The Importance of Uniform Venous Terminology in Reports on Varicose Veins
Abridged and updated version of original publication in Seminars in Vascular Surgery, Volume 23, Issue 2, June 2010, Pages 70-77.
Michael A. VASQUEZ,
The Venous Institute of Buffalo
NY, USA
ABSTRACT
There is a need for a standardized consistent language in vascular surgery that allows easy flow of information and comparison of results among clinicians. Beginning with current nomenclature, a common language serves as a framework for more detailed efforts. Understanding the outcome assessment tools available provides the opportunity for universal outcome reporting. Data collected at widespread points can then be fairly compared, and common goals of therapy can be determined. Common outcomes that have demonstrated verifiable trends and reproducibility should be subjected to the rigors of evidence-based questioning. The resultant standards of care and expectations of therapy are then confidently presented for everyday practice and ongoing research.
INTRODUCTION
Uniform terminology and accurate nomenclature are the bedrock for valuable discourse in medicine. This common language of vascular nomenclature leads to the standardization of outcome reporting, which in turn enables the verified outcomes of evidence-based medicine. With regard to anatomy and procedural technique, nomenclature dispels gray areas in reporting and unifies treatment goals.
ANATOMICAL TERMINOLOGY
History
Vascular anatomic nomenclature is as old as the study of anatomy itself. From an ancient tablet in Athens depicting a man holding a leg with visible varicose veins1 to the identification of blood circulation,2 the advent of arteriography3 and venography,4 to heparin1 and the embolectomy catheter,32
The parallel innovations of disparate physicians and surgeons was initially possible because a common anatomic language existed, namely, the nomenclature of the vascular system. One of the key issues in 21st century discussions of vascular nomenclature is accuracy and uniformity in vessel naming. This is evidenced by support for renaming the superficial femoral vein to reflect its existence as a deep venous structure. Numerous authors have written on the potential for confusion and harm with the current name, as demonstrated in retrospective analysis of physician intent to treat when thrombus was identified in this vein on Doppler imaging.5-8
This issue of confusing vascular nomenclature and attempts to clarify it are not 21st-century phenomena, however. The origins of the name for the saphenous vein have been attributed to a Greek derivation of the word safaina, meaning “evident,” as well as an Arabic derivation in the writings of Avicenna of the term el safin, meaning “concealed.”9 Anatomists throughout time have tried to assign meaning to each of these derivations, summarizing that the Arabic term may refer to the proximal great saphenous vein and the Greek derivation to the palpable course of the vein on the medial thigh.9 In 2002, the saphenous vein was again the focus of a discussion, with Kakkos and Nicolaides addressing this confusion over its naming: “The knowledge of the correct etymological origin of a scientific term is valuable since this could assist its proper usage.”10
In 1986, Kistner wrote about the importance of consistency in establishing diagnoses in venous disease, as well as about possible lingering ambiguity in venous nomenclature: “Historically, the venous system has not been regarded as worthy of detailed evaluation because so little could be done to repair the problems the system harbors.”11 As clinicians and researchers gained knowledge on the form and function of the lower extremity vasculature, it became apparent that existing nomenclature was insufficient in its descriptions.12 Mozes and Gloviczki proposed the framework for a new system of vascular nomenclature: “One reason to adopt a new venous terminology was to provide a system of anatomic names that are based on descriptive topographic terms rather than ill-defined eponyms. Another reason…was the goal to minimize possible errors in the management of patients with venous disease.”13
Bonn wrote on the outcome of a 2001 meeting held by the International Union of Phlebology to review and address inconsistencies in anatomic terminology found in clinical use and in the literature. These included “A deficiency in the nomenclature of the veins of the lower limbs; …the introduction and use of names of veins not present in the existing official anatomic document; …incorrect interpretation of these names, which leads to confusion and inappropriate treatment of venous disease; inadequate listing, specifically of perforating veins, saphenous vein collateral vessels, tributary veins, and some of the deep veins.”6
Consensus on venous anatomy
Many conventional naming methods have proven problematic in the nomenclature of the vascular tree. The importance of a common language of nomenclature has come to light, with a 2002 consensus statement written to clarify the terminology of venous anatomy for academic and clinical applications. Caggiati et al were members of this consensus committee and wrote: “Anatomy of the venous system forms the basis of clinical phlebology and is crucial to the correct evaluation and appropriate treatment of venous disorders.”12 “A common anatomical terminology is the foundation for a common language in phlebologic sciences. Further, such a common language is important for investigation of the venous system and for accurate diagnosis and correct treatment of venous disorders. Universally accepted new terminology will facilitate effective international exchange of information.”12
In conjunction with a 2004 international consensus committee meeting on the subject of nomenclature, Caggiati and colleagues went on to write: “Anatomic terminology is the foundation of medical communication. Effective exchange of information is possible only if a common terminology is used.”14 The 2004 consensus committee “developed a refinement of the nomenclature from 2002, focusing on new terms, on the veins of the pelvis, and on practical recommendations regarding the daily clinical use of the proposed terminology.”14 The committee recommendations included restricting the use of eponyms, confining them to publications with international circulation, and limiting them to “Giacomini’s vein, Cockett’s perforating veins and Santorini’s plexus.”14 Also defined were terms relating to vein size and development.
