State of art in lymphedema management: part 1
Lymphedema and Vascular Malformation,
George Washington University School of
Medicine, USA
Abstract
Chronic lymphedema can be managed effectively using a sequenced and targeted treatment program based on decongestive lymphatic therapy (DLT) with compression therapy and surgery (mostly as an adjunct to DLT). In the maintenance phase, DLT is carried out using the proper combination of compression garments, meticulous personal hygiene and skin care, self-massage based on the principle of manual lymphatic drainage (if applicable), and exercises and activities to promote lymph transport. Pneumatic compression devices/therapy can be applied at home, if desired. When conservative treatment based on DLT fails or delivers suboptimal outcomes, the patient may need additional surgical interventions, either reconstructive or ablative, where applicable. These two surgical therapies are more effective in terms of outcomes when combined postoperatively with manual lymphatic drainage–based DLT. A long-term commitment to postoperative DLT, especially compression therapy, is a critical factor in determining the success of either reconstructive or palliative surgery. Recently, several causal genetic mutations have been identified among primary lymphedema syndromes, which provide possible opportunities for future molecular interventions. This new prospect of gene-oriented management is more promising as a molecular therapy for both primary and acquired lymphedema.
Introduction
Over the last 20 years, the understanding of lymphatic disorders has substantially improved, providing new insights into the lymphatic system’s structure and function for both primary and acquired forms of lymphedema.1-4 This evolution in the evaluation and management of lymphatic disorders was achieved due to advanced diagnostic imaging technology and the subsequent implication of newly developed approaches regarding physical modalities, surgical interventions, and pharmacology.5-8
We can now more clearly understand the differences in etiopathogenesis between primary lymphedema (mostly congenital lymphatic malformations) and secondary lymphedema (acquired conditions).9,10 This paper will present the best and most commonly used therapies available that have been thoroughly evaluated.1,2 These therapies can ultimately be recommended as the most updated guidelines for clinicians who are treating patients with this unique condition worldwide. This paper discusses the contemporary concepts regarding the management of chronic lymphedema, which encompass a broad range of currently available treatment options both old and new. However, the majority of the data available for review are classified as grade 2B or 2C when using the system by Guyatt et al and only a small amount of data are classified as grade 1C or 2A at best from observational studies (Table I).11-13 With consideration of this unique situation, we accept manual lymphatic drainage–based decongestive lymphatic therapy as the main treatment14-17 and surgical management as an additional option for the management of lymphedema.18-21
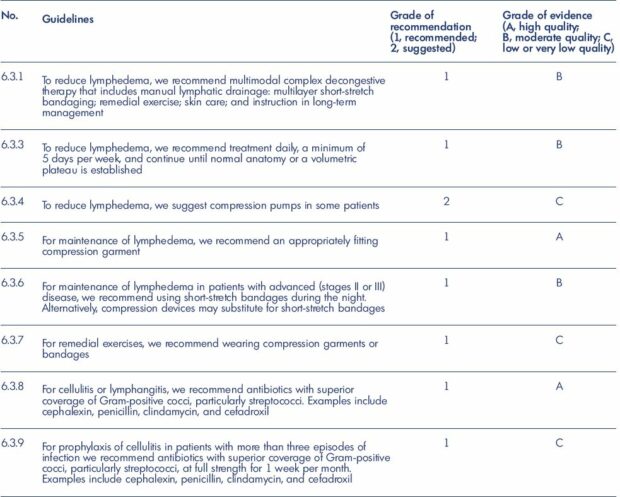
Table I. Guidelines 6.3.0 of the American Venous Forum on lymphedema: medical and physical therapy.
From reference 13: Gamble GL, Cheville A, Strick D. Lymphedema: medical and physical therapy. In: Gloviczki P, ed. Handbook of
Venous Disorders: Guidelines of the American Venous Forum. 3rd Edition. London, UK: Hodder Arnold; 2009:655. © 2009, Edward
Arnold (Publishers) Ltd.
Chronic lymphedema starts as a simple condition of limb swelling following the mechanical failure of the lymphatic system’s mechanism for collecting and transporting lymph. However, such early-stage, but “reversible,” edema may become a chronic degenerative and inflammatory process. The impact of lymphatic fluid accumulation, which is initially limited to the lymphatic system and the lymph nodes, will spread to the entire surrounding soft tissue and skin, resulting in irreversible damage.8,22,23 Chronic lymphedema is a “steadily progressive condition that affects the entire surrounding soft tissue” that results in a disabling and distressing condition, where the major risks include bacterial and fungal infections and subsequent sepsis, chronic inflammation with dermatolipofibrosis, immunodeficiency and wasting phenomenon, and malignancies (eg, Kaposi sarcoma; lymphangiosarcoma) (Figure 1).2,7,24 Therefore, it is mandatory to treat lymphedema at the earliest detectable point in the evolution of the disease. A precise and timely diagnosis to verify its clinical stage is not only critical for proper treatment, but also for the prospective identification of early-stage disease in defined at-risk populations.25-27
Manual lymphatic drainage–based decongestive lymphatic therapy remains a main treatment for the contemporary management of lymphedema.28-31 Further improvements in function and quality of life can be achieved with lifestyle modifications, including specific exercise regimens.32 In addition, incorporating intermittent pneumatic compression may significantly reduce edema and symptoms (Figure 2).33,34 Currently, pharmacological interventions have little applicability in the management of lymphedema35-37; however, antibiotic therapy is necessary for the effective control of infections,38-40 and both growth factor–based and cellular therapies (ie, molecular modifications) continue to show great promise for the future.41-43 During the last 10 years, the use of surgery for lymphedema has increased, mostly by using newly developed/incorporated techniques for both reconstructive (Figure 3)44,45 and excisional (Figure 4)46,47 surgery.
