Stenting as a treatment modality for acute and chronic venous disease
Joe D. T. ESLAND1
Adam M. GWOZDZ2
Olivia SHARP1
Prakash SAHA1
Stephen A. BLACK1
Cardiovascular Division, King’s College
London, Guy’s and St. Thomas’ NHS
Foundation Trust, London, UK
2National Institute for Health Research
(NIHR), Biomedical Research Centre,
King’s College London, Guy’s and
St. Thomas’ NHS Foundation Trust,
London, UK
Abstract
Venous disease is common among the general population, with chronic venous disorders affecting 50% to 85% of the Western population and consuming 2% to 3% of health care funding; therefore, it carries a significant socioeconomic, physical, and psychological burden. The most widely recognized presentation is acute venous thrombosis; however, patients may also experience chronic symptoms related to long-term sequelae, including persistent pain, swelling, and, when severe, ulceration. Due to the high morbidity associated with venous disease and the impact on the patient’s quality of life, an effective treatment is a necessity. Stenting has received increased attention as the treatment of choice for patients with iliofemoral venous disease, particularly when there is an underlying compressive pathology. Initially, stenting was attempted for patients with chronic venous disease and postthrombotic syndrome or nonthrombotic iliac vein lesions, such as May-Thurner syndrome, to overcome chronic symptoms by reestablishing venous patency. More recently, stenting has received increased attention in acute venous disease for acute relief of symptoms and potentially reducing features of postthrombotic syndrome. This review summarizes the use of venous stenting in patients with acute and chronic venous disease, and it provides insight into the rationale for use and reviews on the existing evidence and outcomes. Therefore, this article hopes to provide information on the use of venous stents and give recommendations and indications for its use in both acute and chronic venous disease.
Introduction
Venous disease is common among the general population, with venous disorders affecting 50% to 85% of the Western population and consuming 2% to 3% of health care funding; therefore, it carries a significant socioeconomic, physical, and psychological burden.1 Acute venous thrombosis is the most common and well recognized presentation of venous disease. Although acute venous thrombosis may affect many different vessels, it commonly affects the deep veins of the leg (ie, deep vein thrombosis), which occurs in approximately 1 person in 1000 in the population,2 and presents as a swollen and erythematous limb with skin that is warm to the touch.
Chronic venous disease (CVD) is a spectrum of conditions characterized by the retrograde flow of venous blood back into the affected leg. The pathophysiology of chronic venous disease is attributable to valvular incompetence, venous outflow obstruction, or, most commonly, a combination of both.3 The causes of CVD that are related to deep vein anomalies may be either primary or secondary. In primary CVD, there is an underlying nonthrombotic lesion compressing the vein and leading to outflow obstruction, which may lead to CVD directly or, alternatively, present with an antecedent acute deep vein thrombosis. The etiologies of this are wide and may include pelvic tumors, retroperitoneal fibrosis, intraperitoneal hematomas, or anatomical variants, such as May-Thurner syndrome.
May-Thurner syndrome is characterized by compression of the left common iliac vein by the right common iliac artery, and this irregularity has been observed in 22% to 32% of cadavers.4,5 Secondary CVD tends to follow a deep vein thrombosis, and it can be associated with irreversible damage to the valves6 and a persistent outflow obstruction. Chronically, this damage and obstruction may lead to a postthrombotic syndrome (PTS), which is often associated with pain, itching, restless legs, nocturnal cramps, and, when severe, ulceration,6 resulting in disability and a significantly impaired quality of life.7 PTS occurs in approximately 50% of patients within 2 years of a deep vein thrombosis.8
Recognizing the impact of venous disease has led to increased research efforts to help identify ways of preventing the development of severe chronic venous disease symptoms, which has resulted in the emergence of deep venous stenting to treat specific groups of patients. The aim of this review is to explore the role of venous stenting in the treatment of both acute and chronic venous disease.
Acute venous thrombosis
The current treatment for acute venous disease can be broadly divided into two categories– conservative and interventional. Conservative treatment options include compression therapy and venoactive medications. Interventional medical treatment includes heparin related parenteral treatment followed by bridging to a dose-adjusted vitamin K antagonist (VKA), such as warfarin.9 However, in recent years, newer non–VKA oral anticoagulants (NOACs) have been introduced, and they are becoming a standard treatment in clinical practice.
