The natural progression of chronic venous disorders: An overview of available information from longitudinal studies
Imperial College School of Medicine
Charing Cross Hospital, Fulham Palace Road,
London, W6 8RF
ABSTRACT
Chronic venous disorders remain a common problem worldwide; however, despite increasing research into novel endovenous therapies for the treatment of superficial venous disease, the natural history of primary venous disorders remains poorly understood. The following article provides a review of the longitudinal studies evaluating the progression of chronic venous disorders in the published literature. This includes a summary of the rate of development of venous disease in asymptomatic limbs and the rate of progression of venous disease in terms of hemodynamic, anatomical, and clinical progression including the development of skin changes and venous ulceration.
Venous disorders, including varicose veins and superficial venous insufficiency, are a common pathology, thought to affect between 15% and 40% of the adult population.1-2 They have plagued mankind for thousands of years and there is documentation of their existence from as early as 3500 BC.3 In the last decade, the development of endovenous techniques has led to a rapid increase in the popularity of many novel therapies including thermal and chemical ablation techniques.4-7 Yet, despite the large number of research studies supporting the use of new devices, the etiology and natural history of the progression of venous disease remains poorly understood. Evidence from clinical ultrasonographic and histological studies supports a multicentric theory for the development of venous disorders due to abnormalities in the composition of the vein wall leading to functional changes.8-9 However, the investigation of the progression of venous disease is complex and can be measured in a number of different ways. In recent years, there has been a move away from the use of surrogate end points to grade disease severity, such as the presence or absence of reflux, or the use of hemodynamic parameters such as venous refill times. A greater emphasis is now placed on the clinical assessment of disease severity and its functional impact on the individual patient. The association between the presence of superficial reflux and venous hemodynamic measurements with clinical and functional outcomes has been shown to be weak,2,10,11 and the relationship between these parameters remains poorly understood.