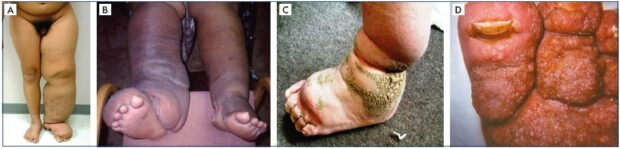
Figure 1. Chronic lymphedema at an advanced stage.
Panels A and B. Advanced condition of chronic lymphedema that cannot be controlled with decongestive lymphatic therapy, and,
with recurrent infection/sepsis, the disease is steadily progressing toward a disabling and distressing condition. Panels C and D.
Unique condition of dermatolipofibrosis with chronic inflammation, which increases the risk of infection and subsequent sepsis, as
well as immunodeficiency and malignancy.
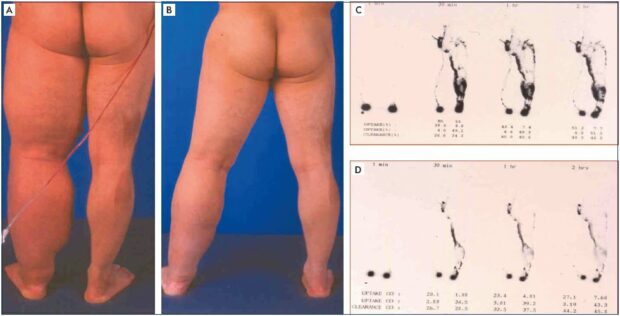
Figure 2. Clinical case of decongestive lymphatic therapy management.
Panel A. Progressive lymphedema condition with recurrent episodes of sepsis before decongestive lymphatic therapy is instituted.
Panel B. Excellent clinical improvement/response to decongestive lymphatic therapy with successful disease control with the initial
intensive care. Panel C. Lymphoscintigraphic findings of lymphatic dysfunction, including the dermal backflow that shows severe
lymphatic obstruction. Panel D. Lymphoscintigraphic findings show an improved lymphedema status following successful decongestive
lymphatic therapy with decreased dermal backflow that is compatible with clinical improvement (1-year follow-up assessment).
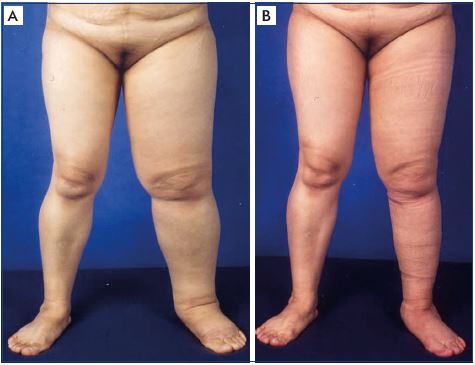
Figure 3. Clinical case of reconstructive surgery.
Panel A. Clinical status of progressive lymphedema despite
maximum decongestive lymphatic therapy–based physical
therapy for more than 1-year. Panel B. Excellent clinical response
to additional care with reconstructive surgery with multiple
lymphovenous anastomoses performed at the popliteal level
(2 weeks postoperation).
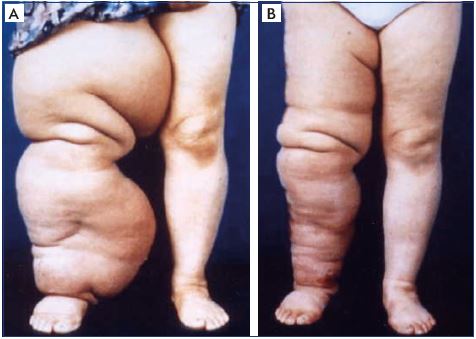
Figure 4. Clinical case of excisional/debulking surgery.
Panel A. Advanced lymphedema that is uncontrollable with
conventional care based on decongestive lymphatic therapy,
resulting in recurrent sepsis. Panel B. Excellent outcome of
debulking surgery to change the local condition, making it
amenable to compression therapy, with efficacy throughout the
postoperative period.
The main treatment goals are to improve the physical condition of the affected limb or area and the patient’s quality of life,48,49 which, despite a psychologically unacceptable physical deformity, will ultimately improve the patient’s social life, functional and psychological state, and the ability to perform normal physical activities, so they can return to a normal or near-normal life.
Primary lymphedema occurs due to abnormal development of the lymphatic system, which frequently has a specific genetic origin that is inherited. Primary lymphedema is only a major clinical sign when patients have either Fms-like tyrosine kinase 4 (FLT4)–related lymphedema (ie, Nonne-Milroy-Meige syndrome [Milroy disease]) or forkhead box protein C2 (FOXC2)–related lymphedema (ie, lymphedema-distichiasis syndrome).51-54 In the majority of complex syndromes, lymphedema is a minor clinical manifestation, while other abnormalities dominate.55-57
Not all gene mutations will result in a phenotype that has a major impact on lymphatic function.58 Some individuals of the same family do not develop the disease due to incomplete penetrance, but they remain (healthy) carriers of the genetic mutation. When the mutation results in defective development (to varying degrees), the individual is no longer a healthy carrier, particularly from the management point of view, as (unknown) modifier genes that act according to a model of genetic susceptibility are closely associated with the activity.53 Indeed, healthy looking limbs, although infrequent, have various locoregional lymph transport abnormalities suggesting a defective development even in the absence of edema. Such a subclinical condition of lymphedema may lead to clinically significant lymphatic transport insufficiency later in life under certain conditions. Additionally, primary lymphedema contributes to many forms of secondary lymphedema due to underlying genetic susceptibility. This unique group of patients with subclinical presentation warrants special consideration in the scope of managing and preventing lymphedema.1,2,9,10
Physical management
Lymphedema can be managed with physical therapy (ie, nonsurgical methods); it combines multiple elements of physical maneuvering known as decongestive physiotherapy.35 Physical therapy has three goals: (i) improve lymphatic function; (ii) soften fibrosclerotic tissues; and (iii) reduce microbial growth on the skin to prevent opportunistic infections. This approach can achieve effective limb volume reduction through a stepwise approach from the initial acute phase of intervention to the subsequent maintenance phase, preserving the integrity of the cutaneous and subcutaneous structures.59-61
A multilayer compression bandage can stimulate lymphatic contractility and subsequent lymph flow through physical activity.62 In addition, external tissue compression can increase interstitial hydrostatic pressure to subsequently reduce lymph formation. When the maximum reduction in edema volume is reached after performing multiple cycles of compression and manual lymphatic drainage, maintaining the therapeutic benefits depends on self-care strategies and the proper use of compression garments.60 The combination of regular exercise and external compression exerted by compression garments can improve lymphedema.63
Another technique that may augment lymph clearance is intermittent pneumatic compression, which provides a distal-to-proximal graduated and sequential compression that results in an adjunctive benefit to the decongestive physiotherapy.64,65 Low-level laser therapy has also been reported to produce both subjective and objective improvements in lymphedema66,67 with both anti-inflammatory and lymphangiogenic effects.68 Lastly, the application of vibration, heat, and external magnetic fields have also been reported to be beneficial, but few data support these reports.69
Decongestive lymphatic therapy is a nonsurgical treatment option to reduce swelling and maintain this reduction over the long term. This method uses compression, massage, and exercise to stimulate lymphatic drainage, which will reduce the swelling, soften the fibrotic tissues, and ultimately improve limb function and mobility. As skin is a barrier to infections, improving its function will reduce the rate and severity of cellulitis (Figure 2).70-73 Decongestive lymphatic therapy is a well-established treatment option for the management of lymphedema. It is an empirical strategy to control edema and it remains the treatment of choice regardless of the disease etiology (primary or secondary) or clinical stage and despite the fact that it is not a cure.
