phlebographic aspect of the I point (identical
characteristics). Image courtesy of M. Greiner.
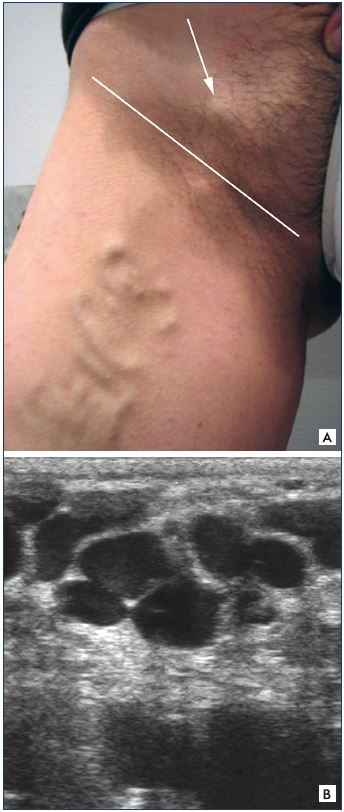
Figure 22. Dystrophic venous network.
Panel A. Clinical aspect of the dystrophic venous network
(arrow) located above the inguinal canal.
Panel B. Ultrasound aspect of this dystrophic venous network
(pathognomonic criterion of an inguinal point).
In our practice,36,37 the presence of a dystrophic venous network, ie, dilated, tortuous, and incontinent, located above the inguinal ligament on either side of the saphenofemoral junction is pathognomonic of the presence of a I point (Figure 22).
Key points
• The inguinal point is an anatomical drainage pathway with inverted flow.
• It is always a drainage pathway for parametrial or uterine varicose veins.
• Its ultrasound appearance is unambiguous, with a pathway that has internal concavity.
• The presence of a dystrophic venous network located just above the inguinal ligament is pathognomonic of the presence of an inguinal leak point.
Conclusions
These few examples illustrate the contribution of ultrasound investigations to the assessment of superficial venous disease. Embryogenesis makes it possible to understand the anatomy and its variations. The expression of venous disease is polymorphic. However, some anatomical and hemodynamic patterns are easily identifiable through stereotyped or even pathognomonic ultrasound semiology. The aim is to achieve the most complete anatomical and hemodynamic mapping of superficial venous disease of the lower limbs.

REFERENCES
1. Schobinger R, Weinstein CE. Varix involving the tibia. J Bone Joint Surg Am. 1962;44-A:371-376.
2. Boutin RD, Sartoris DJ, Rose SC, et al. Intraosseous venous drainage anomaly in patients with pretibial varices: imaging findings. Radiology. 1997;202:751-757.
3. Diaz-Candamio MJ, Lee VS, Golimbu CN, Scholes JV, Rofsky NM. Intrafibular varix: MR diagnosis. J Comput Assist Tomogr. 1999;23:328-330.
4. Peh WC, Wong JW, Tso WK, Chien EP. Intraosseous venous drainage anomaly of the tibia treated with imaging-guided sclerotherapy. Br J Radiol. 2000;73:80- 82.
5. Jung SC, Lee W, Chung JW, et al. Unusual causes of varicose veins in the lower extremities: CT venographic and Doppler US finding. Radiographics. 2009;29:525-536.
6. Mirault T, Lambert M, Vinckier L, Lamotte C, Cousyn M, Hatron PY. Anomalous intraosseous venous drainage: a rare cause of pretibial varicose veins [article in French]. J Mal Vasc. 2010;35:373-376.
7. Lemasle P. Cas clinique in atlas d’échoanatomie – bases biophysiques de l’imagerie sonore. Boulogne sur Seine : LaboratoiresTonipharm. 2011.
8. Kwee RM, Kavanagh EC, Adriaensen ME. Intraosseous venous drainage of pretibial varices. Skeletal Radiol. 2013;42:843-847.
9. Ramelet AA. Maladie veineuse chronique sur anomalie de drainage veineux intraosseux: perforantes osseuses? Phébologie. 2014;67:78-80.
10. Moraes FB, Camelo CP, Brandão ML, Fávaro PI, Barbosa TA, Barbosa RC. Intraosseous anomalous drainage: a rare case of pretibial varicos vein. Rev Bras Ortop. 2016;51(6):716-719.
11. Ramelet AA, Crebassa V, D’Alotto C, et al. Anomalous intraosseous venous drainage: bone perforators? Phlebology. 2016;32(4):241-248.
