The role of MPFF at a dose of 500 mg in the treatment of symptomatic patients with varicose veins
One of several theories put forward as a possible cause of varicose veins (VV) is primary valve dysfunction, which can be congenital or acquired. The role of inflammation in acquired valve dysfunction is predominant and could possibly explain varicose vein etiology.1
THE ROLE OF VARICOSE VEINS IN THE
EVOLUTION OF CHRONIC VENOUS DISEASE
Varicose disease represents the C2 class in the CEAP classification.2 Varicose disease is not the cause of severe morbidity per se (indeed some have seen VV as a simple cosmetic problem). Nevertheless, the complications varicose veins may cause are mainly inflammatory. This may lead to superficial thrombophlebitis and venous ulcers.1
Varicose veins:
the first visible sign of chronic venous disease
Varicose veins are one of the commonest manifestations of chronic venous disease (CVD), occurring in 26% to 38% of women and to 10% to 20% of men.3 The condition starts early in life, as 10% of the schoolchildren in the Bochum study, aged between 10 and 12 years, had slight varicose veins.4
The venous valves play a key role in the genesis of VV, and the detection of reflux usually precedes the clinical appearance of the varicose disease as shown in the children of Bochum. At 10 years, reflux was present in 12%, and there were no VV in these children. At age 30, incidence of reflux increased to 26%, and VV appeared in 12% of the sample,5 while telangiectases appeared later (0% at 10 and 50% at 30).
Varicose veins:
leading to further complications
Varicose veins are likely to increase with age. The prevalence of VV was 10% in 10-year-old Bochum schoolchildren (studies I to IV), and this further increased to 75% at the age of 30. It has now been demonstrated that VV lead to further complications, including leg ulceration. (Figure 1) In the 11-year follow-up of the chemical workers in the Basle study, the prevalence of venous ulcers was related to the severity of varicose veins.
Twenty percent of subjects with severe varicose veins developed ulceration compared with only 0.8% of those with mild varicose veins.6
Superficial thrombophlebitis is thought to be one of the possible complications of VV.7 (Figure 1)
The cause of superficial thrombophlebitis in patients with varicose veins has been postulated as being inflammatory cell infiltration secondary to denudation of the endothelium.1,8
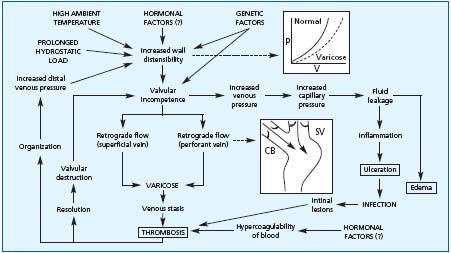
Figure 1.
Complications
of varicose veins
(adapted from
reference 7)
Varicose veins:
often associated with symptoms
Despite the fact that the mechanisms causing symptoms have not been fully elucidated, it is widely reported that symptoms appear at the onset of venous insufficiency, and most of time, long before reflux and the appearance of varicose veins. The CEAP classification contains a C0S clinical class to include those patients with venous symptoms but without obvious signs of venous insufficiency. It is thought that pain related to CVD might mirror the endothelial activation with the cascade of inflammatory mediators released early in the disease process.
A recent cross-sectional study in patients with CVD (C0s to C6) showed that the percentage of patients presenting with any venous symptom significantly increased with CEAP C class.9 In this study, the prevalence of symptoms was 84.6% in patients with varicose veins.
Varicose veins:
the cause of quality of life impairment
Varicose veins affect the quality of life (QoL) not only by the real cosmetic problem they cause but also by the influence they may have on physical disability. In the San Diego study,10 spider veins and varicose veins were found to have a significant effect on the physical component scores of the generic QoL scale SF 36.
In a study of the relationship between signs, symptoms, and quality of life in patients with chronic venous disease, it was demonstrated that symptoms usually attributed to CVD are the factors with the most negative influence on the patient’s QoL.11 In the VEINES study,12 QoL scores decreased in C2 patients but were close to these in C1or C0s. This was attributable to the presence of symptoms that are most often associated with VV.