Compression therapy with bandages, garments, and intermittent pneumatic compression and manual lymphatic drainage are two major components of decongestive lymphatic therapy.71,72 Indeed, compression bandage– based therapy is the single most important component of decongestive lymphatic therapy with or without sequential intermittent pneumatic compression–based mechanical compression.74-77 However, basic hygienic care of the skin, movement exercises, and education for risk reduction, including the prevention of infections, are also essential components of the treatment regimen.
Decongestive lymphatic therapy has an initial phase of intensive decongestion therapy followed by a long-term maintenance phase. The primary goals of intensive treatment are to obtain a significant reduction in limb volume and changes in the tissue, and it includes a 2- to 4-week course of daily skin care, manual lymphatic drainage massage, multilayer compression bandaging, and exercise (Table II). Once intensive treatment is complete, maintenance treatment should be instituted immediately with proper fitting of compression hosiery, because, along with the intensive therapy, the maintenance phase is the cornerstone of contemporary lymphedema treatment.78,79
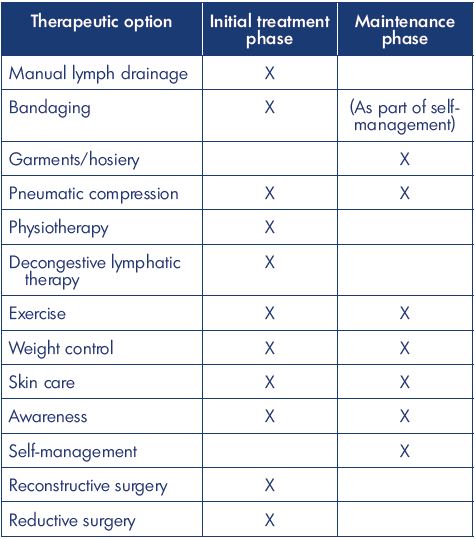
Table II. Useful lymphedema interventions.
Based on data from reference 118: Damstra RJ. Upper limb
lymphedema. In Lee BB, Rockson SG, eds. Lymphedema: A
Concise Compendium of Theory and Practice. 2nd Ed. Springer
International Publishing AG 2011, 2018; 540.
Decongestive lymphatic therapy can be a life-long therapy as the risk of complications and morbidity is minimal and, in the majority of patients, it helps maintain an improveddisease status. However, it is more effective when started in the earlier stages of lymphedema, because, in the later stages of lymphedema, the efficacy is limited and it often fails to prevent progression and complications. Successful decongestive lymphatic therapy requires good treatment compliance and that the patients be motivated to understand their condition, know the options available, and understand the absolute need for using compression daily to maintain the long-term benefits of treatment.80 Therefore, patient involvement in management is essential, especially for home maintenance therapy and should be guided properly for an active involvement in self-management. The long-term success depends on the comprehensive medical care of many accompanying conditions/diseases that can aggravate the lymphedema. Proper management of various comorbid conditions is essential because they can influence the therapeutic outcomes. The most common conditions include hypertension, coronary heart disease, congestive heart failure, obesity, diabetes mellitus, chronic venous insufficiency, malignancies, chronic arthritis, peripheral artery occlusive disease, and peripheral polyneuropathy. Calcium-channel blockers should be avoided since they impair lymphatic pumping.81
There are a few contraindications to each component of decongestive lymphatic therapy, including acute erysipelas, acute thrombophlebitis, phlebothrombosis, decompensated heart failure, and stage IV peripheral artery occlusive disease. High pressure bandaging is risky for any patient with advanced peripheral arterial disease of the limb or advanced cardiac failure.
Manual lymphatic drainage is a technique that physiologically stimulates poorly functioning, if not paralyzed, lymphatic vessels and pathways to facilitate the drainage of interstitial fluid into the initial lymphatic system to reduce lymphatic congestion effectively. In addition, this technique, by improving lymphodynamics during treatment, may reduce fibrosclerosis of the involved soft tissues.82,83
Manual lymphatic drainage uses a massage technique to reroute the accumulated lymph in the swollen region through collateral lymphatic pathways to an area where the lymph can drain normally. The initial step of the process is to decongest the central/proximal areas to make room before massaging the edematous regions. The manual lymphatic drainage massage is an important component of decongestive lymphatic therapy, especially for midline lymphedema treatment where there are few alternatives70; however, it should not be used alone as a sole independent regimen, but rather as one part of the decongestive lymphatic therapy. Indeed, manual lymphatic drainage has not yet been confirmed scientifically with objective data, although it has remained an indispensable component of decongestive lymphatic therapy for decades.84,85 Therefore, depending on local resources, manual lymphatic drainage may be included in the treatment plan despite the lack of evidence for long-term benefits.