DISEASE-RELATED TERMINOLOGY
History
Another recent nomenclature clarification arose with the terms chronic venous disease and chronic venous insufficiency. Although often used interchangeably, the terms have distinct meanings.
Robertson et al wrote that “chronic venous disease of the legs is one of the most common diseases affecting the general adult population. It comprises a wide spectrum of clinical severity, varying from asymptomatic venous incompetence to varicose veins and, in its gravest form, trophic skin changes and ulceration.”15 Bergan and colleagues wrote that “chronic venous disease encompasses the full spectrum of signs and symptoms associated with classes C0 to C6, whereas the term chronic venous insufficiency is generally restricted to disease of greater severity, ie, classes C4 to C6.”16 Porter and Moneta, writing for the International Consensus Committee on Chronic Venous Disorder, wrote that “chronic venous disease is defined as an abnormally functioning venous system caused by venous valvular incompetence with or without associated venous outflow obstruction, which may affect the superficial venous system, the deep venous system, or both.”17 Robertson et al commented on the confusion surrounding chronic venous disease: “the exact prevalence of chronic venous disease remains difficult to determine because of variations in study population, selection criteria and disease definition between different studies.”15
Meissner et al define chronic venous insufficiency as “those manifestations of venous disease resulting from ambulatory venous hypertension, defined as a failure to reduce venous pressure with exercise.”18 Thorisson and colleagues wrote: “chronic venous insufficiency (CVI) of the superficial and/or deep venous systems of the lower extremities is an extremely common condition and is estimated to occur in one of every five Americans. The most common manifestation is varicose veins…the clinical significance of CVI is not merely cosmetic because many patients experience debilitating symptoms ranging from lower extremity pain, swelling, heaviness, warmth, itching, cramping, and muscle fatigue to inflammatory dermatitis and ultimately venous stasis ulcers.”19 Fowkes et al described chronic venous insufficiency as covering “a wide range of conditions, from asymptomatic incompetence of venous valves, through varicose veins, to venous skin changes and leg ulceration,”20 while Eberhardt and Raffetto wrote that “the term chronic venous insufficiency (CVI) describes a condition that affects the venous system of the lower extremities with venous hypertension causing various pathologies including pain, swelling, edema, skin changes, and ulcerations…we will use the term CVI to represent the full spectrum of manifestations of chronic venous disease.”21 Antignani wrote: “chronic venous insufficiency (CVI) is used to describe signs and symptoms of chronic venous hypertension in the lower limbs, a condition generally considered as the pathophysiological trigger of skin changes, the most serious of which is ulceration. Chronic venous disease of the lower limbs ranks as one of the most common conditions affecting humankind.”22
Consensus on venous terms
The 2009 VEIN-TERM consensus document provides thorough definitions of chronic venous disease and chronic venous insufficiency and adds a third global term, namely, chronic venous disorder. Chronic venous disorder “includes the full spectrum of morphological and functional abnormalities of the venous system.”23 Chronic venous disease refers to “any morphological and functional abnormalities of the venous system of long duration manifested either by symptoms and/or signs indicating the need for investigation and/or care.”23 Chronic venous insufficiency (C3-C6) is “a term reserved for advanced chronic venous disease, which is applied to functional abnormalities of the venous system producing edema, skin changes or venous ulcers.” The goal of the consensus committee was to agree on “a common scientific language for reports on the management of chronic venous disease.”23
