Strategies to minimize the effect of neovascularization at the saphenofemoral junction after great saphenous vein surgery: an overview
University Hospital Antwerp,
Belgium
ABSTRACT
The recurrence rate remains high after great saphenous vein (GSV) surgery. One of the major contributing factors is neovascularization at the level of the ligated GSV stump. To mitigate the effect of neovascularization, several approaches are possible, which are reviewed in this article.
Some surgical strategies directly focus on the saphenofemoral junction (SFJ). Instead of simple ligation of the GSV stump, modified techniques have been tested: complete elimination of the GSV stump, hiding (inverting) the GSV stump, increasing the spatial separation between the stump and surrounding superficial veins, adding the construction of a prosthetic or anatomical barrier to the classical ligation, or even completely abandoning SFJ ligation. The recently developed endovenous techniques to obliterate the GSV also aim at limiting neovascularization in the groin by omitting a surgical intervention at this level.
Although some results are promising, more studies will be needed to investigate the effectiveness of all of these techniques to minimize varicose vein recurrence.
INTRODUCTION
The problem of recurrent varicose veins remains incompletely resolved. Some obvious solutions have been proposed to prevent the important causes of recurrence from happening, such as a better understanding of venous anatomy and hemodynamics, adequate preoperative assessment, and most importantly, correct and carefully performed surgery. Although remarkable progress has been made in all the above-mentioned fields, surgical treatment of varicose veins continues to be marred by the development of recurrent reflux, most commonly in the area of the saphenofemoral junction (SFJ), causing recurrent varicose veins from the thigh downwards in the entire leg (Figure 1).1 The recurrence rate remains high, even after “correct” surgery including comprehensive SFJ ligation, above knee stripping of the great saphenous vein (GSV), and ligation of all incompetent perforating veins, and appears to increase with additional years of follow-up.2 Clinical, ultrasonographic, surgical, and histopathological studies have illustrated the existence of a phenomenon called neovascularization (formation of new veins) as a possible explanation for recurrence after correctly performed previous operations (Figure 2).3-5 Such stump-related neovascularization might originate from hypoxia-induced activation of endothelial cells distal to the SFJ stump ligature, which could be mediated by growth factors. As neovascularization is part of the normal healing process, it cannot be inhibited completely. However, we can at least try to mitigate the effect of neovascularization. This may be achieved by several approaches.
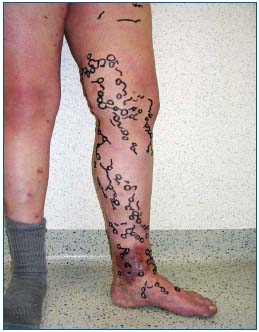
Figure 1. A 33-year-old baker came to the venous clinic because of stubborn recurrent varicose veins (C5s; Ep; As,p; Pr) after having been declared “incurable” by 3 surgeon-colleagues. He had already been operated on for varicose veins in another hospital at
the age of 25 years and 29 years, each time including surgery at
the saphenofemoral junction.
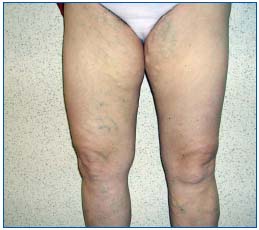
Figure 2. A 59-year-old woman had bilateral varicose vein surgery 8 years ago. She underwent a duplex ultrasound at 2 months,
which showed a perfect postoperative situation in the groin. At a
one-year follow-up, according to a duplex scan, a new vein had
developed on the anterior side of the common femoral vein in the
groin of both legs (classified as “grade 2” neovascularization,
connecting with some tiny, clinically not yet visible, tortuous veins
on the thigh). After 8 years, she presented with typical recurrent
varicose veins (C3s; Ep; As; Pr) from the groin downwards,
encompassing the entire leg, and had to be reoperated on.
a. Elimination of the GSV stump
The axiom “no stump, no neovascularization” is one of the important starting points when trying to reduce the incidence of neovascularization. Complete elimination of the stump can theoretically be accomplished by placing a vascular side clamp on the SFJ and dividing the GSV at its junction with the common femoral vein, leaving a short venotomy that can be closed with a twolayer running suture (with a 5.0 or 6.0 monofilament suture). The immediate result is a completely covered intima, a much smaller foreign body burden than an external ligature, and an intact common femoral vein lumen. However the incidence of recurrent reflux in the above-described technique in the long term has not yet been reported. Up to now, preliminary results have only been published in congress proceedings.