To date, there have been few studies that have investigated the natural history of the progression of primary venous disease. Much of the current information is based on patients self-reporting their symptoms, many of whom subsequently undergo treatment, and the natural history of the condition is infrequently documented. In the current financial climate, the natural history of patients presenting with mild or moderate venous disease becomes increasingly important in order to justify the allocation of scarce resources. The overall societal burden of venous disease is unknown; however, chronic venous ulceration is debilitating for patients and costly for society and therefore preventative treatments are likely to be cost effective.12,13 This article includes a review of the available information from longitudinal studies to date.
LONGITUDINAL STUDIES EVALUATING CLINICAL DISEASE PROGRESSION
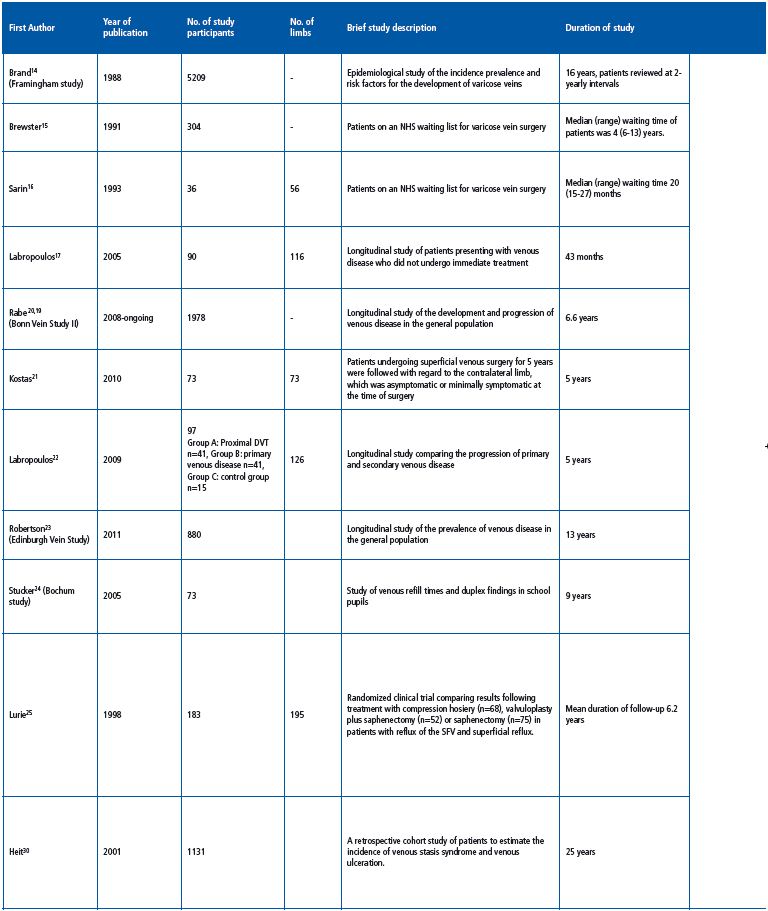
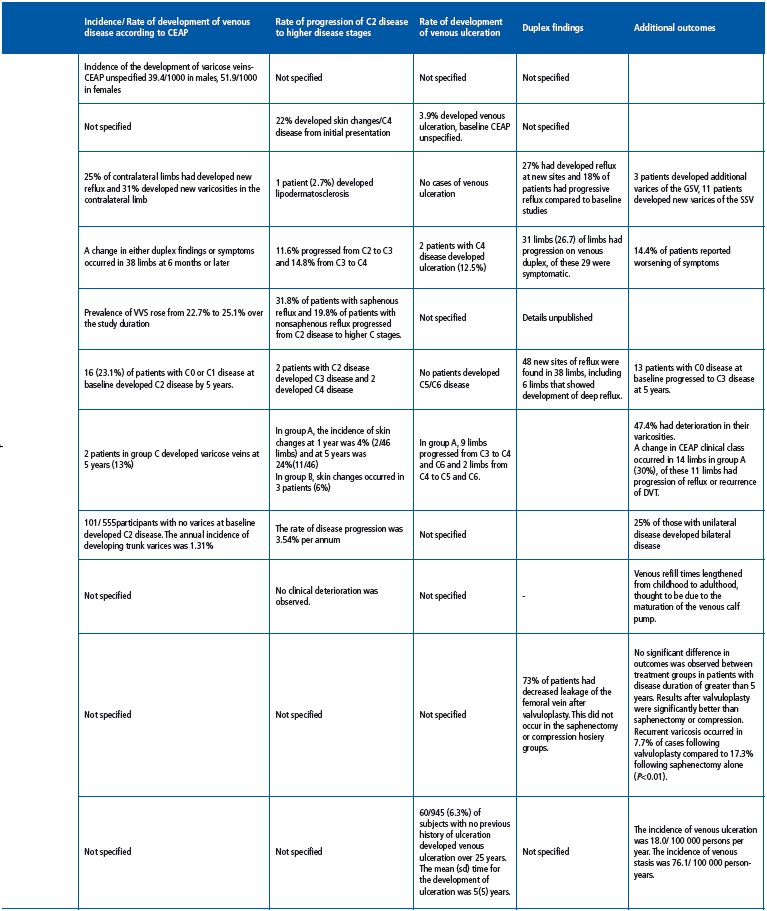
One of the earliest longitudinal epidemiological studies of varicose veins was the Framingham study, which followed 5209 male and female subjects for 16 years at 2-yearly intervals starting in 1966. Subjects were examined for the presence of varicose veins—defined as dilated tortuous veins on the lower limbs—and potential risk factors were recorded. At the beginning of the study, 1720 men and 2102 women had no clinical evidence of varicose veins. Over the 16-year period, 396 men and 639 women developed varicose veins. The development of varicose veins was significantly greater in obese women. Significant differences were also noted in those with a higher systolic blood pressure, and those who were less physically active. The data suggested an incidence of varicose veins of 39.4 per 1000 men and 51.9 per 1000 women with no significant increase with age; however, there was an increase in prevalence with age due to accumulation. The study provided useful information regarding the incidence and potential risk factors associated with the development of varicose veins; however, it did not provide details of the progression of venous disease.