Parenteral anticoagulation with low-molecular-weight heparin prevents thrombus propagation and reduces the risk of embolization,10 but it is unable to remove the thrombus from the deep venous system, which instead undergoes remodeling and recanalization in a process reminiscent of wound healing.11,12 Sometimes this process is incomplete, resulting in a persistent outflow obstruction or stenosis that leads to PTS-related leg ulceration and venous claudication (Figure 1).8,13,14 In addition, there is a subgroup of patients presenting with acute deep vein thrombosis who have an underlying nonthrombotic (“primary”) May-Thurner etiology. This underlying etiology can contribute to venous outflow obstruction, which may lead to a high risk of thrombus extension and recurrence, and it is associated with an increased incidence of PTS.15,16 Overcoming venous outflow obstruction, whether primary or secondary, has been the basis for the development and use of a venous stent.
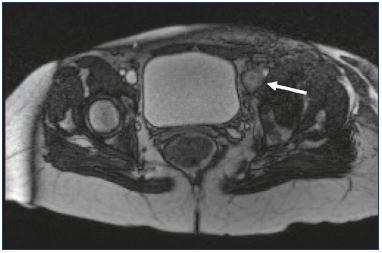
Figure 1. Magnetic resonance venography of a 62-year-old
female with acute left iliofemoral deep vein thrombosis (arrow).
Interventional options
In the acute setting of deep vein thrombosis, research has focused on whether invasive management is more effective than anticoagulation alone. The most common technique for the removal of an acute proximal deep vein thrombosis is catheter-directed thrombolysis (CDT). CDT is an endovascular procedure in which a fibrinolytic drug, typically urokinase or tPA, is infused directly into the thrombus (under fluoroscopic guidance), thus theoretically averting the risks associated with systemic thrombolysis.17,18 Initial data suggested that the procedure was both technically successful in >94% of patients19-23 and safe in this patient group, with the US National Venous Registry reporting infrequent major bleeding (ie, intracranial bleeding [<1%], retroperitoneal hematoma [<1%], and gastrointestinal/ genitourinary/musculoskeletal bleeds [3%])24 and minor bleeding in 16% of the patients (predominantly at the venous insertion site and hematuria).12,25 The best results are seen when the symptoms have been present for <14 days, a thorough history is taken before the procedure, the vein is punctured under ultrasound guidance, and postprocedure observations are taken every 8 hours, and when hemostasis is monitored.25 A venogram check will be done after 12 to 24 hours to assess the degree of thrombus dissolution, thus helping guide ongoing management.18 When compared with conventional therapy alone, the use of CDT for the treatment of acute proximal deep vein thrombosis reduced the incidence of PTS from 55.6% to 41.1% at 24 months. At 6 months, the iliofemoral patency was 65.9% in patients treated with CDT and 47.4% in those receiving conventional therapy.26 The ATTRACT study (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis) is a large, ongoing study that is continuing to address the incidence of PTS in patients with acute proximal deep vein thrombosis treated with CDT.
Stenting, in the context of CDT, has been a secondary measure focused on treating an underlying obstructive lesion revealed by lysis (Figure 2). However, there is a paucity of data to aid in deciding which patients will ultimately occlude without the placement of a venous stent.
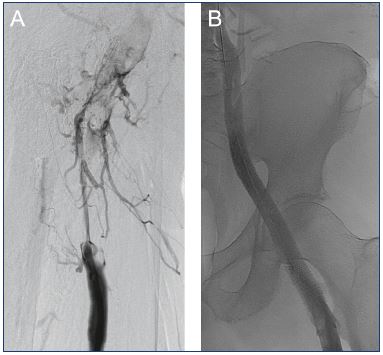
Figure 2. Lower limb venogram of a 62-year-old male with
acute right iliofemoral deep vein thrombosis.
Panel A. Pre-catheter directed thrombolysis (CDT). Panel B.
Completion venogram after the placement of an intravenous
stent and balloon dilatation.
