The role of matrix metalloproteinases (MMPs) and their inhibitors in venous leg ulcer healing
University Hospital,
Freiburg, Germany
SUMMARY
Different hypotheses have been postulated to describe the cause of venous leg ulceration including the “fibrin cuff hypothesis” with pressure-damaged capillary vessels and the “white cell trapping hypothesis” based on accumulated leukocytes. To date, several studies have revealed the important role of matrix metalloproteinases (MMPs) in the process of venous leg ulcer formation and contributed significantly to better understanding of the pathogenesis of venous leg ulcers. MMPs, also known as matrixins, hydrolyze components of the extracellular matrix. Currently over 20 MMP genes have been identified in humans, and most are multidomain proteins. These enzymes had been shown to generate an epidermal and dermal skin defect in lipodermatosclerosis, the stage preceding venous leg ulcers. In addition, misregulation of MMP activity and TIMP-mediated counterregulation in the wound fluid and wound bed contribute to impaired and prolonged wound healing of venous leg ulcers. However, there is also accumulating evidence indicating that several distinct matrixins are also repair-associated and not only prolonging factors. Therefore, the role of matrixins during leg ulcer healing has to be regarded in a differentiated way, and has to consider spatial and temporal factors. This review describes different members of the matrixin family and discusses substrate specificity, domain structure and function, the activation of proMMPs, the regulation of matrixin activity by tissue inhibitors of metalloproteinases (TIMPs), and their pathophysiological implication in severe stages of chronic venous insufficiency.
INTRODUCTION
Proteolytic degradation of the extracellular matrix (ECM) is an important process during venous ulcer formation and healing. Several investigations have provided evidence that matrix metalloproteinases (MMPs), collectively called matrixins, participate strongly in different stages of the ulcerative process, from their formation with the initial epithelial defect until ulcer resolution and repair. The ECM is important for creating the cellular environments required during morphogenesis. MMPs are proteinases that participate in ECM degradation.1,2
Under physiological conditions, the activities of MMPs are precisely regulated at the level of transcription, activation of the precursor zymogens, interaction with specific ECM components, and inhibition by endogenous inhibitors.1,2 It was postulated that a loss of activity control may result in different diseases such as arthritis, atherosclerosis, nephritis, aneurysms, fibrosis, and cancer.3 Specific inhibitors of matrixins are tissue inhibitors of metalloproteinases (TIMPs) that participate in controlling the local activities of MMPs.4,5 The pathological effects of MMPs in chronic venous insufficiency that involve vascular remodeling, and skin tissue instability, as well as inflammatory processes, are of major interest. In the following review, we give an overview of the structure, function, and biochemistry of MMPs and their inhibitors, and provide insight into the pathogenic role of MMPs in lipodermatosclerosis, during the wound healing process of venous leg ulcers, and into modalities to influence their proteolytic properties.
Causes of venous leg ulcers – different hypotheses
Chronic venous insufficiency is associated with venous hypertension, leading, in complicated cases, to venous leg ulceration. The main histological features of advanced stages of chronic venous insufficiency such as lipodermatosclerosis are loss of papillary structures in the dermalepidermal junction zone, dermal pericapillary fibrin cuffs, and fibrosis of the reticular dermis, whereas venous ulcers are characterized by total loss of epidermal and partly dermal cellular and matrix tissue components.6 In the “fibrin cuff hypothesis”, pressure-damaged capillary vessels are made responsible for leakage of fibrinogen.7 In contrast, the “white cell trapping hypothesis” is based on the presence of toxic metabolites and proteolytic enzymes caused by accumulated leukocytes damaging capillary vessels.8 Finally, cytokines released by leukocytes have been suggested to stimulate endothelial cells and fibroblasts to synthesize pericapillary fibrin cuff-forming molecules.6 Impairment of gas and nutrient exchange between plasma and dermis had been suggested as common features, resulting in ulcer formation. Although different hypotheses had suggested underlying factors in the pathophysiology of ulcer formation, there had been great controversy as to how increased tissue pressure may result in such drastic cellular and matrix changes of venous ulcerations. Pericapillary fibrin cuffs are complex structures containing whorls of molecules such as laminin, fibronectin, tenascin, and type I and III collagens.9 They may be synthesized in response to increased venous pressure to protect the vessels and enable them to withstand the higher pressures. Such cuffs may inhibit capillary sprouting, so limiting angiogenesis and neovascularization, as well as acting as a barrier to gases and nutrients. Degradation of the cuffs by proteases may release endothelial cells and pericytes from adhesive environments and so facilitates sprouting and angiogenesis, as well as improving tissue nutrition and oxygenation, thereby supporting wound healing of venous ulcers. However, these alterations depend on the dynamics of matrix turnover, due to proteolytic properties of specific matrix-degrading endopeptidases in advanced stages of venous insufficiency.