Brewster et al published one of the earliest longitudinal studies documenting the details of venous disease progression in 304 patients on an NHS waiting list for superficial venous surgery. The median waiting time for patients on the waiting list was 4 years (range, 6 months to 13 years) and the median reported length of symptom duration was 28 years (range, 2-47 years). Primary varicose veins were observed in 85.5% of patients while 64% of patients reported that they felt that their venous disease had progressed since their initial presentation, and 5.2% suffered an episode of thrombophlebitis. In the time since their initial presentation, 68 patients (22%) had developed skin changes and 12 patients (3.9%) had developed venous ulceration, although the degree of clinical severity at baseline in those who developed ulceration was not reported.15 As with previous published studies, the development of further varicosities did not necessarily correlate with worsening of symptoms.
In a small study of 56 limbs in 36 patients on an NHS waiting list for treatment of uncomplicated varicose veins (43 primary and 13 recurrent), patients underwent noninvasive imaging and clinical examination at initial presentation and again prior to surgery. Superficial venous surgery was delayed by a median of 20 months (range, 15-27 months). An additional 3 patients had developed varicosities along the distribution of the great saphenous vein (GSV), and 11 had developed new varicosities in the small saphenous vein (SSV) distribution prior to treatment. There were no new cases of venous ulceration; however, 1 patient developed lipodermatosclerosis.16 Of the 16 previously clinically normal contralateral limbs, 5 (31%) developed varicosities. Duplex ultrasonography demonstrated that an additional 14 patients developed superficial reflux at new sites (27%) and 18% were shown to have progressive reflux compared to initial duplex scans. New reflux developed in 25% of normal contralateral limbs. No significant deterioration in venous refill times was observed.
Labropoulos et al carried out a longitudinal study comprising 116 limbs from 90 patients presenting with symptomatic chronic venous disease, who—for various reasons—did not undergo immediate treatment for their venous disorder and underwent treatment at a later date.17 All patients underwent two duplex ultrasound scans prior to intervention and reflux was classified as retrograde flow >0.5 s in truncal veins and >350 ms for perforator veins. All changes were documented and the time in between scans was specified. At the time of theinitial duplex scan, 3.4% of patient had C1 disease, 43.8% had C2 disease, 23.3% had C3 disease, 13.8% had C4 disease, and 6.9% and 4.3% had C5 and C6 disease, respectively.17 Over a total of 43 months, 27 of 116 limbs (23.3%) had changes on duplex ultrasonography and patients reported worsening symptoms at some point in 13 of 116 limbs (14.4%), although patients underwent scans at different time intervals. In patients who underwent a second duplex between 1 and 3 months, 1 of 15 limbs exhibited noticeable changes on duplex ultrasonography and 5 of 28 limbs showed duplex changes when scanned after 4- 6 months, although none of these patients had worsening clinical symptoms. In the patients who underwent their second scan at 7-9 months, 6 of 18 limbs showed duplex changes and worsening symptoms were reported in 1 of 18 limbs. In the patients scanned after 10-12 months, 3 of 15 limbs exhibited duplex changes, and worsening symptoms were reported for 1 of 15 limbs. When scanned after 13-18 months, 3 of 12 limbs showed duplex changes and worsening symptoms were reported for 3 of 12 limbs. At 19-24 months and 25-30 months, scans showed duplex changes in 3 of 10 limbs and 2 of 8 limbs, respectively, while worsening symptoms were reported in 3 of 10 limbs and 2 of 8 limbs, respectively. Between 31 and 36 months, 3 of 7 limbs exhibited duplex changes and worsening symptoms were reported in 2 of 7 limbs, while between 37 and 43 months, 1 of 3 limbs had duplex changes and worsening symptoms were reported in 1 of 3 limbs. New reflux was documented at 14 new sites including the GSV (n=2), SSV (n=1), tributaries (n=4), nonsaphenous vein (n=1), perforator veins (n=4), and deep veins (n=2). Extension of reflux in existing refluxing veins was documented in 17 limbs including at the saphenofemoral junction (SFJ) (n=1), GSV (n=7), SSV (n=4), tributaries (n=7), nonsaphenous veins (n=3), perforator veins (n=6), and deep veins (n=3). Overall when compared separately with other veins, saphenous veins and their tributaries were significantly more likely to have undergone change (P<0.01, Fisher exact test). Extension was antegrade in 7 limbs, retrograde in 7 limbs, and in both directions in 3 limbs. Worsening swelling (C2 to C3 change) was reported in 7 cases, worsening skin changes (C3 to C4) in 4 cases, and there were 2 cases of venous ulceration (C4 to C6). Overall 73% of limbs did not undergo any change; in those that did, this was usually after 6 months from the original scan. Approximately half of the patients who had significant changes on duplex ultrasonography reported a change in symptoms that occurred after 1 year. In addition, a number of patients who did not have noticeable changes on duplex ultrasonography were observed to have progressive clinical disease.