First report of venous stenting
In 1995, Berger et al first reported a case of stenting for acute deep vein thrombosis.27 A 51-year-old man presented with May-Thurner syndrome. Following treatment with CDT and a subsequent angioplasty, two premounted Palmaz intravascular stents were placed in tandem into the left common iliac vein. At the 6-month follow-up, the patient maintained a good clinical response and the stent was patent. The authors proposed that this intervention warranted further investigation.
Catheter-directed thrombolysis and venous stenting
Stent patency
In studies on venous stenting following CDT for a nonthrombotic iliac vein lesion (NIVL), a widely used primary outcome measure is long-term stent patency. It has been proposed that a residual outflow obstruction will increase the likelihood of further or recurrent venous thrombosis21 and PTS.15 Early data on stent patency were limited by small studies and short follow-up times. In patients with iliofemoral stenting for May-Thurner syndrome following an acute deep vein thrombosis, primary patency at 1 year was 83% to 91.8% (n=155).14,21-23 The primary patency was 84.5% to 90.2% at 2 years,22,23 and 83% to 85% at 5 years.14,23
In a 2015 study by Park et al, 56 patients with an acute iliofemoral deep vein thrombosis were treated with CDT.22 Completion venography was used to assess the degree of stenosis, and, in patients with a severe stenotic segment or with May-Thurner syndrome, a stent was inserted. This venography data resulted in 37 patients receiving stents. The 5-year primary patency rate was 77.8% in stented patients and 42.1% in nonstented patients (P=0.02). Deep vein thrombosis recurrence was also higher in the nonstented group (57.9% vs 27.1%; P=0.027), with recurrence occurring between 1 and 127 months of follow up.28
Incidence of postthrombotic syndrome
A small number of studies have examined the incidence of PTS in patients receiving a venous stent following an acute deep vein thrombosis. PTS is reported to be as low as 11.5% at 5 years by Xue et al,23 but the incidence was much higher in a separate study by Alhadad et al, which reported an incidence of 41% at the 6.2-year follow-up (range, 4.6-10.3).29 In a single-centered, prospective study of 87 patients treated with stenting after a venous stenosis was found following CDT (defined as residual luminal narrowing >50%, absent anterograde flow, or presence of collateral vessels), 12% and 6% developed PTS at 3 months and 1 year, respectively.30 In the study by Park et al,22 30 patients (54%) were assessed for PTS at 57 months (mean) using the Villalta scale. There was a tendency for a lower score in the stenting group when compared with the nonstenting group, although this did not reach statistical significance.28
Surgical thrombectomy and venous stenting
Only a small number of studies have assessed the role of venous stenting following surgical thrombectomy; however, these studies focused on patients presenting with acute deep vein thrombosis, and they had small patient numbers (n=11-29). Of note, thrombolysis was often used along with a thrombectomy, either before treatment16 or after if there was a residual thrombus.31,32
In two studies investigating patients with May-Thurner syndrome, the stent patency rate at 1 year varied widely. In patients with proximal deep vein thrombosis (iliofemoral [n=2]; iliopopliteal [n=1]; iliotibial [n=8]), patency was reported as 64% at 1 year,16 whereas, in a study of patients with only iliofemoral deep vein thrombosis (n=26), the patency rate was 96%.15 Where patients with varied etiologies for venous occlusion were included non-discriminately in the study group, patency rate for those with iliocaval deep vein thrombosis was 79% at 1 year,32 and 74% in patients with iliofemoral clots (mean follow-up, 68 months; range, 3-129 months).31
Technical success in all papers was 100%, although there was significant heterogeneity between the different types of procedures.15,16,31,32 Such heterogeneity also made interpreting postprocedure complications difficult. Except for a 2010 paper by Hölper et al that reported rethrombosis in 20% of patients within 7 days,31 it was a rare early complication in other studies, only affecting between 3% to 9% of the patients.15,16,32 The development of PTS was assessed using the Villalta score in a single study (n=11) by Husmann et al. The study showed that, at 3 and 6 months, only a single patient had developed features of PTS, and, in those followed-up after 12 months (n=9), there were no further cases of PTS reported.31
Surgical thrombectomy may be associated with a lower rate of stenting than catheter-directed lytic techniques, particularly mechanical techniques, which may be related to the fact that a residual clot after performing these techniques is misinterpreted as a stenosis/disease that requires a stent. Rates of stenting as high as 100% have been reported when purely mechanical thrombectomy was used.33 Regardless, there is a paucity of high-quality studies examining the long-term efficacy of stenting in this group of patients.