The cornerstone of physical therapy for lymphedema regardless of its etiology is compression therapy, which increases tissue pressure and subsequently decreases the transmural pressure gradient to reduce the lymphatic load by reducing microcirculatory filtration.86 Compression therapy is generally initiated with a multicomponent bandage with high stiffness; short-stretch bandaging combined with exercises is ideal during the initial management phase and should be guided by specifically trained therapists. Following the initial decongestion phase, the maintenance phase must be well organized and use the best combination of compression garments, selfmanagement, skin care, and exercises because this phase requires a life-long commitment.87
The optimal degree and duration of compression remains debatable. Recent data support an optimal pressure range around 30 mm Hg for the upper extremities and 50 to 60 mm Hg for the lower extremities; however, higher pressures may be counterproductive.88 Lower compression pressure are more user friendly, which would improve compliance (Table III). Self-management must be adjusted with the proper combination of compression bandages or Velcro devices, movement exercises, and/or self-massage to fit with individual needs best. Less bulky bandages and Velcro devices seem to allow better movement and subsequently better outcomes than the widely used heavy set multilayer and multicomponent bandages.89,90 A recent study reported that self-adjustable Velcro devices might reduce edema more effectively than inelastic lymph bandages.90
Compression hosiery/stockings are made for the maintenance phase to maintain the effect achieved through the initial intensive treatment phase. Fitted garments with higher compression classes (30 to 40 mm Hg) are ideal, but they become a limiting factor, especially in patients with advanced age, obesity, and/or arthritis.
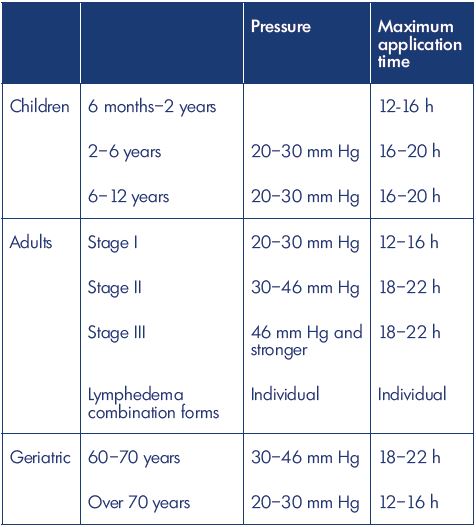
Table III. Compression bandaging depends on the age of the
patient and the stage of the lymphedema.
Based on data from reference 119: Földi E, Földi M, Rockson
SG. Complete decongestive physiotherapy In Lee BB, Rockson
SG, eds. Lymphedema: A Concise Compendium of Theory and
Practice. 2nd Ed. Springer International Publishing AG 2011,
2018;406.
For many decades, pneumatic compression with multichamber devices has been effectively incorporated into multidisciplinary therapeutic programs as an adjunctive therapy to effectively remove excess fluid from the extremities.33,34,64 However, this device remains controversial due to concerns that the pressures generated by the device may damage the skin lymphatics, tempering earlier enthusiasm for the benefits of this technique.91,92 Recent studies have shown that intermittent pneumatic compression relieves symptoms, reduces episodes of cellulitis in patients with lower extremity lymphedema,93 and increases tissue elasticity.94
Sequential intermittent pneumatic compression can be recommended as an adjunct treatment,35 particularly for patients whose isotonic exercise capacity is highly compromised or absent, which means that the lymphedema can only be treated with passive physical therapy (eg, elderly, bedridden patients, patients with serious disabilities, etc).96,97 However, sequential intermittent pneumatic compression should be used as an adjunct treatment for mixed lymphovenous edema and it should not be used in preference to exercise and compression garments. Furthermore, clinical evidence shows that the formation of new tissue channels as functional pathways by intermittent pneumatic compression promotes the clearance of edema fluid in patients with lymphedema in the limbs.95
Medical and pharmacological management
a range of pharmacological treatments has been available for decades to try to improve lymphatic function, such as α-benzopyrones, which include coumarin derivatives, and γ-benzopyrones (ie, flavonoids), which includes flavones, flavonols (eg, diosmin), and flavanes (eg, hesperidin). The proposed mechanism of action is that benzopyrones reduce vascular permeability,98 which reduces the lymphatic load. Additionally, benzopyrones may increase tissue macrophage activity,99 thereby encouraging proteolysis with favorable effects on fluid clearance and tissue composition.100 Such drugs are all designed to help patients with lymphedema by reducing protein and extracellular fluid accumulation,101 stimulating lymph contractility and flow,102 and reducing protein concentration and fibrotic induration in tissues by stimulating tissue macrophage activity to increase proteolysis.99,100 However, there has been little, if any, data to support the use of these drugs, with the exception of the flavonoid/benzopyrone groups that have demonstrated significant and objective improvements.98,103,104
Recent data show that the hepatotoxic effects of coumarin (5, 6, benzo-α-pyrone), which prohibit its use for the treatment of lymphedema, are a consequence of a genetic and metabolic problem relating to the breakdown of coumarin.105 A new test that screens for genetic polymorphisms can identify people who have a functional, nonpolymorphic cytochrome P450 2A6 (CYP2A6) enzyme, a liver enzyme responsible for the metabolism of coumarin to noncytotoxic metabolites.106 A better understanding of genetics and genomics will help determine which patients will respond well and overcome the adverse outcomes; this new pharmacogenomic test106 helps limit the use of benzopyrones (particularly coumarin) to those patients with a functional, nonpolymorphic CYP2A6 enzyme to reduce the risk of hepatic toxicity.