The aim of the Bonn Vein study was to investigate the prevalence of deep and superficial reflux in the general population.20 The most recent evidence from the Bonn Vein Study—which surveyed over 1978 patients in Germany—showed that over 6.6 years, progression of C2 disease to higher C classes was 31.8% in patients with saphenous reflux and 19.8% in patients with nonsaphenous reflux.18 The prevalence of varicose veins rose from 22.7% to 25.1%, and the prevalence of CVI increased from 14.6% to 16%. Risk factors for disease progression were identified using a multivariate analysis and included increasing age, obesity, and arterial hypertension.19
73 patients with primary superficial venous disease who underwent unilateral varicose vein surgery were followed up after 5 years with regard to the contralateral limb, which was either asymptomatic or minimally symptomatic at the time of initial treatment.21 Patients underwent clinical examination and were graded according to the Clinical-Etiological-Anatomical- Pathophysiological (CEAP) classification. Duplex ultrasonography was performed to establish the presence of retrograde flow >0.5 s in either deep or superficial veins. At initial recruitment, 5 of 73 patients had mild symptoms in the contralateral limb and 56 patients (77%) had no evidence of venous disease, 21% of patients were graded as C1, and 4 patients (5%) were graded as C2. A total of 12 limbs had isolated superficial reflux, and 6 limbs had deep reflux on duplex ultrasonography. At 5 years, 48 new sites of reflux were found in 38 limbs, most frequently in the superficial veins; however, 6 limbs developed deep venous reflux. In the limbs where reflux had propagated, this was antegrade in 10 limbs and retrograde in 8. In the majority of patients, changes in detectable reflux were associated with clinical changes, with 5 limbs progressing from C0 to C1, 5 from C0 to C2, 2 from C0 to C3, and 5 from C0 to C2/3. Progression from C1 to C2 was observed in 6 limbs, while 3 limbs progressed from C1 to C3, and 2 from C2 to C3. There were 2 patients who were initially classified as C2 who developed C4 skin changes. The study found that the progression of CVDwas significantly affected by orthostatism and obesity, and that progression was reduced to some extent with the use of elastic compression stockings. Conversely, parity and estrogen treatments were not associated with progression of CVD. Overall, approximately a third of limbs with mild or asymptomatic reflux developed clinical signs over 5 years. This is similar to the 27% reported by Labropoulos.17
The progressions of primary and secondary venous disorders are noticeably different. A prospective comparison of the rate of progression of primary and secondary venous disease was performed over a 5 year period in 41 patients with a proximal DVT (group A), compared with a cohort of 41 patients with primary venous disease (group B) and followed up at 5 years with duplex ultrasonography. These were also compared with 15 control cases with no evidence of venous disease (group C). At 5 years, CEAP scores for the 3 groups were as follows: group A, C0 n=6, C1 n=0, C2 n=0, C3 n=29, C4 n=8 C5 n=1, C6 n=2; group B, C0/C1 n=0, C2 n=29, C3 n=18, C4 n=3, C5/C6 n=0; group C, C0 n=25, C1 n=3, C2 n=2 C3/C4/C5/C6 n=0. All patients were encouraged to wear compression stockings with 30 to 40mm Hg of graduated compression. Results confirmed that patients with a previous history of DVT developed significantly more skin changes compared with those with primary venous disease (P=0.019), and those with no venous disease (P<0.01) and that the progression was more rapid in patients with a previous history of DVT. Skin changes also occurred significantly more frequently in patients with combined reflux and venous obstruction (P=0.12).22
Data from this cohort study included 1566 randomly selected adults between 18 and 64 years of age who were examined at baseline and then at 13 years as part of the Edinburgh Vein Study. Recorded measurements included a questionnaire of lifestyle factors, CEAP grade, and duplex ultrasonography. Of the 1566 patients, 880 were followed up at 13 years. A total of 325 patients had truncal varicosities at baseline, and at 13 years, 154 patients had deterioration in their varicosities (47.4%) while 62 had stayed the same (19.1%). Of the 555 patients with no truncal varices at baseline, 101 developed C2 varices during the 13-year follow-up. The annual incidence of developing trunk varices was 1.35%, the rate of disease progression was 3.54% per annum, and the number of patients with unilateral disease who developed bilateral disease was 25.3%. A total of 109 patients showed improvement (33.5%), of which 16.6% had undergone surgery or sclerotherapy. Patients who had not undergone treatment were all reported to have mild disease and differences in grade may be attributable to interobserver variability.