UNIFORM CLASSIFICATION
History
The end of the 20th century and the first part of the 21st century have been a time of compilation, contemplation, and advancement in regard to venous disease and therapy. While treatments were being offered to patients for many vascular conditions, the outcomes were only sporadically evaluated. After pioneering three valvular reconstructive procedures, Kistner found that diagnostic and reporting methods were not standardized between facilities, preventing inter-institutional trials from taking place and diluting the outcome data.4
Consensus
In 1994, Kistner joined with other vascular specialists to address these issues of universality on testing and reporting; this committee developed the Clinical-Etiologic-Anatomic-Pathophysiologic (CEAP) classification system for venous disease diagnosis and outcome that remains today’s standard.4 Eklöf wrote that the CEAP classification was created to be a common language for venous disease, which lacked precise descriptions. The realization that diagnostic methods and treatment options cannot exist in myriad isolated incarnations has focused the attention of the scientific community on coalescing this information to provide a framework of commonality in clinical practice and research. The CEAP classification was adopted24 and over time has become “the accepted standard for classifying chronic venous disorders.”25 The basic design of the CEAP classification is to function as a universally accepted system for quantifying all degrees of chronic venous disease, leading to a common platform for clinical intervention and scientific inquiry.24 The CEAP classification meets the desired criteria for an assessment in the range of systems and symptoms included, the nature of the severity measurements, and the inclusion of a disability score.24
TOOLS TO ASSESS TREATMENT OUTCOMES
Mozes and Gloviczki wrote that a common language in treatment and outcome, based on a common anatomic language, would lead to beneficial standardization of contributing factors to venous disease.13 The goal of this standardization is clarity in outcome reporting and the ability to compare results: “Outcome scoring systems have made a difference in scientific assessment of venous disease. There remains, however, the confusion over the different names used for leg veins. The confusion includes the terminology of saphenous veins (long, great or greater, small, short or lesser), the myriad of eponyms of the perforators, and, most importantly, the superficial femoral vein (a deep vein) (Tables 1-3).”13
Universal agreement on nomenclature and the anatomy of chronic venous disease will result in uniformity in reporting standards and outcome assessments. A common language must be in place for the outcome assessment to be relevant in international settings. Meissner et al wrote about the importance of evaluating outcomes: “The scientific future of studies of chronic venous disease and its management depends on using proper outcome assessment methods, and this should be a major priority of all those engaged in the study of venous disease. However, measuring outcomes in CVD is complex and more difficult than most other vascular diseases.”18 They also discussed what is required to achieve true standardization: “Bolstering clinical assessment by objective improvement in universally accepted venous tests could have a tremendous impact on venous outcomes assessment…[and] [r]equires that the normal range for each venous test parameter be standardized…. In addition, what constitutes a significant change in each of these parameters also needs to be established, to provide an objective basis for claiming improvement in response to an intervention. Finally, test protocols must be standardized and variability established for this approach to have universal application.”18
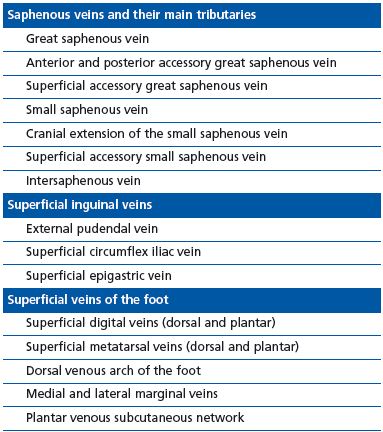