Chronic deep venous obstructive syndrome
Historic treatment
Chronic deep venous obstructive syndrome responsible for CVD or chronic venous insufficiency (CVI) has historically been treated conservatively with compression and anticoagulation alone. However, these treatments provide variable results, and invasive surgical treatment has been suggested as an alternative approach to treating patients with severe venous claudication.18,34,35 Surgery options include vein-patch angioplasty with excision of intraluminal bands, the division of the right common iliac artery, relocation behind the left common iliac vein or vena cava, and femorofemoral bypass (Palma’s bypass) by grafting the contralateral saphenous vein to the ipsilateral common femoral vein with the creation of a temporary arteriovenous fistula. Long-term patency rates vary from 40% to 88%, and experience with these techniques is limited to only a few centers.36 As a result, over the last 20 years, endovascular stenting has received increased attention for CVD.
Initial reports on stenting for CVD related to deep vein obstruction
The first large study in stenting for CVD was in 2000 by Neglén et al.37 They investigated patency of 139 consecutive lower extremities in 137 patients after femoral vein cannulation, percutaneous balloon angioplasty, and iliac vein stenting. A total of 68 patients had PTS and 61 had May-Thurner syndrome (52% and 60% 1-year primary patency rates, respectively). They recommended stenting in all venoplasties with wide-diameter stents (16 mm) and found an improvement in pain and swelling with a postintervention thrombosis rate of just 4% (8% for PTS and 0% for May-Thurner syndrome). The authors further noted that 50% of all venous ulcers healed.37 A follow-up publication, in 2003, featured outcomes of 447 lower extremities that were treated for obstruction alone or obstruction with reflux affected by CVI. They performed angioplasty and stenting without adjuvant treatment for venous reflux disease and found that this treatment led to a marked reduction in CVI symptoms: 50% of their patients were pain-free, 33% of swelling was relieved, and 55% of lower limb ulcers healed. These results suggested an important role of stenting for the treatment of CVI symptoms.38
Indications of stenting in CVD
Hartung et al39 explored patency rates in 89 patients with nonmalignant obstructive iliocaval disease and found that there was a 98% technical success rate for patients treated with balloon angioplasty and stenting. Of these, 52 patients had primary disease with NIVL, 35 patients had disease secondary to PTS, and 2 patients had congenital abnormalities. The combined primary, primary-assisted, and secondary patency rates were 89%, 94%, and 96%, respectively, at 1 year, with restenosis predominantly found in patients with PTS.
The study by Neglén et al reflected on long-term outcomes of stenting in 982 nonmalignant obstructive lesions of femoroiliocaval veins. The study included NIVL and PTS patients and demonstrated a 6-year primary, primary-assisted, and secondary patency of 79%, 100%, and 100%, respectively, in patients with nonthrombotic disease and 57%, 80%, and 86%, respectively, in patients with thrombotic disease. Severe in-stent restenosis was qualified as >50% obstruction and occurred in 1% of NIVL and 10% of PTS limbs. There was also a significant reduction in pain scores and leg swelling, with an ulcer healing rate of 58%; the authors emphasized the benefit of using stent technology in patients with CVD.40 In this study, stenting was most commonly performed for left sided lesions, for nonocclusive obstruction, and in women; stenting had better outcomes in patients with NIVL vs PTS. Stent occlusion generally occurred if the obstruction was thrombotic in origin, suggesting that restenosis was likely to be associated with a thrombotic event rather than progressive occlusion. Occlusion was also more common if there was an associated recent trauma, if the stent was extended to the common femoral vein, if the obstruction was complete, and if the stenting was performed at a younger age. History of thrombophilia, additional procedures, and sex were not associated with worse stent outcomes.