LONGITUDINAL STUDIES EVALUATING VENOUS HEMODYNAMICS
The Bochum study surveyed 73 pupils over a period of 9 years at the beginning, middle, and end of their schooling. A fourth survey was also performed at 11 years. Pupils underwent clinical examination, duplex ultrasonography, and digital PPG and were classified according to the CEAP classification. Overall venous refill times appeared to lengthen from childhood to adulthood; this was thought to be due to maturation of the venous calf pump during adolescence. Interestingly, no clinical deterioration was observed in this cohort.24
LONGITUDINAL STUDIES EVALUATING THE EFFECTS OF INTERVENTION ON DISEASE PROGRESSION
This study evaluated 195 limbs in 183 patients with primary chronic venous insufficiency and reflux affecting the femoral vein, the SFJ, and the GSV.25 Venous ulceration lasting for more than 6 months but less than 3 years was reported in 99 limbs. Patients underwent ascending and descending venograms and duplex scanning, and were randomized to receive one of three treatment options including elastic compression hosiery (n=68), surgical treatment of venous insufficiency/ saphenectomy (n=75), or deep vein reconstruction/ valvuloplasty with saphenectomy (n=52). Clinical results were recorded based on the clinical severity scoring system recommended by the subcommittee on the reporting standards for venous disease.26 Over a mean follow-up period of 6.2 years, no significant difference was observed between the results of the three interventions in patients who had a disease duration of less than 5 years. However, in patients with a disease history of greater than 5 years, the results of valvuloplasty were significantly better, and the incidence of recurrentvaricosities was significantly lower after valvuloplasty in comparison with superficial vein repair alone. Recurrent varicosities were observed in 18 patients (14.2%). The authors concluded that valvuloplasty significantly improved the results of superficial venous surgery and that successful treatment was associated with an improvement in valvular function.25
LONGITUDINAL STUDIES EVALUATING RISK FACTORS AND THE DEVELOPMENT OF VENOUS ULCERATION
It is known that disease progression is related to the severity of venous reflux and duration of disease.27 The sensation of leg swelling in otherwise mild disease was found to be an indicator of likely disease progression and poorer prognosis.28 The number of patients with superficial reflux who are likely to progress to edema, skin changes, lipodermatosclerosis, and venous ulceration is unknown; however, the overall incidence of edema and skin changes in the general UK population is thought to be approximately 1% per year.27 The incidence of venous ulceration is 1% in the UK and the majority of those presenting with venous ulceration have had venous disease for more than 20 years.15 29
A retrospective population cohort study was conducted in 1131 patients, including 263 patients who had venous ulceration over a 25-year period, to evaluate the incidence of venous stasis syndrome and venous ulceration. The incidence of venous stasis was 76.1 per 100 000 person-years and the incidence of venous ulceration was 18 per 100 000 person-years. Of the 945 patients who had venous reflux alone and no previous history of ulceration, 60 (6.3%) developed venous ulceration and the mean (±SD) time from the diagnosis of venous stasis to the development of a venous ulcer was 5 (±5) years with a range of 14 days to 24 years.30 Venous ulceration increased with age in linear fashion and was higher in women than men.