Indeed, a combined approach107 with conventional physical therapy108 and benzopyrones as an additional medical treatment gets new attention,109 despite the fact that the systemic use of benzopyrone is an unsettled issue due to its hepatic toxicity. Nevertheless, until now, pharmacology has provided few therapeutic options for the management of lymphedema, except the use of antibiotics to treat and prevent recurrent episodes of soft-tissue infections, which is critical for the complete eradication of pathogens in patients with lymphedema who have a poor ability to clear pathogens and an impaired immune system trafficking mechanism due to abnormal biology of the lymphedematous tissues.105
Infections and inflammation of skin and soft tissues are more common among patients with lymphedema due to lymph stasis, which allows microorganisms that are retained in the tissue fluid to grow. In these patients, not only do commensal bacteria (eg, Staphylococcus epidermidis and coagulase-negative strains, S aureus, and Corynebacterium) proliferate and become pathogenic, but also other pathogenic microbes originating from the perineal region (eg, Enterococcus, Enterobacter, Acinetobacter, Proteus, Escherichia coli, and Pseudomonas) cause various conditions of infection: lymphangitis, erysipelas, and necrotizing fasciitis (Figure 5).110,111
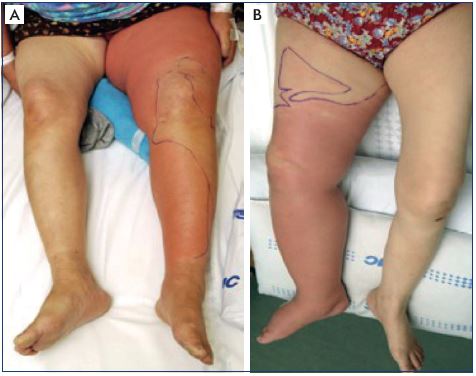
Figure 5. Clinical case of infection resulting in cellulitis.
Panels A and B. Display of two different patients with severe
cellulitis affecting almost the entire lower extremity up to the
upper thigh subsequently causing systemic sepsis (5B) to require
in-hospital intensive care with the antibiotics.
The decreased ability of the immune system to neutralize and eradicate the microorganisms penetrating the integuments means that these organisms change into a persisting form with a decreased metabolism.112,113 Therefore, acute episodes of a unique inflammatory condition, known as dermatolymphangioadenitis (DLA), should be treated with a wide-spectrum antibiotic therapy for 3 to 7 days, and low-dose (benzathine) penicillin should be further administered on a long-term basis to prevent the revival of dormant microbes and decrease the frequency of DLA as a chronic form (Figure 1).38-40
There has been a growing interest in the role of inflammation in the pathogenesis of lymphedema114 because, in experimental models, the targeted inhibition of these inflammatory pathways significantly improved the structure and function of the lymphatic system.15,116
Inhibition of transforming growth factor β (TGFβ) expression improved lymphatic function by diminishing inflammation, the migration of T helper 2 type (Th2) cells, and the expression of profibrotic Th2-type cytokines.41 Hence, proper inhibition of lymphangiogenesis by Th2-type cytokines is considered a potent means of improving lymphangiogenesis by manipulating the antilymphangiogenic pathways.42 Also, excessive generation of immature lymphatic vessels, which are essential for the pathogenesis and maintenance in lymphedema, is dependent upon an interaction between CD4 and macrophages, and lymphedema can be improved by inhibiting the activation of T helper 1 type and T helper 17 type cells.43 These two lines of investigation show a promising future for pharmacological approaches to improve the treatment and prevention of lymphedema.

REFERENCE
1. Lee BB, Andrade M, Bergan J, et al; IUP. Diagnosis and treatment of primary lymphedema. Int Angiol. 2010;29(5):454- 470.
2. Lee BB, Andrade M, Antignani PL, et al; IUP. Diagnosis and treatment of primary lymphedema. Int Angiol. 2013;32(6):541- 574.
3. Lee BB. Lymphedema-angiodysplasia syndrome: a prodigal form of lymphatic malformation. Phlebolymphology. 2005;47:324-332.
4. Lee BB, Kim YW, Seo JM, et al. Current concepts in lymphatic malformation. Vasc Endovasc Surg. 2005;39(1):67-81.
5. Lee BB, Kim DI, Whang JH, Lee KW. Contemporary issues in management of chronic lymphedema – personal reflection on an experience with 1065 patients. Commentary. Lymphology. 2005;38:28-31.
6. Lee BB. Contemporary issues in management of chronic lymphedema: personal reflection on an experience with 1065 patients. Lymphology. 2005;38(1):28-31.
7. Lee BB, Antignani PL, Baroncelli TA, et al. IUA-ISVI consensus for diagnosis guideline of chronic lymphedema of the limbs. Int Angiol. 2015;34(4):311-332.
8. Lee BB, Laredo J. Pathophysiology of primary lymphedema. In: Neligan PC, Piller NB, Masia J, eds. Complete Medical and Surgical Management. Boca Raton, FL: CRC Press; 2016:177-188.
9. Lee BB, Villavicencio JL. Primary lymphoedema and lymphatic malformation: are they the two sides of the same coin? Eur J Vasc Endovasc Surg. 2010;39(5):646-653.
10. Lee BB, Laredo J, Neville R. Primary lymphedema as a truncular lymphatic malformation. In: Lee BB, Bergan J, Rockson SG, eds. Lymphedema: A Concise Compendium of Theory and Practice. 1st edition. London, UK: Springer-Verlag; 2011:419-426.
11. Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines. Chest. 2006;129(1):174-181.
12. Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
13. Gamble GL, Cheville A, Strick D. Lymphedema: medical and physical therapy. In: Gloviczki P, ed. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 3rd edition. London, UK: Hodder Arnold; 2009:655.