12. Verneuil A. Traité de chirurgie de Duplay et Reclus. 1890 ; tome 2 :221.
13. Servelle M. Pathologie vasculaire. Les affections veineuses. Masson, Paris. 1978;44-75.
14. Thiery L. La veine du nerf sciatique. Impact et thérapeutique. Phlébologie. 1988;41:687-689.
15. Trigaux JP, Vanbeers BE, Delchambre FE, de Fays FM, Schoevaerdts JC. Sciatic venous drainage demonstrated par varicography in patients with a patent deep venous system. Cardiovasc Intervent Radio. 1989;12:103-106.
16. Lemasle P, Uhl JF, Lefebvre-Vilardebo M, et al. Veine du nerf sciatique et maladie variqueuse : aspects écho-anatomiques et hémodynamiques. Phlébologie. 2001;54:219-228.
17. Ricci S. Observation ultrasonique du nerf sciatique et de ses branches à la fosse poplitée : toujours visible, jamais observé. Phlébologie. 2005;58(2):197- 202.
18. Ricci S, Georgiev M, Jawien A, et al. Sciatic nerve varices. Eur J Vasc Endo Vasc Surg. 2005;29:83-87.
19. Gillot C. Le prolongement postaxial de la petite veine saphène. Etude anatomique – Considérations fonctionnelles – Intérêt pathologique. Phlébologie. 2000;53:295-325.
20. Gillot C. Dispositifs veineux poplités : hypothèses et certitudes. Phlébologie. 1998;51:65-74.
21. Gillot C. Atlas anatomique des dispositifs veineux superficiels des membres inférieurs. Editions phlébologiques françaises : Paris, 1998.
22. Gillot C. Variations et valvulation du système tronculaire fémoro-poplité. Phlébologie. 1991;44:537-76.
23. Del Pinãl F, Taylor GI. The venous drainage of nerves; anatomical study, and clinical implications. Br J Plast Surg. 1990;43:511-520.
24. Lemasle P. Atlas d’écho-anatomie veineuse superficielle. La varicose pelvienne de la femme (tome 5), 2006 Laboratoires IPSEN – 2008 Laboratoires TONIPHARM, Paris.
25. Labropoulos N, Tassiopoulos AK, Gasparis AP, Pappas PJ. Veins along the course of the sciatic nerve. J Vasc Surg. 2009;49(3):690-696.
26. Lemasle P. De la clinique aux ultrasons : veine du nerf sciatique. Phlébologie. 2003;56:219-228.
27. Hobbs JT. The pelvic congestion syndrome. Br J Hosp Med. 1990;43:200- 206.
28. Villavicencio JL, Gillespie D, Durholt S, et al. Diagnosis and treatment of the pelvic venous disorders: pelvic congestion and pelvic dumping syndromes. In: Cann CC, ed. Surgical Management of Venous Disease. 1st ed. Baltimore, MD, USA: Williams and Wilkins; 1997;462-483.
29. Greiner M. Syndrome de congestion pelvienne : diagnostic et traitement. Phlébologie. 2005;58:293-298.
30. Lefebvre D, Bastide G, Vaysse Ph, et al. Connexions veineuses intra et extra-pelviennes. Etude anatomique. Phlébologie. 1989;42(3):385-389.
31. Franceschi C, Bahnini A. Points de fuite pelviens viscéraux et varices des membres inférieurs. Phlébologie. 2004;57:237-242.
32. Franceschi C, Bahnini A. Treatment of lower extremity venous insufficiency due to pelvic leak points in women. Ann Vasc Surg. 2005;19:284-288.
33. Perrin MR, Labropoulos N, Leon LR Jr. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;2:327-323.
34. Lemasle P. Stratégie diagnostique dans la prise en charge de la varicose pelvienne de la femme. Phlébologie. 2009;62(3):21-39.
35. García-Gimeno M, Rodríguez-Camarero S, Tagarro-Villalba S, et al. Duplex mapping of 2036 primary varicose veins. Duplex mapping of 2036 primary varicose veins. J Vasc Surg. 2009;49(3):681-689.
36. Lemasle P, Greiner M. Place de l’exploration ultrasonore dans le bilan de l’insuffisance veineuse pelvienne de la femme in Ultrasons et phlébologie. Société Française de phlébologie. Editions phlébologiques française. Paris 2017;2:24.
37. Lemasle P, Greiner M. Duplex ultrsound investigation in pelvic congestion syndrome. technique and results. Phlebolymphology. 2017;24(2):79-87.
38. Pieri A, Vannuzzi A, Duranti A, et al. Rôle central de la valvule pré-ostiale de la saphène interne dans la genèse des varices tronculaires des membres inférieurs. Phlébologie. 1995;48(2):227- 238.
39. Mühlberger D, Morandini L, Brenner E. Venous valves and major superficial tributary veins near the saphenofémoral junction. J Vasc Surg. 2009;49:1562- 1569.