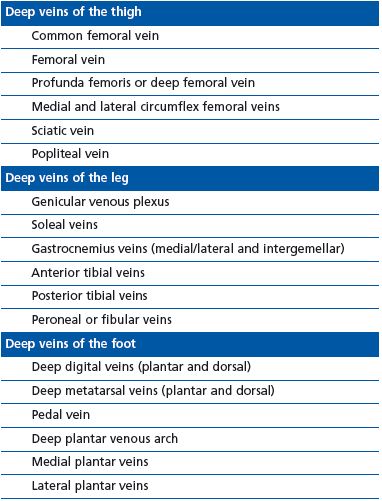
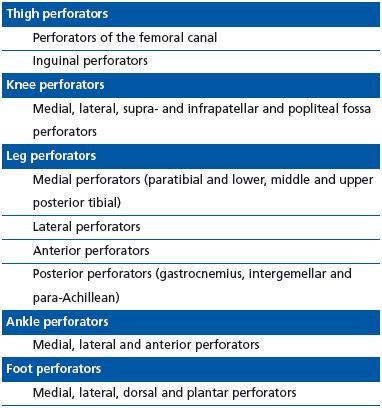
extremity.14
Venous diseases, and varicosities in particular, are being diagnosed with increasing frequency worldwide, with varicosities being the most frequently diagnosed vascular anomaly.26 The use of outcome assessments in venous disease has grown in importance with the explosion of technology and procedures offered for chronic venous insufficiency. To place these procedures on an international level playing field for accurate comparison, O’Donnell wrote: “The adoption of a surgical strategy that corrects the abnormal superficial venous system alone (saphenous veins and perforators) in the face of deep venous reflux requires that the therapeutic outcomes of such a strategy be judged with objective criteria. The improved clinical status of a limb as defined by ulcer healing and by lack of ulcer recurrence, especially when expressed in a life table format, clearly is an objective outcome measurement.”27
After years of debate and trial-and-error attempts to identify the standard for outcome reporting, the consensus is that scoring instruments should provide uniform, accurate, reproducible measurements and be amenable to change to reflect the chosen therapy.28 According to Dayal and Kent, they should “define essential terms and make recommendations regarding the following: clinical classification of disease; criteria for improvement, deterioration, and failure; a grading system for risk factors; categorization of operations and interventions; [and] complications encountered with grades for severity or outcome.”28 Results should be reported in a common language, which will lead to easy comparison of outcomes across studies.29
Because of the variability in presentation of chronic venous disease, outcome reporting instruments have been difficult to devise. Meissner et al wrote: “The ideal clinical outcome measure for CVD would include the full spectrum of disease and be sufficiently sensitive to allow stabilization, improvement or deterioration to be precisely quantified.”30
One example of a useful outcome reporting method in chronic venous disease is the CEAP classification. But due to the static nature of its items, the CEAP cannot serve the purpose of assessing the treatment outcome. It only measures severity at a single time point. Like many other chronic conditions, venous disease encompasses a continuum of symptoms and severity, and change in status following therapy is an ongoing process. Some investigators have proposed combining the CEAP classification with other outcome assessment measures to increase its specificity for longitudinal assessment.31,32
An assessment tool complementary to the CEAP: the VCSS
The Venous Clinical Severity Score (VCSS) is a longitudinal measure of nine categories universally considered relevant in the diagnosis and management of chronic venous disease. This score has a strong relationship with the CEAP classification, indicating that “to some extent both scores are based on common characteristics.”26 That validation study by Kakkos et al26 found that the VCSS and the CEAP classification were equally sensitive in outcome assessment over time. While the VCSS was determined to be useful in assessing postprocedural outcome in the short term and in the long term, the CEAP classification was found to be valuable in staging venous disease throughout the treatment period.26
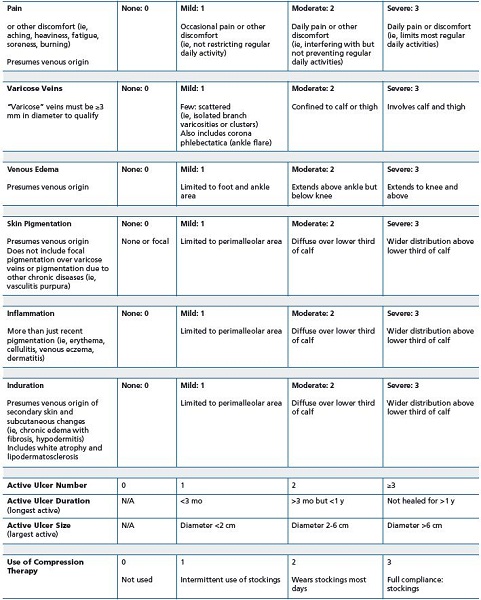
The VCSS has also demonstrated good correlation with the results of ultrasonography, and its simplicity makes it easy to administer and score.31,33 Recently, a valuable application for the VCSS has arisen in the form of its visual descriptive power. The “visual language of [the] VCSS” is a common framework for consistent physician scoring of venous disease (Figure 1). Similarity in scoring and in descriptions of venous sequelae adds to the structure of the language of chronic venous disease.