14. Mortimer PS. Therapy approaches for lymphedema. Angiology. 1997;48(1):87- 91.
15. Földi E. The treatment of lymphedema. Cancer. 1998;83(12):2833-2834.
16. Leduc O, Leduc A, Bourgeois P, Belgrado JP. The physical treatment of upper limb edema. Cancer. 1998;83(12):2835-2839.
17. Szolnoky G, Lakatos B, Keskeny T, et al. Intermittent pneumatic compression acts synergistically with manual lymphatic drainage in complex decongestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology. 2009;42(4):188-194.
18. Lee BB, Kim YW, Kim DI, Hwang JH, Laredo J, Neville R. Supplemental surgical treatment to end stage (stage IV-V) of chronic lymphedema. Int Angiol. 2008;27(5):389-395.
19. Lee BB, Laredo J, Neville R. Reconstructive surgery for chronic lymphedema: a viable option, but. Vascular. 2011;19(4):195- 205.
20. Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85(1):64-74.
21. Campisi C, Boccardo F, Zilli A, Maccio A, Gariglio A, Schenone F. Peripheral lymphedema: new advances in microsurgical treatment and long-term outcome. Microsurgery. 2003;23(5):522-525.
22. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87(2):198-210.
23. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92(3):1005-1060.
24. Joh JH, Lee BB, Chun YS, Chung WK, Lee HY. Angiosarcoma after excisional surgery for chronic lymphedema. J Vasc Surg Venous Lymphat Disord. 2016;4(3):336-339.
25. Lee BB, Bergan JJ. New clinical and laboratory staging systems to improve management of chronic lymphedema. Lymphology. 2005;38(3):122-129.
26. Lee BB. Classification and staging of lymphedema. In: Tredbar LL, Morgan CL, Lee BB, Simonian SJ, Blondeau B, eds. Lymphedema: Diagnosis and Treatment. London, UK: Springer; 2008:21-30.
27. International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document. 2013;46(1):1-11.
28. Casley-Smith JR, Mason MR, Morgan RG, Casley-Smith JR. Complex physical therapy of the lymphedematous leg. Int J Angiol. 1995;4(3):134-142.
29. Johansson K, Albertsson M, Ingvar C, Ekdahl C. Effects of compression bandaging with or without manual lymph drainage treatment in patients with postoperative arm lymphedema. Lymphology. 1999;32(3):103-110.
30. Szolnoky G, Mohos G, Dobozy A, Kemény L. Manual lymph drainage reduces trapdoor effect in subcutaneous island pedicle flaps. Int J Dermatol. 2006;45(12):1468-1470.
31. Kasseroller RG. The Vodder School: the Vodder method. Cancer. 1998;15(83):2840-2842.
32. Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breastcancer- related lymphedema. N Engl J Med. 2009;361(7):664-673.
33. Zelikovski A, Haddad M, Reiss R. The “Lympha-Press” intermittent sequential pneumatic device for the treatment of lymphoedema: five years of clinical experience. J Cardiovasc Surg. 1986;27(3):288-290.
34. Dittmar A, Krause D. A comparison of intermittent compression with single and multi-chamber systems in treatment of secondary arm lymphedema following mastectomy [article in German]. Z Lymphol. 1990;14(1):27-31.
35. Rockson SG, Miller LT, Senie R, et al. American Cancer Society Lymphedema Workshop. Workgroup III: diagnosis and management of lymphedema. Cancer. 1998;83(12):2882-2885.
36. Ramelet AA. Pharmacologic aspects of a phlebotropic drug in CVI-associated edema. Angiology. 2000;51(1):19-23.
37. Hoult JR, Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol. 1996;27(4):713-722.
38. Gabillot-Carré M, Roujeau JC. Acute bacterial skin infections and cellulitis. Curr Opin Infect Dis. 2007;20(2):118- 123.
39. Dreyer G, Medeiros Z, Netto MJ, Leal NC, de Castro LG, Piessens WF. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Trans R Soc Trop Med Hyg. 1999;93(4):413-417.
40. Jamal S, Manokaran G, Tripathi FM, Zaleska M, Stelmach E, Olszewski WL. The effectiveness of long-acting penicillin (penidur) in preventing recurrences of dermatolymphangioadenitis (DLA) and controlling skin, deep tissues, and lymph bacterial flora in patients with “filarial” lymphedema. Lymphology. 2005;38:66- 80.
41. Avraham T, Daluvoy S, Zampell J, et al. Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;177(6):3202-3214.
42. Savetsky IL, Ghanta S, Gardenier JC, et al. Th2 cytokines inhibit lymphangiogenesis. PLoS One. 2015;10(6):e0126908.
43. Ogata F, Fujiu K, Matsumoto S, et al. Excess lymphangiogenesis cooperatively induced by macrophages and CD4+ T cells drives the pathogenesis of lymphedema. J Invest Dermatol. 2016;136(3):706-714.
44. Lee BB, Laredo J, Neville R. Current Status of lymphatic reconstructive surgery for chronic lymphedema: it is still an uphill battle! Int J Angiol. 2011;20(2):73-79.
45. Lee BB, Laredo J, Neville R: Current dilemma with controversy. In: Lee BB, Bergan J, Rockson SG, eds. Lymphedema: a Concise Compendium of Theory and Practice. 1st edition. London, UK: Springer-Verlag; 2011:381-386.
46. Kim DI, Huh SH, Hwang JH, Joh JH. Excisional surgery for chronic advanced lymphedema. Surg Today. 2004;34(2):134-137.
47. Lee BB, Laredo J, Neville R. Contemporary indication and controversy on excisional surgery. In: Lee BB, Bergan J, Rockson SG, eds. Lymphedema: a Concise Compendium of Theory and Practice. 1st edition. London, UK: Springer-Verlag; 2011:403-408
48. Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68(3)273-282.
49. Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(30:465- 470.