A meta-analysis by Wen-da et al in 2015 looked at 14 studies exploring the use of stents in chronic venous disease related to deep vein obstruction.41 It included a total of 1987 patients, of which, 43.2% underwent stenting for chronic PTS sequelae and 56.8% for chronic symptoms or signs related to NIVL. Of the included limbs, 81.2% involved the left side. They found that the 30-day thrombosis rates were higher (4.0% vs 0.8%) and the rates of ulcer healing lower (70.3% vs 86.9%) in patients with PTS vs NIVL. These findings were consistent with the results of Neglén et al, where it was shown that thrombotic lesions have a higher restenosis rate that is likely associated with a thrombotic event rather than progressive restenosis. This meta-analysis further concluded that occlusions before stenting and long lesions extending into the common femoral vein that damaged the inflow tract were associated with higher rates of restenosis. At the same time, sex (unless in younger patients), thrombophilia, and stents crossing the inguinal ligament did not influence patency. There were minimal complications (fewer in NIVL than in PTS), and the procedure was deemed safe.41
An ongoing study in our department is looking at the 1-year patency rates of using dedicated stents for deep venous obstruction. We have studied 347 stents in 140 patients with 57 patients receiving stenting for acute deep vein thrombosis post–CDT and 81 patients receiving stenting for chronic outflow obstruction; 9 of these patients had ulcers. The diagnosis was based on clinical presentation combined with Doppler ultrasound and magnetic resonance venography (Figure 3). Our results show an overall primary, primary-assisted, and secondary patency rate of 67%, 80%, and 82%, respectively, reflecting the potential of using first-generation dedicated venous stents for venous disease. Our data and the studies mentioned above show that, when conservative treatment is not entirely effective, venous stenting is an appropriate and safe alternative therapy for chronic venous disease, resulting in clinical relief of pain and swelling, acceptable ulcer healing and recurrence rates, rare severe peri-operative complications, and good long-term patency.42
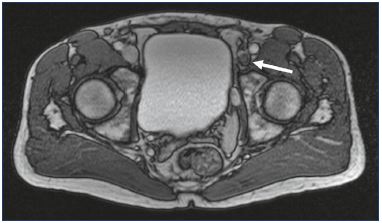
Figure 3. Magnetic resonance venography of a 53-year-old
male with postthrombotic syndrome of the left lower limb.
Iliofemoral deep vein thrombosis resulting in vein occlusion
(arrow).
Stenting as a treatment for symptoms of CVI
In a large follow-up study by Neglén et al in 2007, adjunct procedures to control saphenous venous reflux were performed, including great saphenous vein ablation and small saphenous vein stripping, in 197 limbs. Additional procedures did not affect patency rates or improve the quality of life scores, suggesting that stenting alone can be used to treat certain groups of patients with symptoms of chronic venous obstruction.40
A study by Raju et al in 2010 looked at the use of stenting alone for iliac vein obstruction and deep venous reflux in 528 limbs from 504 patients. Patients who were unresponsive to conservative measures with significant symptoms of pain (VAS ≥5/10), marked swelling, stasis, and skin changes, including ulcers, or a combination of signs and symptoms received stents. Large-caliber stents (14 to 18 mm) were used, and all lesions without skip areas were covered. There was a significant improvement in both pain and swelling in 78% and 55% of patients, respectively. Complete relief from pain and swelling was felt in 71% and 36% of patients, respectively. Cumulative secondary stent patency was 88% at 5 years. The study further suggested a role of iliac vein stenting for symptomatic relief of outflow obstruction and reflux (Figure 4).43
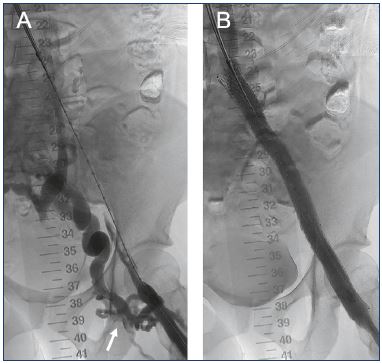
Figure 4. Lower limb venogram of a 37-year-old female with
postthrombotic syndrome in the left lower limb following
iliofemoral deep vein thrombosis.
Panel A. Preintervention with extensive collateralization observed
(arrow). Panel B. Completion venogram after the placement of
an intravenous stent and balloon dilatation.
Summary and recommendations
This paper reported on the use of venous stenting in the lower limbs and focused largely on two distinct groups of patients: (i) patients presenting with an acute deep vein thrombosis, where the underlying primary veno-occlusive pathology in the majority of studies was the May-Thurner syndrome; and (ii) patients presenting with CVI, where the etiology was either May-Thurner syndrome or PTS. Therefore, the findings are the most relevant to these specific patient groups.