Extracellular matrix and its turnover
The ECM is often viewed as the “scaffolding” that supports the cellular components of various tissues. It is now recognized that in addition to their structural functions, the components of the ECM serve as modulators of cell growth and tissue differentiation. The latter results in changing dynamics between the cellular and stromal matrix elements. Changes in matrix composition require the removal of the previous extracellular components. This is accomplished through the action of proteases, which selectively degrade matrix macromolecules and may alter cell-matrix attachments.10 Matrix removal and synthesis occur simultaneously in an orderly and progressive fashion. The end stage of differentiation is a homeostasis achieved between new matrix formation and matrix turnover. Turnover of the ECM is a unique biological problem because of the high collagen content of most ECM structures and the resistance of these triple helical macromolecules to cleavage of most proteases. It is well recognized that connective tissue remodeling, either physiological or pathological, is in most cases a highly organized process that involves the selective action of MMPs, that collectively degrade major components of the ECM.11
The family of matrix metalloproteinases
MMPs play an important role in the remodeling of the ECM. Recent studies have increased the list of biological processes in which MMPs appear to be involved, and in several cases pointed to processes that do directly involve matrix remodeling. These enzymes constitute a family of several zinc-dependent endopeptidases which are expressed at low levels in normal adult tissues. They are upregulated during different normal and pathological remodeling processes such as embryonic development, tissue repair, inflammation, tumor invasion, and metastasis.12-14 MMPs are known to be proteases that can cleave collagen macromolecules, which are of significant importance in maintaining the architecture and integrity of skin. MMPs belong to a growing family of soluble and membrane-bound endopeptidases which degrade important structural proteins. MMPs generally consist of a prodomain, a catalytic domain, a hinge region, and a hemopexin domain (Figure 1). The catalytic domain, which contains the active Zn2+ and stabilizing Ca2+-binding site, is required for proteolytic activity and for membrane binding.15 Proteolytic properties of these enzymes are controlled by transcriptionally regulated protein synthesis as well as by posttranslational modification of the synthesized proteins. Most MMPs are constitutively expressed in vitro at low levels by different cell types, such as keratinocytes, fibroblasts, macrophages, endothelial cells, mast cells, eosinophils, and neutrophils.16,17
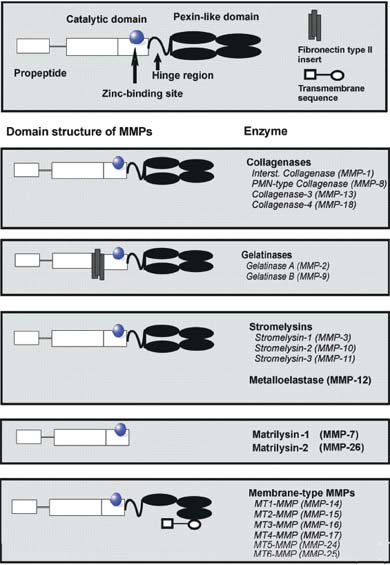
Figure 1.
The matrixins
displays major
domain structures.
Each matrix
metalloproteinase
(MMP) subtype
consists of a
propeptide, a catalytic
domain containing
the zinc-binding site,
and a hinge region
connected to four
pexin-like domains.