These previous studies showed the negative impact VV may have on the QoL of patients, most probably related to the presence of the associated symptoms. This has important consequences on the daily life of patients with CVD, even right from the onset of the disease, and long before the occurrence of reflux and VV.
OBJECTIVE OF TREATMENT OF CVD
Bearing in mind that varicose disease is most often painful and that it is likely to progressively lead to severe complications, there is a twin goal to be achieved by any treatment of VV:
– first of all, to rapidly and powerfully relieve patients from symptoms and pain in order to help them recover a better quality of life.
– secondly, to protect VV patients from further complications. For this reason, the biochemical processes involved in the CVD development are the main target of pharmacological treatment, since they are theoretically modifiable by systemic therapy.
How does MPFF at a dose of 500 mg act in the prevention
of CVD development?
MPFF at a dose of 500 mg: an ability to inhibit leukocyte
activation
It has been observed that firm leukocyte attachment to the endothelial wall and subsequent migration of leukocytes into the interstitium is a mechanism of tissue damage during inflammation, and that attenuation of this phenomenon during ischemia-reperfusion could explain the positive effects of MPFF at a dose of 500 mg on clinical edema.13,14 In an ischemiareperfusion model by Korthuis,15 MPFF at a dose of 500 mg significantly inhibited leukocyte adhesion and migration through the venous endothelium as well as the protein leakage observed in this model. During restoration of venous blood flow, the number of rolling, adherent, and migrating leukocytes as well as cells exhibiting apoptosis in the parenchyma significantly decreased in animals pretreated with MPFF at a dose of 500 mg compared with control subjects.
The molecular mechanisms in leukocyte adhesion and activation in CVD patients involve the increased expression of several types of cell adhesion molecules at the leukocyte surface, particularly L-selectins ( CD62L) and integrins (CD11b). The expression of these leukocyte adhesion molecules were substantially decreased on monocytes and neutrophils after a 60-day treatment with MPFF at a dose of 500 mg in patients with CVD (C2 to C4).16 (Figure 2) This implies that MPFF at a dose of 500 mg would prevent the inflammatory process in CVD.
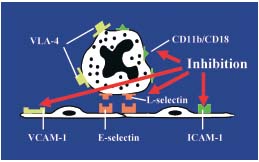
Figure 2. Inhibition of the leukocyte activation by MPFF at a dose of 500 mg
(adapted from reference 16)
MPFF at a dose of 500 mg: an action at the core of the
disease, ie, the leukocyte-endothelium
interaction.
In patients with CVI as well as after prolonged standing, plasma concentrations of endothelial adhesion molecules, vascular cell adhesion molecule (VCAM) and interstitial cell adhesion molecule (ICAM) are increased. In the study by Shoab,16 plasma level of VCAM-1 and ICAM-1 significantly decreased in C2 to C4 patients pretreated with MPFF at a dose of 500 mg. This reflects the ability of MPFF at a dose of 500 mg to prevent the interaction between the endothelium and the leukocytes which is at the core of the CVD progression.
MPFF at a dose of 500 mg: a protective effect against valve
damage
Acquired valve dysfunction can occur due to inflammation, as evidenced by monocyte infiltration.17 It seems possible that activated leukocytes can migrate into the endothelium of proximal surfaces of the vein valves as well as proximal vein walls and promote destruction of supporting structures and remodeling of the valves with consequent valvular insufficiency. Immunohistochemical studies have demonstrated monocyte/macrophage infiltration into the valve leaflets and venous wall of patients with varicose veins.17
In a recent study by Schmid Schönbein et al, performed on a pharmacological model of hypertension,18 MPFF at a dose of 500 mg was shown to limit the leukocyte infiltration into the vein valve and to inhibit the expression of leukocyte adhesion molecules.
As a result, MPFF at a dose of 500 mg attenuated the vein and valve destruction and significantly reduced the reflux rate in a dose-dependent manner. By acting at the core of the disease, ie, the leukocyteendothelium interaction, MPFF at a dose of 500 mg is the only phlebotropic drug with a protective effect on the valve endothelium. MPFF at a dose of 500 mg delays the reflux appearance and is thus likely to prevent the evolution of the CVD towards complications.