Irrespective of surgical technique, the technical success of venous stenting appeared high in all studies, with acceptable safety and effectiveness when compared with traditional therapies.21,22 Stenting for venous disease starts with careful patient selection, and most reported studies suggest that stenting is indicated for chronic venous disease when the obstruction is >50%, when superficial collaterals form, and when there is reflux in the deep and/or superficial veins.34,36,41 A thorough preoperative preparation in both chronic and acute presentations is required, when possible, to ensure a positive outcome. Preoperative imaging includes ultrasonic angiology with duplex followed by imaging of the deep veins for appropriate planning.34 Further requirements include patient medical optimization, a thorough anesthetic assessment, and rationalizing of anticoagulant treatment. Anticoagulation with warfarin is usually bridged with treatment-dose heparin.9
Intraoperative techniques are essential for a successful outcome, such as selecting an appropriate stent, timing of stenting postthrombolysis or thrombectomy in acute presentations, and considering an arteriovenous fistula.36 The most common access sites are the femoral vein, the popliteal vein, and the internal jugular vein.40,41 Ultrasound guided puncture has shown the safest results, and it is a necessity for lesions in the external iliac and/or common femoral vein. Concerning the most appropriate stent that can be used, preference is usually given to flexible stents with a high radial force. Further preference is given to self-expandable stents rather than balloon-expandable stents to prevent kinking at the confluence of the internal and external iliac vein. The most common stent that has been used is a Wallstent (Boston Scientific, Galway, Ireland) and preference is given to large (14 to 16 mm) and long (>6 cm) self-expanding metallic stents with an overlap where multiple stents are requirement. Some newer dedicated venous stents, such as the Sinus XL stent, Sinus Venous stent (both Optimed, Ettlinger, Germany), the Zilver Stent (Cook, Limerick, Ireland), and the Veniti Vici System (Veniti, St. Louis, MO, USA), have been launched.34 Intraoperative intravascular ultrasound or venography has also been shown to improve results.34,41 There is no consensus on whether an arteriovenous fistula is required for preventing rethrombosis.31,44
Postoperative care is equally essential, and it involves a well-defined interval-imaging regime to check for rethrombosis. At our center, we aim to perform a venous duplex scan at 1 day, 2 weeks, 3 months, 6 months, and 1 year postoperatively to ensure high secondary patency. Similarly, a rationalized antiplatelet or anticoagulant approach is also recommended. For NIVL, antiplatelet therapy may suffice; however, for PTS, anticoagulation is generally recommended due to a higher risk of rethrombosis.45
Acute venous disease
Stent patency was the most closely studied outcome measure in the literature, with rates reported at approximately 80% or above in the first 5 years following CDT. When patency was assessed in a stented vs an unstented group, the results were significantly better in patients who had iliac vein stenting.28 These results are encouraging, but have yet to demonstrate whether improved patency rates translate into better patient outcomes.
An attempt was made in the literature to measure the incidence of PTS in those with venous stenting. Following a deep vein thrombosis, PTS may occur in up to 50%7 of patients, and this increases to 70% when the causal factor is May-Thurner syndrome16,29; however, the assessment of PTS was not consistent between the studies.23,29 In the two studies that did use the validated Villalta Scale for assessment of PTS,46 the sample size was small in one study,16 and there was a loss to follow-up and a variable follow-up period in the other.28
In our experience, stenting for acute venous disease can be done in unison with thrombolysis in the acute period of clot onset (ie, within 14 days). Therefore, in an ideal setting, we recommend conducting stenting with thrombolysis as soon as possible after the diagnosis of acute deep vein thrombosis, guided by the patient’s clinical suitability to undergo surgery.