Gelatinases contain
fibronectin type II
repeats.
Membrane-type
(MT)-MMPs have a
transmembrane
domain.
The first MMP discovered was a collagenase in the tail of a tadpole undergoing metamorphosis. To date, different vertebrate MMPs have been identified, of which 23 are found in humans. MMPs are also found in sea urchin,18 Hydra,19 and Arabidopsis.20 The sequence homology with collagenase 1 (MMP-1), the zinc-binding motif HEXGHXXGXXH in the catalytic domain, and the cysteine switch motif PRCGXPD in the propeptide that maintains MMPs in their zymogen form (proMMP) are the signatures used to assign proteinases to this family. The only exception is MMP-23, which lacks the cysteine switch motif. However, the amino acid sequence of the catalytic domain is related strongly to MMP-1. To date, the MMP family consists of several structurally related members, which can be classified according to the primary structure and substrate specificity into distinct subgroups of collagenases, gelatinases, stromelysins, and membrane-type matrix metalloproteinase (MT-MMP).12 On the basis of substrate specificity, sequence similarity, and domain organization, vertebrate MMPs can be divided into six groups (Table I). MMP-1, MMP-8, MMP-13, and MMP-18 (Xenopus) belong to the group of collagenases. The key feature of these enzymes is their ability to cleave interstitial collagens I, II, and III at a specific site three-fourths from the N-terminus. Collagenases can also degrade a number of other ECM and non-ECM molecules.
Gelatinase A (MMP-2) and gelatinase B (MMP-9) belong to a further group named gelatinases. Gelatinases contain three repeats of type II fibronectin domain, which are inserted in the catalytic domain and bind to gelatin, collagens, as well as laminin.21 They readily degrade the denatured collagens and gelatins. MMP-2, but not MMP-9, digests type I, II, and III collagens.22,23 Although MMP-2 null mice develop without any apparent abnormality,24 mutations in human MMP-2 resulting in the absence of active enzyme are linked with an autosomal recessive form of multicentric osteolysis. This disease belongs to a rare genetic disorder that causes destruction and resorption of the affected bones.25 Stromelysin 1 (MMP-3) and stromelysin 2 (MMP-10) both have similar substrate specificities, but MMP-3 has a proteolytic efficiency higher than that of MMP-10. Besides digesting ECM components, MMP-3 activates a number of proMMPs. The action of MMP-3 on a partially processed proMMP-1 is critical for the generation of fully active MMP-1.26 MMP-11 is called stromelysin 3. The matrilysins are characterized by the lack of a hemopexin domain. Matrilysin 1 (MMP-7) and matrilysin 2 (MMP-26),27 also called endometase,28 belong in this group. Apart from ECM components, MMP-7 digests cell surface molecules such as pro-defensin, Fas-ligand, and E-cadherin. Matrilysin 2 (MMP-26) also degrades a number of ECM components. Membrane-type (MT)-MMPs have a transmembrane domain, which is constituted by a transmembrane sequence in the carboxy-terminal. They have short spanning domains and a cytoplasmic tail downstream of the hemopexin domain. To date six membrane-type MMPs (MT-MMPs) have been identified. Four membranetype MMPs are type I transmembrane proteins (MMP-14, MMP-15, MMP-16, and MMP-24), and two are glycosylphosphatidylinositol (GPI) anchored proteins (MMP-17 and MMP-25). With the exception of MT4-MMP, they are all able to activate proMMP-2. These enzymes can also degrade a number of ECM molecules, and MT1-MMP shows collagenolytic activity on type I, II, and III collagens.29 During postnatal development, MT1-MMP null mice exhibit skeletal abnormalities, since these mice probably lack collagenolytic activity.30 MT1-MMP plays an essential role in angiogenesis.31 MT5-MMP is brain-specific and is mainly expressed in the cerebellum.32 MT6-MMP (MMP-25) is expressed almost exclusively in peripheral blood leukocytes and in anaplastic astrocytomas and glioblastomas, but not in meningiomas.33,34 Seven MMPs are not classified in the above categories. Metalloelastase (MMP-12) is mainly expressed in macrophages35 and is essential for macrophage migration.36 Besides elastin, it degrades a number of other proteins.