A SUMMARY OF THE CLINICAL EVIDENCE ON THE DEVELOPMENT AND PROGRESSION OF VENOUS DISEASE
A number of longitudinal studies have reported the clinical development and progression of venous disease. Regarding the development of venous disease in asymptomatic patients (ie, patients not experiencing any symptomatic discomfort), Kostas et al reported results over a 5-year period where 22% of patients developed new C1 disease, 31% developed new C2 disease, and 27% developed new C3 disease, while Sarin et al reported that 25% of patients on a waiting list developed new varicosities over a median of 20 months and 1 patient (3%) developed lipodermatosclerosis. Regarding the progression of venous disease in patients with early clinical stages, Kostas et al reported that 3% of patients progressed from C2 to C4 disease.21 In the Bonn Vein Study II, the rate of clinical progression from C2 to higher C stages was 31.8% in patients with saphenous reflux and 19.8% in those with nonsaphenous reflux over an average of 6.6 years.18 Data from the Edinburgh Vein study suggested that 47.4% of patients with truncal varicosities showed clinical deterioration over a 13-year period and the rate of disease progression was 3.54% per annum.23 Brewster et al noted that 22% of patients reported skin changes while waiting an average of 4 years for treatment of superficial venous disease. Labropoulos et al reported 7 cases of progression from C2 to C3, 4 cases of progression from C3 to C4, and 2 cases of venous ulceration (C4 to C6) over a period of 43 months.
A SUMMARY OF THE ANATOMICAL AND HEMODYNAMIC EVIDENCE FOR THE PROGRESSION OF VENOUS DISEASE
Although the presence of superficial venous reflux and abnormal venous hemodynamics are associated with symptoms, the degree of symptoms reported and the severity of venous disease frequently correlate poorly with quantitative anatomical or hemodynamic findings. Therefore, it is difficult to interpret the relevance of the observed anatomical progression of venous reflux demonstrated on duplex ultrasonography or the deterioration in hemodynamic function. Nevertheless, the evidence is summarized below.
Sarin et al reported that 27% of patients developed sites of new reflux in limbs where venous reflux previously existed and 25% of normal contralateral limbs had developed new reflux over this time, although no significant deterioration in venous refill times were observed. Labropoulos et al observed that over a total of 43 months, 23.3% of limbs had changes on duplexultrasonography and an extension of existing reflux was documented in 14.7% of limbs. Kostas et al reported 48 new sites of reflux in 38 limbs at 5 years (52%) and 6 limbs developed deep venous reflux compared with baseline.
A SUMMARY OF THE FACTORS AFFECTING THE PROGRESSION OF VENOUS DISEASE
Although the etiology of venous disease is incompletely understood, a number of factors appear to be related to the progression of venous disease. There is good evidence that obesity and arterial hypertension28 significantly affect disease progression. In addition, there is evidence that prolonged standing increases the rate of disease progression, and that the use of elastic compression hosiery may reduce disease progression.21 The presence of deep venous reflux, a past history of venous thromboembolism, and the presence of lipodermatosclerosis, corona phlebectatica, or varicose eczema are associated with an increased risk of developing venous ulceration.31 In patients with secondary venous disease following acute deep venous thrombosis, the natural history of the disease is better understood. Studies suggest that approximately 30% of patients will go on to develop postthrombotic syndrome, with 3% to 6% developing venous ulceration,32 and that disease progression occurs more rapidly than in those with primary venous disorders.22
Improving the diagnosis and treatment of patients with venous ulceration has been shown to significantly improve patient outcomes. Evidence from a Swedish study confirmed that through education and a coordinated multidisciplinary approach—including early diagnosis, thorough investigation, and early use of superficial venous surgery—venous ulceration was reduced by 46% between 2002 and 2005.33
PRACTICAL GUIDANCE
Based on the available evidence, the Society of Vascular Surgery and the American Venous Forum have produced practical guidance regarding the management of primary venous disorders.34 According to this guidance, there is weak evidence for compression hosiery for patients with symptomatic varicose veins and it is not recommended as the primary treatment if apatient is a candidate for saphenous vein ablation. Compression treatment is recommended for patients with primary venous ulceration, with ablation of superficial reflux to reduce the risk of ulcer recurrence. Thermal ablation (radiofrequency or laser) is recommended for the treatment of great saphenous vein reflux in preference to high ligation and stripping, although foam sclerotherapy is also suggested as a treatment option. The treatment of tributaries with phlebectomy or foam sclerotherapy is also recommended. The routine treatment of perforating veins in C2 patients is not supported, although the selective treatment of perforators in patients with ulceration is suggested.