MPFF at a dose of 500 mg: a therapeutic efficacy
right from the first symptoms and in the longterm:
Effects of MPFF at a dose of 500 mg on symptoms of CVD
In the Reflux assEssment and quality of lIfe improvEment, with micronized Flavonoids (RELIEF) study (n=4527, intention-to-treat population), patients receiving 2 tablets of MPFF at a dose of 500 mg daily showed progressive improvement in the symptoms of CVI.19 After 6 months, patients in the per-protocol population showed significant improvement from baseline in the study outcome measures (ankle circumference, pain, leg heaviness, cramps, sensation of swelling; P <0.012). In the 1-year trial of 2 tablets of MPFF at a dose of 500 mg daily in 170 patients,20 a significant reduction from baseline values in physician-assessed clinical symptoms (functional discomfort, cramps, and evening edema), ankle and calf circumference, and patient overall assessment of symptom severity was demonstrated at each 2-month evaluation (P <0.001). The rapid reductions observed during the first 2 months of treatment represented approximately 50% of the total improvement ultimately observed after 1 year of treatment. Continuing improvement in all parameters, albeit less rapid, was reported at each time point from month 2 to month 12.
Effects of MPFF at a dose of 500 mg on leg edema
The reduction in leg edema with MPFF at a dose of 500 mg, 2 tablets daily, was demonstrated in the Blume study in which the reduction was assessed by volumetric measurements after 6 weeks of treatment.21 In this study, the reduction in the mean volume was 263 mL (8%) in all patients, and was 392 mL (12%) in patients with leg edema associated with varicose veins. In both trials, the reduction in leg volume was highly significant (P <0.001).
Edema, measured by leg circumference, significantly decreased compared with baseline in patients in the RELIEF study.19
MPFF at a dose of 500 mg: improves QoL by reducing
symptoms
During the course of a 6-month period of treatment with MPFF at a dose of 500 mg, changes in the QoL scores were comparable across the different CEAP subgroups.22 Only patients with symptoms had greater improvement in QoL than patients without symptoms. These changes in QoL scores resulted mainly from the alleviation of symptoms: improvement between D180 vs D0 in pain, heaviness, swelling and cramps was significant (P <0.001 for all symptoms).11 However, the associated signs (telangiectases, varices, edema, or skin changes) did not show such a direct impact on QoL.19
In this study, the dramatic improvement of QoL observed in patients treated with MPFF at a dose of 500 mg can be interpreted as a result of symptom alleviation.
MPFF at a dose of 500 mg: its therapeutic value in leg
ulcer healing
The preliminary results of a meta-analysis of five comparable, prospective, randomized, controlled studies identified from medical literature databases and from the files of the manufacturer in which 723 patients were pooled23 confirm that venous ulcer healing is accelerated by adding MPFF at a dose of 500 mg, 2 tablets daily, to conventional treatments: the rate of complete ulcer healing at 6 months was in favor of the MPFF at a dose of 500 mg group (61.3% vs 47.7%; OR =2.02; CI =1.05-3.89; P =0.035).
CONCLUSION
Pharmacological attenuation of leukocyte activation and leukocyte-endothelium interactions by MPFF at a dose of 500 mg at the level of the venous valve, venous wall, and the microcirculation is an explanation of facilitation it brings to ulcer healing as well as the improvement in symptoms it allows. MPFF at a dose of 500 mg also shows promise in preventing the continuing deterioration of venous function produced by valve insufficiency.13
REFERENCES
2. Porter JM, Moneta GL. International Consensus Committee on chronic venous disease. Reporting standards in venous disease: an update. J Vasc Surg. 1995;21:635-645.
3. International Task Force. The management of chronic venous disorders of the leg: an evidence-based report of an international task force. Phlebology. 1999;14(Suppl.1): 23-34.