50. Michelini S, Failla A, Moneta G, Zinicola V, Romaldini, Puglisi D. International classification of lymphedema functioning and disability evaluation. Eur J Lymphology. 2007;17(51):16-19.
51. Brice G, Child AH, Evans A, et al. Milroy disease and the VEGFR-3 mutation phenotype. J Med Genet. 2005;42(2):98-102.
52. Gordon K, Schulte D, Brice G, et al. Mutation in vascular endothelial growth factor-C, a ligand for vascular endothelial growth factor receptor-3, is associated with autosomal dominant Milroy-like primary lymphedema. Circ Res. 2013;112(6):956-960.
53. Brice G, Mansour S, Bell R, et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet. 2002;39(7):478-483.
54. Fang J, Dagenais SL, Erickson RP, et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedemadistichiasis syndrome. Am J Hum Genet. 2000;67(6):1382-1388.
55. Bull LN, Roche E, Song EJ, et al. Mapping of the locus for cholestasis-lymphedema syndrome (Aagenaes syndrome) to a 6.6-cM interval on chromosome 15q. Am J Hum Genet. 2000;67(4):994-999.
56. Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43(10):929-931.
57. Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41(12):1272-1274.
58. Ferrell RE, Finegold DN. Research perspectives in inherited lymphatic disease: an update. Ann N Y Acad Sci. 2008;1131:134-139.
59. Rockson SG. Lymphedema therapy in the vascular anomaly patient: therapeutics for the forgotten circulation. Lymphat Res Biol. 2005;3(4):253-255.
60. Szuba A, Cooke JP, Yousuf S, Rockson SG. Decongestive lymphatic therapy for patients with cancer-related or primary lymphedema. Am J Med. 2000;109(4):296-300.
61. Badger CM, Peacock JL, Mortimer PS. A randomized, controlled, parallelgroup clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer. 2000;88(12):2832-2837.
62. Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol. 1980;239(6):H775-H783.
63. Cheema BS, Kilbreath SL, Fahey PP, Delaney GP, Atlantis E. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148(2):249-268.
64. Brennan MJ, Miller LT. Overview of treatment options and review of the current role and use of compression garments, intermittent pumps, and exercise in the management of lymphedema. Cancer. 1998;83(12):2821- 2817.
65. Szuba A, Achalu R, Rockson SG. Decongestive lymphatic therapy for patients with breast carcinomaassociated lymphedema. A randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer. 2002;95(11):2260-2267.
66. Piller N, Thelander A. Treatment of chronic postmastectomy lymphedema with low level laser therapy: a 2.5 year follow-up. Lymphology. 1998;31(2):74-86.
67. Carati CJ, Anderson SN, Gannon BJ, Piller NB. Treatment of postmastectomy lymphedema with low-level laser therapy: a double blind, placebo-controlled trial. Cancer. 2003;98(6):1114-1122.
68. Jang DH, Song DH, Chang EJ, Jeon JY. Anti-inflammatory and lymphangiogenetic effects of low-level laser therapy on lymphedema in an experimental mouse tail model. Lasers Med Sci. 2016;31(2):289-296.
69. Ohkuma M. Treatment of peripheral lymphedema by concomitant application of magnetic fields, vibration and hyperthermia: a preliminary report. Lymphology. 2002;35(2):87-90.
70. Földi E, Földi M, Weissleder H. Conservative treatment of lymphoedema of the limbs. Angiology. 1985;36(3):171- 180.
71. Vodder E. Die manuelle lymphdrainage und ihre medizinischen anwendungsgebiete. Erfahrungsheilkunde. 1966;16.
72. Földi M, Strößenreuther R. Grundlagen der Manuellen Lymphdrainage. 4th edition. München, Germany: Elsevier; 2007.
73. Bernas M, Witte MH. Consensus and dissent on the ISL consensus document on the diagnosis and treatment of peripheral lymphedema. Lymphology. 2004;37(4):165-167.
74. Adams KE, Rasmussen JC, Darne C, et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Opt Express. 2010;1(1):114-125.
75. Stout N, Partsch H, Szolnoky G, et al. Chronic edema of the lower extremities: international consensus recommendations for compression therapy clinical research trials. Int Angiol. 2012;31(4):316-329.
76. Hwang JH, Kim TU, Lee KW, Kim DI, Lee BB. Sequential intermittent pneumatic compression therapy in lymphedema. J Korean Acad Rehab Med. 1997;21(1):146-153.
77. Pflug JJ. Intermittent compression in the management of swollen legs in general practice. Lancet. 1975;215(1285):69-76.
78. Brunner U, Frei-Fleischlin C. Gegenwärtiger Stand der kombinierten physikalischen Entstauungstherapie beim primären und sekundären Lymphödem der Beine. VASA. 1993;Band 22(1).
79. Földi E, Baumeister RGH, Bräutigam P, Tiedjen KU. Zur diagnostik und therapie des lymphödems. Dtsch Ärztebl. 1998;95(13):A740-A747.
80. Mortimer P, Todd J. Lymphoedema: Advice on Self-management and Treatment. 3rd edition. Beaconsfield, Bucks, UK: Beaconsfield Publishers, LTD; 2007.
81. von der Weid PY, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295(5):H1989-H2000.
82. Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol. 2005;44(5):449-457.
83. Johansson K, Ingvar C, Albertsson M, Ekdahl C. Arm lymphoedema, shoulder mobility and muscle strength after breast cancer treatment? A prospective 2-year study. Adv Physther. 2001;3(2):55-66.
84. Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Christiaens MR. Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: a review. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):3-9.
85. Devoogdt N, Christiaens MR, Geraerts I, et al. Effect of manual lymph drainage in addition to guidelines and exercise therapy on arm lymphoedema related to breast cancer: randomised controlled trial. BMJ. 2011;343:d5326.