Chronic venous disease
Current experience with the use of stenting for chronic venous disease shows that it is safe, effective, and a reproducible procedure with the right equipment and technique. Study outcomes all show a very good patency rate after 1 year, with low rates of restenosis and major complications. As such, for chronic conditions, such as postthrombotic lesions, elastic lesions, and May-Thurner syndrome, stenting is rapidly becoming the treatment of choice.41,42
At the same time, there is clearly a difference in the results between PTS and NIVL patients. Early 30-day thrombotic incidence is reported as 0.8% in the NIVL group vs 4% in the PTS group, and long-term patency is lower in the PTS group vs the NIVL group.41 These results may be related to the pathogenesis of restenosis, which is likely due to an acute thrombotic event rather than a progressive stenosis. Similarly, the healing rate for ulcers is significantly lower in the PTS group (70.3%) than in the NIVL group (86.9%), with a global recurrence of 8.7%, which may be due to PTS lesions being more extensive; therefore, preventing the progress of ulcer healing. There is, however, no difference between access site complications, stent migration, back pain, retroperitoneal bleeding, and contrast extravasation.40,41 As such, further research is required to explore the timing of stenting, the type of stent that can be used, and the optimal intraoperative method for PTS.34,36
Conclusion
Stenting has been shown to be a safe and effective method of recanalization of veins in both the acute and chronic setting. It has high patency rates and acceptable complication rates. It remains to be seen if the early benefits translate into long-term improvements in morbidity. Although this seems a sensible inference, a large randomized controlled trial is needed. Robust, long-term follow-up is also necessary, and validated assessment tools should be used to assess outcome, particularly for PTS, where the Villalta score, despite its drawbacks, appears to be the gold-standard assessment.
REFERENCES
1. Eklof B, Perrin M, Delis KT, Rutherford RB, Gloviczki P. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(2):498- 501.
2. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
3. Raju S, Neglén P. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360(22):2319-2327.
4. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937-943.
5. Ferris EJ, Lim WN, Smith PL, Casali R. May-Thurner syndrome. Radiology. 1983;147(1):29-31.
6. Tsai S, Dubovoy A, Wainess R, Upchurch GR Jr, Wakefield TW, Henke PK. Severe chronic venous insufficiency: magnitude of the problem and consequences. Ann Vasc Surg. 2005;19(5):705-711.
7. Baldwin MJ, Moore HM, Rudarakanchana N, Gohel M, Davies AH. Post‐thrombotic syndrome: a clinical review. J Thromb Haemost. 2013;11(5):795-805.
8. Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28(4):465- 476.
9. Black SA, Cohen AT. Anticoagulation strategies for venous thromboembolism: moving towards a personalised approach. Thromb Haemost. 2015;114(4):660-669.
10. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidencebased clinical practice guidelines. Chest. 2008;133(suppl 6):454S-545S.
11. Comerota AJ, Gravett MH. Iliofemoral venous thrombosis. J Vasc Surg. 2007;46(5):1065-1076.
12. Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39-49.
13. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239(1):118-126.
14. Jeon UB, Chung JW, Jae HJ, et al. May-Thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long-term stent patency and changes in the iliac vein. AJR Am J Roentgenol. 2010;195(3):751-757.
15. Zhu QH, Zhou CY, Chen Y, et al. Percutaneous manual aspiration thrombectomy followed by stenting for iliac vein compression syndrome with secondary acute isolated iliofemoral deep vein thrombosis: a prospective study of single-session endovascular protocol. Eur J Vasc Endovasc Surg. 2014;47(1):68-74.
16. Husmann MJ, Heller G, Kalka C, et al. Stenting of common iliac vein obstructions combined with regional thrombolysis and thrombectomy in acute deep vein thrombosis. Eur J Vasc Endovasc Surg. 2007;34(1):87-91.
17. Vedantham S. Catheter-directed thrombolysis for deep vein thrombosis. Curr Opin Hematol. 2010;17(5):464- 468.
18. Saha P, Black S, Breen K, Patel A, Modarai B, Smith A. Contemporary management of acute and chronic deep venous thrombosis. Br Med Bull. 2016;117(1):1-14.
19. Kölbel T, Lindh M, Holst J, et al. Extensive acute deep vein thrombosis of the iliocaval segment: midterm results of thrombolysis and stent placement. J Vasc Interv Radiol. 2007;18(2):243-250.
20. Kwak HS, Han YM, Lee YS, Jin GY, Chung GH. Stents in common iliac vein obstruction with acute ipsilateral deep venous thrombosis: early and late results. J Vasc Interv Radiol. 2005;16(6):815-822.