4. Schultz-Ehrenburg U, Weindorf N,Matthes-U, et al. Epidemiological study on the pathogenesis of varicose veins (Bochum I-III) [in French]. Phlébologie. 1992;45:497-500.
5. Schultz-Ehrenburg U, Reich S, Robak- Pawelczyk B, Altmeyer P, Stücker M. Prospective epidemiological study of developing varicose veins over a period of two decades (Bochum I-IV). Abstract presented at the 2003 UIP World Chapter Meeting. August 27-31, 2003, in San Diego, California, USA.
6. Widmer LK, Holz D, Morselli B, Zbinden O, et al. Progression of varicose veins in 11 years. Observations on 1441 working persons of the Basle Study. Unpublished paper. Basle: Angiology Division of University Dpt of Medicine, 1992. Results summarized in: International Task Force: the management of chronic venous disorders of the leg. Phlebology. 1999;14(Suppl. 1):23-34.
7. Vanhoutte PM, Corcaud S, de Montrion C. Venous disease: from pathophysiology to quality of life. Angiology. 1997;48:559-567.
8. Wali MA, Eid RA. Intimal changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2002;38:63-74.
9. Carpentier PH, Cornu-Thénard A, Uhl JF, Partsch H, Antignani PL. Appraisal of the information content of the C classes of CEAP clinical classification of chronic venous disorders: a multicenter evaluation of 872 patients. J Vasc Surg. 2003;37:827-833.
10. Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37:1047-1053.
11. Perrin M, Arnould B, Regnault A, Launois R. Relationship between symptoms, signs, reflux, and quality of life in patients with chronic venous disease: a multicentre study. Oral presentation at the 16th Annual Meeting of the American Venous Forum (AVF), Orlando, USA, February 26- 29, 2004.
12. Kurz X, Lamping DL, Baccaglini U, Zuccarelli F, Spreafico G, Abenhain L, and the VEINES Study Group. Do varicose veins affect quality of life? Results of an international population-based study. J Vasc Surg. 2001;34:641-648.
13. Bergan JJ, Schmid Schönbein G, Takase S. Therapeutic approach to chronic venous insufficiency and its complications: place of MPFF at a dose of 500 mg. Angiology. 2001;Suppl. 1:S43-S47.
14. Friesenecker B, Tsai AG, Intaglietta M. Cellular basis of inflammation, edema and the activity of MPFF at a dose of 500 mg. Int J Microcirc Clin Exp. 1995;15(Suppl. 1):17-21.
15. Korthuis RJ, Gute DC. Adhesion molecule expression in postischemic microvascular dysfunction: activity of a micronized purified flavonoid fraction. J Vasc Res. 1999;36(Suppl. 1):15-23.
16. Shoab SS, Porter JB, Scurr JH, et al. Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease-a pilot study. J Vasc Surg. 2000;31:456-461.
17. Ono T, Bergan JJ, Schmid-Schönbein GN, Takase S. Monocyte infiltration into venous valves. J Vasc Surg. 1998;27:158-166.
18. Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schönbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. In press.
19. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids. Angiology. 2002;53:245-256.
20. Guillot B, Guilhou JJ, de Champvallins M, et al. A long term treatment with a venotropic drug: results on efficacy and safety of MPFF at a dose of 500 mg in chronic venous insufficiency. Int Angiol. 1989;8 (Suppl. 4):67-71.
21. Blume J, Langenbahn H, de Champvallins M. Quantification of edema using the volometer technique; therapeutic application of MPFF at a dose of 500 mg in chronic venous insufficiency. Phlebology. 1992;7(Suppl. 2):37-40.
22. Arnould B, Regnault A, Perrin M. Change in the quality of life in patients with chronic venous disease: results of a 6-month study using MPFF at a dose of 500 mg. European Venous Forum abstracts, June 25-27, 2004. Phlebology. 2004;19:2.
23. Ramelet AA, Coleridge Smith PD, Gloviczki P. Factors affecting venous leg ulcer healing. Abstracts of the 21st World Congress of the International Union of Angiology. Int Angiol.2004;23 (Suppl.1):158.