CONCLUSION
Chronic venous insufficiency is a complex disorder with an incompletely understood multifactorial etiology. The majority of patients presenting with venous disorders request treatment, so the natural history of the disorder is difficult to evaluate. At present there is a lack of published evidence from longitudinal studies that have evaluated the natural history of disease progression. Combining data from different studies is difficult due to the heterogeneity of the outcome measures reported in different studies and a lack of understanding of the relationship between them. However, there is promising data from large long-term studies, including the Bonn Vein and Edinburgh Vein studies, which are likely to allow a better understanding of disease progression in the future. Nevertheless, based on the data currently available in the published literature, it could be suggested that in patients with uncomplicated varicose veins, disease progression to higher C stages is likely to be somewhere between 3.5% and 7% per annum15-17,21,28,35 and is subject to a number of patient and environmental factors. The development of venous ulceration usually occurs in patients who have had venous disease for over 20 years, and skin changes and deep venous incompetence are associated with a significantly higher risk of venous ulceration. The rate of progression from C4 disease in patients with skin changes to venous ulceration is unknown, but based on the available evidence, it is estimated to be in the in the region of 1% to 2% per annum.15,17,18,25 These figures become highly important when considering the prevalence of venous disease. A recent study evaluating the societal cost of C4- C6 disease in European countries and the USA confirms that the costs run to hundreds of millions of euros each year for the treatment of superficial reflux, the treatment of venous ulcers, and the cost of days lost from work due to venous disorders.36 A better understanding of the rate of progression of venous disease, and the ability to identify patients at risk of venous ulceration will help with the allocation of healthcare resources and ensure the appropriate management of patients with moderate venous disorders, and, therefore, further data from ongoing longitudinal studies is awaited

REFERENCES
1. Callam MJ. Epidemiology of varicose veins. Br J Surg. 1994;81(2):167-173.
2. Evans CJ, Fowkes FG, Ruckley CV, et al. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53(3):149-153.
3. van den Bremer J, Moll FL. Historical overview of varicose vein surgery. Ann Vasc Surg. 2010;24(3):426-432.
4. Edwards AG, Baynham S, Lees T, et al. Management of varicose veins: a survey of current practice by members of the Vascular Society of Great Britain and Ireland. Ann R Coll Surg Engl. 2009;91(1):77-80.
5. Goodwin R, Ding K, Seymour L, et al. Treatment-emergent hypertension and outcomes in patients with advanced non-small-cell lung cancer receiving chemotherapy with or without the vascular endothelial growth factor receptor inhibitor cediranib: NCIC Clinical Trials Group Study BR24. Ann Oncol. 2010;21(11):2220-2226.
6. Van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49(1):230-239.
7. Winterborn RJ, Corbett CR. Treatment of varicose veins: the present and the future—a questionnaire survey. Ann R Coll Surg Engl 2008;90(7):561-564.
8. Labropoulos N, Giannoukas AD, Delis K, et al. Where does venous reflux start? J Vasc Surg. 1997;26(5):736-742.
9. Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23(2):85-98.
10. Evans CJ, Allan PL, Lee AJ, et al. Prevalence of venous reflux in the general population on duplex scanning: the Edinburgh vein study. J Vasc Surg. 1998;28(5):767-776.
11. Shepherd AC, Gohel MS, Lim CS, et al. A study to compare disease-specific quality of life with clinical anatomical and hemodynamic assessments in patients with varicose veins. J Vasc Surg. 2010;53(2):374-382.
12. Gohel MS, Barwell JR, Earnshaw JJ, et al. Randomized clinical trial of compression plus surgery versus compression alone in chronic venous ulceration (ESCHAR study)— haemodynamic and anatomical changes. Br J Surg. 2005;92(3):291-297.
13. Gohel MS, Epstein DM, Davies AH. Cost-effectiveness of traditional and endovenous treatments for varicose veins. Br J Surg. 2010;97(12):1815- 1823.