86. Moffatt C, Partsch H, Schuren J, et al; International Lymphedema Framework. Best practice for the management of lymphoedema – 2nd edition. Compression therapy: a position document on compression bandaging. http://www.lympho.org/portfolio/ compression-therapy-a-positiondocument- on-compression-bandaging/. Published 2012. Accessed January 26, 2018.
87. Partsch H, Stout N, Forner-Cordero I, et al. Clinical trials needed to evaluate compression therapy in breast cancer related lymphedema (BCRL). Int Angiol. 2010;29(5):442-453.
88. Partsch H, Damstra RJ, Mosti G. Dose finding for an optimal compression pressure to reduce chronic edema of the extremities. Int Angiol. 2011;30(6):527- 533.
89. Lamprou DA, Damstra RJ, Partsch H. Prospective, randomized, controlled trial comparing a new two-component compression system with inelastic multicomponent compression bandages in the treatment of leg lymphedema. Dermatol Surg. 2011;37(7):985-991.
90. Damstra RJ, Partsch H. Prospective, randomized controlled trial comparing the effectiveness of adjustable compression Velcro wraps versus inelastic multilayer compression bandages in the initial treatment of leg lymphedema. J Vasc Surg Venous Lymphat Disord. 2013;1(1):13-19.
91. Eliska O, Eliskova M. Are peripheral lymphatics damaged by high pressure manual massage? Lymphology. 1995;28(1):21-30.
92. Foeldi E. Massage and damage to lymphatics. Lymphology. 1995;28(1):1-3.
93. Blumberg SN, Berland T, Rockman C, et al. Pneumatic compression improves quality of life in patients with lowerextremity lymphedema. Ann Vasc Surg. 2016;30:40-44.
94. Zaleska M, Olszewski WL, Durlik M. The effectiveness of intermittent pneumatic compression in long-term therapy of lymphedema of lower limbs. Lymphat Res Biol. 2014;12(2):103-109.
95. Zaleska M, Olszewski WL, Cakala M, Cwikla J, Budlewski T. Intermittent pneumatic compression enhances formation of edema tissue fluid channels in lymphedema of lower limbs. Lymphat Res Biol. 2015;13(2):146-153.
96. Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M, Gradalski T. Tissue fluid pressure and flow during pneumatic compression in lymphedema of lower limbs. Lymphat Res Biol. 2011;9(2):77-83.
97. Mayrovitz HN. Interface pressures produced by two different types of lymphedema therapy devices. Phys Ther. 2007;87(10):1379-1388.
98. Compression for lymphoedema. Lancet. 1986;327(8486):896.
99. Cluzan RV, Alliot F, Ghabboun S, Pascot M. Treatment of secondary lymphedema of the upper limb with cyclo 3 fort. Lymphology. 1996;29(1):29-35.
100. Casley-Smith JR, Morgan RG, Piller NB. Treatment of lymphedema of the arms and legs with 5,6-benzo-[α]-pyrone. N Engl J Med. 1993;329(16):1158-1163.
101. Loprinzi CL, Kugler JW, Sloan JA, et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med. 1999;340(5):346- 350.
102. Roztocil K, Pretovsky I, Olivia I. The effects of hydroethylrutosides on capillary filtration rate in the lower limbs of man. Eur J Clin Pharmacol. 1993;11(6):435- 438.
103. Clement DL. Management of venous edema: insights from an international task force. Angiology. 2000;51(1):13-17.
104. Moseley A, Piller N, Douglass J, Esplin M. Comparison of the effectiveness of MLD and LPG. J Lymphoedema. 2007;2(2):30-36.
105. Pecking AP, Fevrier B, Wargon C, Pillion G. Efficacy of MPFF at a dose of 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer). Angiology. 1997;48(1):93-98.
106. Farinola N, Piller N. Pharmacogenomics: its role in re-establishing coumarin as treatment for lymphedema. Lymphat Res Biol. 2005;3(2):81-86.
107. Raunio H, Rautio A, Gullstén H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharmacol. 2001;52(4):357-363.
108. Michelini S, Campisi C, Failla A, Boccardo F, Moneta G. Combination treatment of lymphedema. Our experiences [article in Italian]. Minerva Cardioangiol. 1998;46(10):395-396.
109. Campisi C, Boccardo F. Lymphedema and microsurgery. Microsurgery. 2002;22(2):74-80.
110. Olszewski WL. Episodic dermatolymphangioadenitis (DLA) in patients with lymphedema of the lower extremities before and after administration of benzathine penicillin: a preliminary study. Lymphology.1996;29(3):126-131.
111. Karonidis A, Chen HC. Preservation of toes in advanced lymphedema: an important step in the control of infection. Ann Plast Surg. 2010;64(4):446-450.
112. Celestin R, Brown J, Kihiczak G, Schwartz RA. Erysipelas: a common potentially dangerous infection. Acta Dermatovenerol Alp Panonica Adriat. 2007;16(3):123-179.
113. Olszewski WL. Episodic dermatolymphangioadenitis (DLA) in patients with lymphedema of the lower extremities before and after administration of benzathine penicillin: a preliminary study. Lymphology. 1996;29(3):126-131.
114. Singh R, Ray P, Das A, Sharma M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol. 2009;58(pt 8):1067-1073.
115. Tabibiazar R, Cheung L, Han J, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3(7):e254.
116. Nakamura K, Radhakrishnan K, Wong YM, Rockson SG. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One. 2009;4(12):e8380.
117. Tian W, Rockson SG, Jiang X, et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med. 2017;9(389):pii: eaal3920.
118. Damstra RJ. Upper Limb Lymphedema. In Lee BB, Rockson SG, eds. Lymphedema: A Concise Compendium of Theory and Practice. 2nd Ed. Springer International Publishing AG 2011, 2018; 540.
119. Földi E, Földi M, Rockson SG. Complete decongestive physiotherapy In Lee BB, Rockson SG, eds. Lymphedema: A Concise Compendium of Theory and Practice. 2nd Ed. Springer International Publishing AG 2011, 2018; 406.