Newly issued recommendations by the Society for Vascular Surgery and American Venous Forum include the use of CEAP in both clinical and research applications. Revised VCSS is recommended for assessment of therapeutic results in all levels of venous disease.34
Revision of the VCSS
In 2007, through the American Venous Forum, an international ad hoc working group was created to revise the VCSS.35 (Table 4). The intention was to update the terminology, simplify the application, and clarify ambiguities. The additional objective was to protect the strengths of the VCSS, while acknowledging the limitations. Revisions to each of the clinical descriptors were made using, where applicable, quality of life language to address patients at the lower end of the disease spectrum. The pain component now contains common patient symptoms (aching, heaviness, fatigue, soreness, and burning) that establish a venous origin. The effect on different types of daily activities is clarified. The varicose vein component has modified the vein size criteria to greater than three mm to maintain consistency with the revised CEAP. Telangiectasias and reticular veins remain without a score; however, corona phlebectatica (ankle flare) has been added to the mild category. The edema component presumes a venous origin and now reflects anatomic distribution and extent. Skin pigmentation has guideline criteria for anatomic distribution and extent and excludes non-venous causes. Inflammation has been expanded to include more than just recent pigmentation changes or underlying infection. Erythema, cellulitis, venous eczema, and dermatitis have been incorporated, as well as anatomic distribution and extent. Induration has been modified to reflect more severe venous disease. Chronic edema, with fibrosis, hypodermitis, white atrophy and lipodermatosclerosis, has been added. The ulcer categories have been refined to include size and duration to reflect the largest and longest active ulcers. The compressive therapy category led to the most discussion and has now eliminated leg elevation to reflect that the category comprises only the wearing of compression garments. This revised VCSS is currently undergoing validation testing internationally.
Another assessment tool complementary to the CEAP: the REVAS scale
Another outcome assessment used in conjunction with the CEAP classification is REVAS (recurrent varices after surgery). While the CEAP classification alone is effective in staging chronic venous disease, there is a need for a tool specifically designed to score and assess recurrent varicosities after therapy. Although useful in following the course of venous disease after therapy, the VCSS is not designed to specifically address factors important in scoring recurrent varicosities, including the effect of initial treatment and the type of follow-up provided.36
The use of REVAS and the CEAP classification together provides the type of information that is necessary for a complete picture of the nature of recurrent varicosities in the context of chronic venous disease. This allows for clearer, more accurate reporting of this common postprocedural problem.36
Eklöf wrote a commentary on REVAS in which he explained that the goal was “to create a classification for REVAS to be used as a complement to [the] CEAP [classification], which was expanded to define the sites, nature, and sources of recurrence, as well as the magnitude of the reflux and possible contributory factors. Factors responsible for recurrence and recommendations for primary prevention were debated.”36
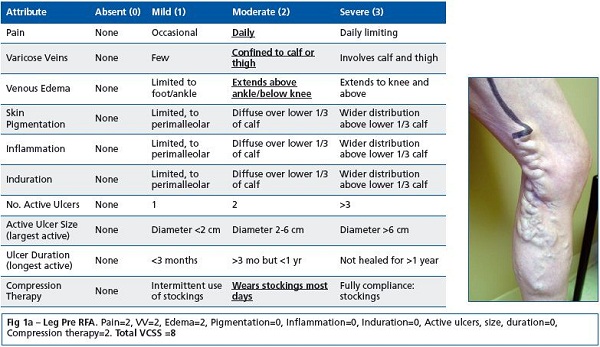
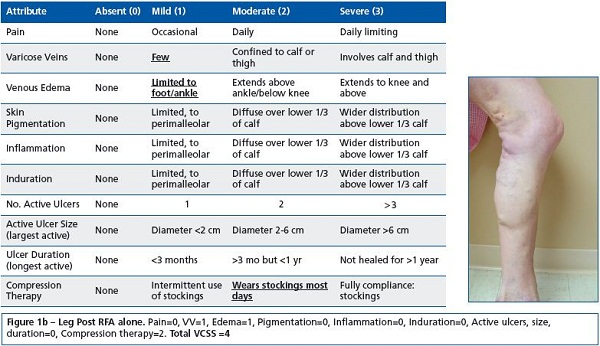
A NEED FOR COMMON OUTCOME MEASURES
Progression to the next step in assessment involves having common outcome measures that can be subjected to rigorous examination. According to Meissner et al, “An evidence-based approach to the treatment of any disease requires a mechanism for ensuring that patient populations are comparable and a means of quantifying outcome.”30
Regardless of the instrument chosen for outcome assessment, the manner in which results are tabulated and presented is of paramount importance in determining the effect of therapy. Rutherford wrote: “Results mean everything…. The results of therapy for vascular diseases have little meaning if presented in isolation, no matter how uniform and valid the criteria used for reporting them…. So the proper comparison of outcomes goes much farther than standardized reporting practices, as essential as these are. It requires not only reporting outcomes in a standard fashion but including comparable data on all factors known to affect those outcomes.”