21. Matsuda A, Yamada N, Ogihara Y, et al. Early and long-term outcomes of venous stent implantation for iliac venous stenosis after catheter-directed thrombolysis for acute deep vein thrombosis. Circ J. 2014;78(5):1234- 1239.
22. Park JY, Ahn JH, Jeon YS, Cho SG, Kim JY, Hong KC. Iliac vein stenting as a durable option for residual stenosis after catheterdirected thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May-Thurner syndrome. Phlebology. 2014;29(7):461-470.
23. Xue G, Huang XZ, Ye M, et al. Catheterdirected thrombolysis and stenting in the treatment of iliac vein compression syndrome with acute iliofemoral deep vein thrombosis: outcome and follow-up. Ann Vasc Surg. 2014;28(4):957-963.
24. Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol. 2010;30(4):669-674.
25. Baekgaard N, Klitfod L, Broholm R. Safety and efficacy of catheter-directed thrombolysis. Phlebology. 2012;27(suppl 1):149-154.
26. Enden T, Haig Y, Kløw NE, et al. Longterm outcome after additional catheterdirected thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38.
27. Berger A, Jaffe JW, York TN. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995;21(3):510- 514.
28. Srinivas BC, Patra S, Reddy B, Nagesh CM, Agarwal N, Manjunath CN. Outcome of venous stenting following catheter directed thrombolysis for acute proximal lower limb venous thrombosis: a prospective study with venous Doppler follow-up at 1-year. Cardiovasc Interv Ther. 2015;30(4):320-326.
29. Alhadad A, Kölbel T, Herbst A, Holst J, Alhadad H, Gottsäter A. Iliocaval vein stenting: long term survey of postthrombotic symptoms and working capacity. J Thromb Thrombolysis. 2011;31(2):211-216.
30. Engelberger RP, Fahrni J, Willenberg T, et al. Fixed low-dose ultrasound-assisted catheter-directed thrombolysis followed by routine stenting of residual stenosis for acute ilio-femoral deep-vein thrombosis. Thromb Haemost. 2014;111(6):1153- 1160.
31. Hölper P, Kotelis D, Attigah N, Hyhlik- Dürr A, Böckler D. Longterm results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39(3):349-355.
32. Hartung O, Benmiloud F, Barthelemy P, Dubuc M, Boufi M, Alimi YS. Late results of surgical venous thrombectomy with iliocaval stenting. J Vasc Surg. 2008;47(2):381-387.
33. Lichtenberg M, Stahlhoff F, Boese D. Endovascular treatment of acute limb ischemia and proximal deep vein thrombosis using rotational thrombectomy: a review of published literature. Cardiovasc Revasc Med. 2013;14(6):343-348.
34. de Graaf R, Arnoldussen C, Wittens CH. Stenting for chronic venous obstructions a new era. Phlebology. 2013;28(suppl 1):117-122.
35. Berntsen CF, Kristiansen A, Akl EA, et al. Compression stockings for preventing the postthrombotic syndrome in patients with deep vein thrombosis. Am J Med. 2015;129(4):447.e1-447.e20.
36. Mussa FF, Peden EK, Zhou W, Lin PH, Lumsden AB, Bush RL. Iliac vein stenting for chronic venous insufficiency. Tex Heart Inst J. 2007;34(1):60-66.
37. Neglén P, Berry MA, Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20(6):560-571.
38. Neglén P, Thrasher TL, Raju S. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38(5):879-885.
39. Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38(1):118-124.
40. Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979-990.
41. Wen-da W, Yu Z, Yue-Xin C. Stenting for chronic obstructive venous disease: a current comprehensive meta-analysis and systematic review. Phlebology. 2016;31(6):376-389.
42. Saha P, Karunanithy N, Cohen A, Hunt B, Breen K, Black S. One-year clinical outcomes following deep venous reconstruction using dedicated venous stents. J Vasc Surg Venous Lymphat Disord. 2016;4(1):152.
43. Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51(2):401-408.
44. Hartung O, Otero A, Boufi M, et al. Midterm results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42(6):1138-1144.
45. Lang KJ, Saha P, Roberts LN, Arya R. Changing paradigms in the management of deep vein thrombosis. Br J Haematol. 2015;170(2):162-174.
46. Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post‐thrombotic syndrome. J Thromb Haemost. 2009;7(5):884-888.