b. Hiding or destroying the stump endothelium
Based on the idea of hiding the stump endothelium, Frings et al6 published satisfying preliminary results of a study in which they ligated the stump with a nonresorbable suture and then buried the stump with a running polypropylene suture. Recently, the same authors reported the results of color duplex venous imaging two years after operation in 152 of 500 initially included limbs.7 They could show that recurrent reflux in the groin was reduced by oversewing the ligated SFJ with a running polypropylene suture (3% versus 11% in limbs without endothelial closure). However, in a prospective randomized study with follow-up up to 5 years after surgery, Haas et al8 could not confirm any beneficial effect of such inverting suture of the stump endothelium. On duplex ultrasound control, they still found neovascular vessels in 9% of 279 limbs operated on with this technique. Also, other investigators have chosen to focus only on the stump endothelium, destroying it with chemical or heat cauterization, or in some instances reducing the amount of endothelium exposed by placing a second ligature near the free end of the GSV stump, all without conclusive results.9,10
c. Spatial separation of the stump
It also seems important to achieve greater spatial separation between the deep veins underlying the fossa ovalis and the superficial venous system, by careful ligation of all tributaries in the groin beyond their junction and routinely stripping the thigh portion of the GSV.11-13 Hence, the rationale for stripping the aboveknee GSV lies not only in the fact that thigh perforators are disconnected, but also that the most important conduit for recurrent reflux is eliminated by stripping. Some authors have even suggested that stripping of the anterior (and/or posterior) accessory great saphenous vein should be performed as far distally as possible.14 This additional maneuver further reduces the potential conduits for reflux originating in newly formed refluxing veins in the groin, in the case of postoperative neovascularization. The importance of adding this to the classical surgical strategy should be confirmed in future prospective studies.
d. Barrier techniques
Interposition of a physical barrier (prosthetic or anatomical) between the ligated GSV stump on the common femoral vein (CFV) and the surrounding superficial veins can prevent tiny neovascular veins developing at the stump from connecting with superficial veins in the groin and thigh. Various barrier techniques have recently been studied in primary as well as in recurrent varicose veins. Glass15 was the first to report apparently good results on clinical follow-up 4 years after patch implantation and closure at the SFJ. Earnshaw et al16 studied the results of a comparable barrier technique using a polytetrafluoroethylene (PTFE) patch (small patch, 1 x 2 cm), not only on clinical examination, but also with duplex ultrasound scanning after one year. This study indicated that patch saphenoplasty was safe, but did not abolish neovascularization after one year. In another study at our center, we compared two groups of limbs with and without a silicone implant (2 x 3 cm) and closure of the cribriform fascia one year after the operation with duplex scanning (Figures 3a,b,c).17 Silicone patch saphenoplasty significantly lowered the incidence of neovascularization (Figure 4).
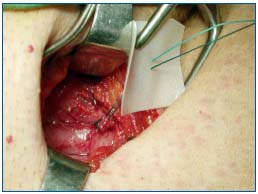
Figure 3a. Silicone patch saphenoplasty at the saphenofemoral
junction: (a) the saphenous stump has been ligated with a
nonresorbable suture and a silicone patch is fixed to the ligated
stump.
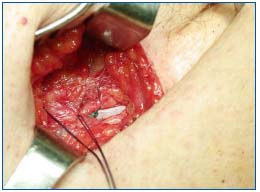
Figure 3b. after the patch has been tucked under the cribriform
fascia, covering in this way the anterior half of the common femoral vein, the cribriform fascia is closed with separate stitches.
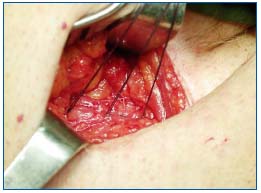
Figure 3c. situation before closing Scarpa’s fascia: the saphenous stump is not visible anymore as it has been covered with a
prosthetic and anatomical barrier.
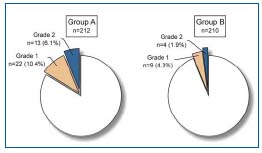
Figure 4. Incidence of the different degrees of neovascularization on duplex examination in group A (without silicone patch) and group B (with silicone patch) 12 months postoperatively. Grade 1: tiny new vein(s) < 4 mm at the previously ligated saphenofemoral junction; Grade 2: tortuous new communicating vein(s) with a diameter > 4 mm and with pathological reflux.