The journey from an assessment instrument to a standardized reporting practice to a universal results measurement that is useful in clinical practice and research methods is a long exacting process. Along the way, there are key elements to consider to ensure reliability and a clear focus on the desired objectives.
Several categories of assessment tools are now in use, including physician- and patient-completed surveys, disease-specific and generic surveys, and tools that measure quality of life or that grade objective changes following intervention.35 The choice of instrument is based on the focus of the assessment, whether a straightforward measure of success or failure following an intervention or a detailed analysis of patient perception of change over time. Recently, the case has been made for inclusion of physician- and patient generated measures to assess the full effect of treatment for chronic venous disease.35,37
CONCLUSION
Consistent nomenclature provided the language foundation that has allowed communication to progress in a common direction. This communication later focused on the development and achievement of common therapeutic goals, which over time manifested as the reporting of outcomes. When results could be stratified in a universal format, these findings then could be subjected to the rigorous scrutiny of evidence-based medicine. This process is the seed for future therapies and continued conversations.
In his presidential address to the Midwestern Vascular Surgical Society, Glover spoke about the potential influence of this generation of vascular surgeons on the next. He articulated the value of future research, emphasized the inevitability of change in vascular surgery, and quoted one of his mentors, Vanderbilt anatomy professor Sam Clark: “Come, let us work, and in this little time do some new thing that no one on this earth has ever thought to do. Split from the world’s eternal truth some atom of the everlasting! Then let us die, and leave for coming generations one bit of knowledge by which we’ll be remembered until some later one shall show the truth we found was but a grain gleaned from some vast store we’d hardly touched and we shall be forgot and he remembered—but we, out where absolute is near, shall smile, seeing how little a beach of sand resembles the granite cliff from which it weathered.”38

REFERENCES
1. Yao JST. Presidential address: Venous disorders: Reflections of the past three decades. J Vasc Surg. 1997;26:727-735.
2. Scultetus AH, Villavicencio JL, Rich NM. Facts and fiction surrounding the discovery of the venous valves. J Vasc Surg. 2001;33:435-441.
3. Strandness DE, Eidt JF. Peripheral vascular disease. Circulation. 2000;102:IV-46-IV-51.
4. Eklöf B. The dynamic approach to venous disease: Following in the footsteps of Gunnar Bauer and Robert Kistner. J Vasc Surg. 2005;42:369-376.
5. Hewitt RL, Ayettey AS, Akers DL, Yates RD. New nomenclature for femoral vessels. J Vasc Surg. 1996;24:906-907.
6. Hammond I. The superficial femoral vein [letter]. Radiology. 2003;229:604- 606.
7. Georgiev M. Regarding “Nomenclature of the veins of the lower limbs: An international interdisciplinary consensus statement [letter].” J Vasc Surg. 2004;39:1144.
8. Bundens WP, Bergan JJ, Halasz NA, Murray J, Drehobl M. The superficial femoral vein: A potentially lethal misnomer. JAMA. 1995;274:1296-1298.
9. Caggiati A, Bergan JJ. The saphenous vein: Derivation of its name and its relevant anatomy. J Vasc Surg. 2002;35:172-175.
10. Kakkos SK, Nicolaides AN. Regarding “The saphenous vein: Derivation of its name and its relevant anatomy [letter].” J Vasc Surg. 2002;35:1307.
11. Kistner RL. Diagnosis of chronic venous insufficiency. J Vasc Surg 1986;3:185-188.
12. Caggiati A, Bergan JJ, Gloviczki P, Jantet G, Wendell-Smith CP, Partsch H; International Interdisciplinary Consensus Committee on Venous Anatomical Terminology. Nomenclature of the veins of the lower limbs: An international interdisciplinary consensus statement. J Vasc Surg. 2002;36:416-422.