14. Brand FN, Dannenberg AN, Abbott R, D KW. The Epidemiology of Varicose Veins:The Framingham Study. Am J Prev Med. 1988;4(2):96-101.
15. Brewster SF, Nicholson S, Farndon JR. The varicose vein waiting list: results of a validation exercise. Ann R Coll Surg Engl. 1991;73(4):223-226.
16. Sarin S, Shields DA, Farrah J, et al. Does venous function deteriorate in patients waiting for varicose vein surgery? J R Soc Med. 1993;86(1):21-23.
17. Labropoulos N, Leon L, Kwon S, et al. Study of the venous reflux progression. J Vasc Surg. 2005;41(2):291-295.
18. Rabe E, Pannier F, Ko A, et al. Incidence of Varicose Veins, Chronic Venous Insufficiency, and Progression of Disease in the Bonn vein Study II. J Vasc Surg. 2010;51(3):791.
19. Pannier F, Rabe E. Progression of Chronic Venous Disorders-Results from the Bonn Vein Study. Abstract presented at: American Venous Forum, 23rd annual meeting; 2011; San Diego, CA.
20. Maurins U, Hoffmann BH, Losch C, et al. Distribution and prevalence of reflux in the superficial and deep venous system in the general population— results from the Bonn Vein Study, Germany. J Vasc Surg. 2008;48(3):680- 687.
21. Kostas TI, Ioannou CV, Drygiannakis I, et al. Chronic venous disease progression and modification of predisposing factors. J Vasc Surg. 2010;51(4):900-907.
22. Labropoulos N, Gasparis AP, Pefanis D, et al. Secondary chronic venous disease progresses faster than primary. J Vasc Surg. 2009;49(3):704-710.
23. Robertson L, Boghossian S, Evans C, et al. Incidence and Risk Factors for Development of Varicose Veins in the General Population: Edinburgh Vein Study. Abstract presented at: American Venous Forum, 23rd annual meeting; 2011; San Diego, CA.
24. Stucker M, Reich S, Robak-Pawelczyk B, et al. Changes in venous refilling time from childhood to adulthood in subjects with apparently normal veins. J Vasc Surg. 2005;41(2):296-302.
25. Lurie F, Makarova NP. Clinical Dynamics of Varicose Disease in Patients with High Degree of Venous Reflux During Conservative Treatment and After Surgery: 7-Year Follow-Up. Int J Angiol 1998;7(3):234-237.
26. Reporting standards in venous disease. Prepared by the Subcommittee on Reporting Standards in Venous Disease, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1988;8(2):172-181.
27. Tran NT, Meissner MH. The epidemiology, pathophysiology, and natural history of chronic venous disease. Semin Vasc Surg. 2002;15(1):5- 12.
28. Pannier F, Rabe E. Progression of Chronic Venous Disorders-Results from the Bonn Vein Study. Abstract presented at: American Venous Forum, 23rd annual meeting; 2011; San Diego, CA.
29. Hoare MC, Nicolaides AN, Miles CR, et al. The role of primary varicose veins in venous ulceration. Surgery. 1982;92(3):450-453.
30. Heit JA, Rooke TW, Silverstein MD, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25- year population-based study. J Vasc Surg. 2001;33(5):1022-1027.
31. Robertson L, Lee AJ, Gallagher K, et al. Risk factors for chronic ulceration in patients with varicose veins: a case control study. J Vasc Surg. 2009;49(6):1490-1498.
32. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7.
33. Nelzén O. The Pacific Vascular Symposium 6 Extended abstracts. J Vasc Surg 2010;52(14 S):39S-44S.
34. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011;53(suppl 5):2S-48S.
35. Boghossain S, Robertson L, Evans C, et al. Deterioration in Trunk Varicosisties in the General Population Over a 13 year period: Edinburgh Vein Study. Abstract presented at: American Venous Forum, 23rd annual meeting, 2011; San Diego, CA.
36. Rabe E, Pannier F. Societal costs of chronic venous disease in CEAP C4, C5, C6 disease. Phlebology 2011;25(suppl 1):64-67.