In particular, in groins being reoperated on for recurrent SFJ reflux, patch saphenoplasty appears to be a valuable adjunctive measure to reduce the incidence of rerecurrence.18-20 In a recent study at our center, we found that patch saphenoplasty significantly improved the clinical and duplex ultrasound results five years after repeat surgery.20 Recurrent thigh varicosities were observed in 58% of limbs in the group without a patch and in 26% of those with a patch. Duplex scans revealed important neovascular vessels in 45% versus 9% of limbs. In redo procedures, it is of particular importance to fix the patch well to the CFV, as there is usually no cribriform fascia left to cover the patch and keep it where it should stay (covering the saphenous stump in close apposition to the CFV). Only by maintaining the patch at the right location, can it act as an efficacious barrier to contain neovascularization. Of course, the obvious disadvantage of the use of a prosthetic implant in the groin is the risk of infection. This may appear as an acute infection in the immediate postoperative course or even many years after the initial procedure due to a sudden exacerbation of a “silent” infection. In very obese patients (BMI > 35), often with preexisting mycotic infection in the groin, it is therefore wiser to refrain from implantation of such a prosthetic patch. Postoperative symptomatic stenosis of the common femoral vein due to excessive scar tissue formation around the patch has also been observed after silicone patch saphenoplasty in exceptional cases (unpublished observation).
To contain neovascularization and hence prevent recurrence in the groin, the use of an anatomical barrier to cover the ligated saphenous stump has also been proposed.15,21,22 The easiest approach to construct such an anatomical barrier consists of simply suturing the opening in the cribriform fascia, once the SFJ has been ligated. At our center, the results of duplex ultrasound scanning one year after closure of the cribriform fascia in GSV surgery (first varicose vein operations) were comparable with those obtained after silicone patch saphenoplasty (unpublished observation). A more complex barrier technique, first described by Sheppard21 consists of the use of a flap of pectineus fascia at the SFJ. In a randomized controlled trial, Gibbs et al22 could not confirm the previously suggested benefit of this particular technique. Further critical evaluation of the effects of a simplified barrier technique without the use of foreign material is mandatory.
e. Abandoning SFJ ligation?
Finally, what about comprehensive SFJ ligation, the “sacred cow”? Although we have always been taught that an accurate groin dissection with detachment of all tributaries is the ideal method to prevent recurrence in the groin, in fact, the reverse could become true during the forthcoming years. On one hand, the usefulness of stripping the GSV (above knee) rests on clear-cut experimental clinical evidence.12,13,23-27 On the other hand, the importance of ligating all tributaries of the GSV in the groin has always been assumed since the introduction of “high ligation” in the groin, but never really proved. Chandler et al28 tried to define the role of extended SFJ ligation, while studying radiofrequency ablation of the GSV (VNUS®). They compared no ligation of the SFJ in the groin with extended SFJ ligation in combination with radiofrequency obliteration.28 They found no notable differences between both groups. These results questioned the axiom stating that SFJ ligation with ligation of all tributaries is an essential component of the treatment of GSV insufficiency. Perhaps complete (endovenous) exclusion of the thigh portion of the GSV from the superficial venous system could be sufficient to achieve equal therapeutic benefits in cases of a refluxing main GSV trunk. The quite revolutionary idea of abandoning SFJ ligation in the management of primary varicose veins associated with GSV reflux will certainly have to be further examined. Prospective randomized long-term follow-up studies will have to clarify this important issue.
a. Endovenous radiofrequency obliteration
The recently developed endovenous treatment methods do not seem to be associated with neovascularization in the groin and could, therefore, become the future method of choice for treatment of primary varicose veins. A prospective multicenter randomized trial showed significant early advantages to endovenous radiofrequency GSV obliteration with the VNUS® closure method compared with conventional high ligation and stripping.29 An earlier return to work and routine daily activities, and a significantly better quality of life early after the intervention (and maybe a lower incidence of recurrence) could provide advantages in the form of reduced indirect costs of the procedure. However, the direct costs of endovenous obliteration remain more than twice that of surgery.30 The recently reported results of GSV radiofrequency obliteration after 3 and 5 years continue to be promising, and duplex ultrasound findings confirm the absence of neovascular veins in the groin.31-33
b. Endovenous laser treatment34,35 Duplex scans showing occlusion of the vein one year after the procedure, also showed that the vein remained occluded at further controls up to 3 years after treatment.36 Potential advantages of the endovenous laser are the small diameter and flexibility of the laser fiber and the faster withdrawal rates, which result in less heat-related damage to adjacent nontarget perivenous tissue compared with radiofrequency.