13. Mozes G, Gloviczki P. New discoveries in anatomy and new terminology of leg veins: Clinical implications. Vasc Endovasc Surg. 2004;38:367-374.
14. Caggiati A, Bergan JJ, Gloviczki P, Eklöf B, Allegra C, Partsch H; International Interdisciplinary Consensus Committee on Venous Anatomical Terminology. Nomenclature of the veins of the lower limb: Extensions, refinements, and clinical application. J Vasc Surg. 2005;41:719-724.
15. Robertson L, Evans C, Fowkes FGR. Epidemiology of chronic venous disease. Phlebology. 2008;23:103-111.
16. Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklöf B. Chronic venous disease. N Engl J Med. 2006;355:488-498.
17. Porter JM, Moneta GL; International Consensus Committee on Chronic Venous Disorder. Reporting standards in venous disease: An update. J Vasc Surg. 1995;21:635-645.
18. Meissner MH, Moneta G, Burnand K, et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(suppl S):4S-24S.
19. Thorisson HM, Pollack JS, Scoutt L. The role of ultrasound in the diagnosis and treatment of chronic venous insufficiency. Ultrasound Q. 2007;23:137-150.
20. Fowkes FG, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology. 2001;52(suppl 1):S5-S15.
21. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2005;111:2398-2409.
22. Antignani PL. Classification of chronic venous insufficiency: A review. Angiology. 2001;52(suppl 1):S17-S26.
23. Eklöf B, Perrin M, Delis KT, Rutherford RB, Gloviczki P. Updated terminology of chronic venous disorders: The VEINTERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49:498-501.
24. Eklöf B, Rutherford RB, Bergan JJ, et al; American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J Vasc Surg. 2004;40:1248- 1252.
25. Meissner MH, Gloviczki P, Bergan J, et al. Primary chronic venous disorders. J Vasc Surg. 2007;46(suppl S):54S-67S.
26. Kakkos SK, Rivera MA, Matsagas MI, et al. Validation of the new venous severity scoring system in varicose vein surgery. J Vasc Surg. 2003;38:224-228.
27. O’Donnell TF. Lessons from the past guide the future: Is history truly circular? J Vasc Surg. 1999;30:775-786.
28. Dayal R, Kent KC. Standardized reporting practices. In: Rutherford RB, ed. Vascular Surgery. 6th ed. Philadelphia, PA: WB Saunders; 2005.
29. Kundu S, Lurie F, Millward SF, et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: Joint Statement of the American Venous Forum and the Society of Interventional Radiology. J Vasc Surg. 2007;46:582-589.
30. Meissner MH, Natiello C, Nicholls SC. Performance characteristics of the Venous Clinical Severity Score. J Vasc Surg. 2002;36:889-895.
31. Ricci MA, Emmerich J, Callas PW, et al. Evaluating chronic venous disease with a new venous severity scoring system. J Vasc Surg. 2003;38:909-915.
32. Rutherford RB, Padberg FT, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307- 1312.
33. Vasquez MA, Wang J, Mahathanaruk M, Buczkowski G, Sprehe E, Dosluoglu HH. The utility of the Venous Clinical Severity Score in 682 limbs treated by radiofrequency saphenous vein ablation. J Vasc Surg. 2007;45:1008- 1015.
34. Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, Lohr JM, McLafferty RB, Meissner MH, Murad MH, Padberg FT, Pappas PJ, Passman MA, Raffetto JD, Vasquez MA, Wakefield TW. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53:2S-48S.
35. Vasquez M, Rabe E, McLafferty R, Shortell C, Marston W, Gillespie D, Meissner M, Rutherford R. Revision of the Venous Clinical Severity Score: Venous Outcomes Consensus Statement: Special Communication American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52:1387-1396.
36. Eklöf B. Invited commentary to: Perrin MR, Labropoulos N, Leon LR Jr. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43:327-334.
37. White JV. Proper outcomes assessment: Patient-based and economic evaluations of vascular interventions. In: Rutherford RB, ed. Vascular Surgery. 6th ed. Philadelphia, PA: WB Saunders; 2005.
38. Glover JL. Presidential address: Vascular surgery: The third generation. J Vasc Surg 1990;11:615-623.