c. Ultrasound-guided foam sclerotherapy
Foam slerotherapy under duplex ultrasound guidance was introduced as a third alternative treatment method.37 The increased efficacy of foam, in comparison with classical sclerotherapy with liquid sclerosants, is attributed to its displacement of blood from the treated vein and its increased contact time between the sclerosant and the vein. The foam is clearly imaged by duplex ultrasound so that precise filling of an incompetent vein can be assured. Foam sclerotherapy, therefore, enables treatment of varicose veins with larger diameter and even main superficial trunks.38 Promising results have also been obtained in patients with recurrent varicose veins. Minor complications are similar to conventional liquid sclerotherapy. The advantage of foam sclerotherapy is that it requires no general or regional anesthesia to perform and takes much less time than other current techniques. The most significant concern with this technique has been deep vein thrombosis.
d. External valvuloplasty of the SFJ
Repair of the terminal valve of the GSV, hence reconstituting competence of the main trunk seems to be an attractive physiological alternative to high ligation of the SFJ and stripping. Lane et al39 recently reported promising results after external valvular stenting of the SFJ by means of Venocuff implantation. This technique has the potential advantage in that the GSV is not transected in the groin, which might reduce the stimulus for postoperative neovascularization.
CONCLUSION
During the past decades, our knowledge about the causes of recurrent varicose veins after surgery has grown considerably. In particular, surgeons have become more aware of neovascularization at the ligated GSV stump being one of the important phenomena potentially leading to recurrence. Important efforts have been made to try to find the optimal method to avoid recurrence, focusing on either the SFJ or the main trunk of the GSV.
However, the final solution has not yet been found. At the level of the SFJ, simple closure of the opening in the cribriform fascia might be the easiest way to cover (and isolate) the ligated stump in a first GSV operation. In groins being operated on for recurrent SFJ reflux, covering the stump with a prosthetic barrier might be preferred. At the level of the main trunk of the GSV, results of alternative treatment techniques are promising, but remain inconclusive. Critical investigation using well-established criteria and longterm follow-up periods (of at least 5 years) are, therefore, needed to evaluate the effectiveness of the abovementioned (and other) procedures.

REFERENCES
2. Fischer R, Linde N, Duff C, et al. Late recurrent saphenofemoral junction reflux after ligation and stripping of the greater saphenous vein. J Vasc Surg. 2001;34:236-240.
3. De Maeseneer MGR. The role of postoperative neovascularization in recurrence of varicose veins. Acta Chir Belg. 2004;104:281-287.
4. Van Rij AM, Jones GT, Hill GB, Jiang P. Neovascularization and recurrent varicose veins: more histologic and ultrasound evidence. J Vasc Surg. 2004;40:296-302.
5. Stücker M, Netz K, Breuckmann F, et al. Histomorphologic classification of recurrent saphenofemoral reflux. J Vasc Surg. 2004;39:816-822.
6. Frings N, Glowacki P, Nelle A, Van-Than- Phuong T. Prospektive Studie zur Verhinderung der Neoangiogenese nach Magna-crossektomie – erste Ergebnisse. Zentralbl Chir. 2001;126:528-530.
7. Frings N, Nelle A, Tran P, Fischer R, Krug W. Reduction of neoreflux after correctly performed ligation of the saphenofemoral junction. A randomized trial. Eur J Vasc Endovasc Surg. 2004;28:246-252.
8. Haas E, Burkhardt T, Maile N. Rezidivhäufigkeit durch Neoangiogenese nach modifizierter Krossektomie. Phlebologie. 2005;34:101-104.
9. Gorny P, Reinharez D, Hutinel B, et al. Chirurgie post-stripping en ambulatoire: une étude sur 124 interventions et une discussion sur le néogénèse. Phlébologie. 1994;47:265-272.
10. Sarahay M, Shields DA, Georgiannos SN. Endothelial activation in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 1998;15:342-349.
11. Bradbury AW, Stonebridge PA, Ruckley CV, Beggs I. Recurrent varicose veins: correlation between clinical and hand-held Doppler ultrasonographic examination, and anatomical findings at surgery. Br J Surg. 1993;80:849-851.
12. Sarin S, Scurr JH, Coleridge Smith PD. Stripping the long saphenous vein in the treatment of primary varicose veins. Br J Surg. 1994;81:1455-1458.
13. Winterborn RJ, Foy C, Earnshaw JJ. Causes of varicose vein recurrence: late results of a randomized controlled trial of stripping the long saphenous vein. J Vasc Surg. 2004;40:634-639.
14. Earnshaw JJ. Improving the results of varicose vein surgery. In: Earnshaw JJ, Murie JA, eds. The evidence for vascular surgery. Cheltenham, UK: Tfm publishing; 1999:131-135.
15. Glass GM. Prevention of recurrent saphenofemoral incompetence after surgery for varicose veins. Br J Surg. 1989;76:1210.
16. Earnshaw JJ, Davies B, Harradine K, Heather BP. Preliminary results of PTFE patch saphenoplasty to prevent neovascularization leading to recurrent varicose veins. Phlebology. 1998;13:10-13.
17. De Maeseneer MG, Giuliani DR, Van Schil PE, De Hert SG. Can interposition of a silicone implant after sapheno-femoral ligation prevent recurrent varicose veins? Eur J Vasc Endovasc Surg. 2002;24:445-449.
18. Bhatti TS, Whitman B, Harradine K, et al. Causes of re-recurrence after polytetrafluoroethylene patch saphenoplasty for recurrent varicose veins. Br J Surg. 2000;87:1356-1360.
19. Creton D. Surgery for recurrent saphenofemoral incompetence using expanded polytetrafluoroethylene patch interposition in front of the femoral vein: long-term outcome in 119 extremities. Phlebology. 2002;16:93-97.
20. De Maeseneer MG, Vandenbroeck CP, Van Schil PE. Silicone patch saphenoplasty to prevent repeat recurrence after surgery to treat recurrent saphenofemoral incompetence: long-term follow-up study. J Vasc Surg. 2004;40:98-105.
21. Sheppard M. A procedure for the prevention of recurrent saphenofemoral incompetence. Aust N Z J Surg. 1978;48: 322-326.
22. Gibbs PJ, Foy DM, Darke SG. Reoperation for recurrent saphenofemoral incompetence: a prospective randomized trial using a reflected flap of pectineus fascia. Eur J Vasc Endovasc Surg. 1999;18:494-498.
23. Jones L, Braithwaite BD, Harradine K, Earnshaw JJ. Neovascularization is the principal cause of varicose vein recurrence: results of a randomized trial of stripping the long saphenous vein. Eur J Vasc Endovasc Surg. 1996;12:442-445.
24. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins. J Vasc Surg. 1999;29:589-592.
25. Rutgers PH, Kitslaar PJ. Randomized trial of stripping versus high ligation combined with sclerotherapy in the treatment of the incompetent greater saphenous vein. Am J Surg. 1994;168:311-315.
26. Neglén P, Einarsson E, Eklof B. The functional long-term value of different types of treatment for saphenous vein incompetence. J Cardiovasc Surg. 1993;34:295-301.
27. Jakobsen BH. The value of different forms of treatment for varicose veins. Br J Surg. 1979;66:182-184.
28. Chandler JG, Pichot O, Sessa C, et al. Defining the role of extended saphenofemoral junction ligation: a prospective comparative study. J Vasc Surg. 2000;32:941- 953.
29. Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg. 2003;38:207-214.
30. Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration vs conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958-965.
31. Nicolini P; Closure Group. Treatment of primary varicose veins by endovenous obliteration with the VNUS closure system: results of a prospective multicenter study. Eur J Vasc Endovasc Surg. 2005;29:433-439.
32. Merchant RF, Pichot O, Closure Study Group. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg. 2005;42:502-509.
33. Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189-195.
34. Proebstle TM, Lehr HA, Espinola-Klein C, et al. Endovenous treatment of the greater saphenous vein with a 940 nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser-generated steam bubbles. J Vasc Surg. 2002;35:729-736.
35. Proebstle TM, Doendue G, Kargl A, Knop J. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29: 357-361.
36. Min RJ, Khilnani N, Zimmet S. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
37. Cabrera Garrido JR, Cabrera Garcia- Olmedo, Dominguez MA, et al. Elargissement des limites de la sclérotherapie : nouveaux produits sclérosants. Phlébologie. 1997;50:181-188.
38. Raju B, Mc Collum CN. Microfoam injection sclerotherapy. In: Greenhalgh RM. Vascular and endovascular challenges. London, UK: Biba Publishing; 2004:382-389.
39. Lane RJ, Coroneos JC. The treatment of varicose veins with external stenting to the saphenofemoral junction. J Vasc Endovasc Surg. 2002;36:179-192.