MMP-19 was identified by cDNA cloning from liver37 and as a T-cell–derived autoantigen from patients with rheumatoid arthritis.38 Enamelysin (MMP-20), which degrades amelogenin, is located within newly formed tooth enamel. MMP-22 was first cloned from chicken fibroblasts,39 and a human homologue has been identified on the basis of EST sequences. However, the function of this enzyme is still unclear.
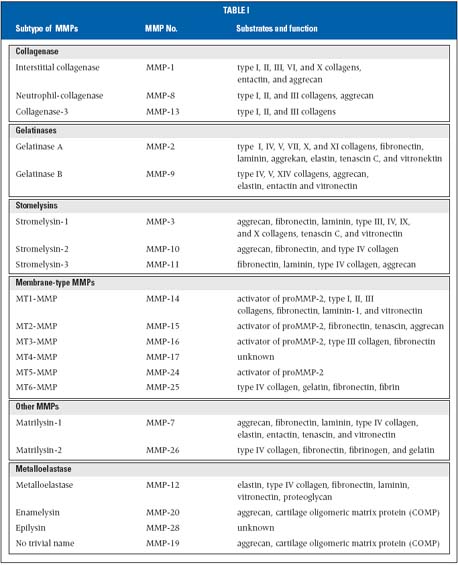
Table I. Selected substrates of different matrix metalloproteinases (MMPs). Interstitial collagenases cleave preferentially different interstitial collagen subtypes. Stromelysins digest basement membrane proteins as substrates, whereas gelatinases process primarily cleaved matrix proteins into smaller fragments.
The so-called cysteine array MMP (MMP-23) is mainly expressed in reproductive tissues.40 This enzyme lacks the cysteine switch motif in the prodomain and the hemopexin domain. Instead, it has a cysteine-rich domain followed by an immunoglobulin-like domain. Epilysin (MMP-28) is the latest addition to the MMP family, which mainly is expressed in keratinocytes.41,42 Expression patterns in intact and damaged skin suggest that MMP-28 might function in tissue hemostasis and wound repair.41-43 MMPs are induced at transcriptional level by a variety of mediators such as interleukin-1 and – 6 (IL-1 and IL-6), tumor necrosis factor á(TNF-á), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and transforming growth factor-â (TGF-â).44
Stepwise proteolytic activation
Proteolytic activation of MMPs is stepwise and it may have evolved to accommodate finer regulatory mechanisms to control destructive enzymes.45 MMPs are secreted in catalytically latent forms which are subsequently activated in the pericellular and extracellular environment. MMPs can be activated by proteinases or in vitro by chemical agents,45 such as thiol-modifying agents (HgCl2, 4- aminophenylmercuric acetate, and N-ethylmaleimide), oxidized glutathione, chaotropic agents, SDS, and reactive oxygens. There is accumulating evidence indicating the major activation step to be based on the formulated “cysteine switch hypothesis.”45 Upon activation, the Zn2+- binding site is converted to a catalytic propetide. Proteolytic attack on the propeptide domain destabilizes the Cys-Zn2+ bond and leads to a spontaneous opening of the switch. The stepwise process of MMP activation by loss of the propeptide region may be initiated by exogeneous proteolytic cleavage.46 Activation of interstitial collagenases (MMP-1), stromelysin-1 and -2 (MMP-3 and MMP-10), matrilysin (MMP-7), and gelatinase B (MMP-9) can be initiated by plasmin, kallikreins, cathepsins B or G, or by an autocatalytic process.47 Investigations of proMMP-3 activation with a mercurial compound have indicated that the initial cleavage occurs within the propeptide. This reaction seems to be intramolecular rather than intermolecular.48 The subsequent removal of the rest of the propeptide is due to intermolecular reaction of the generated intermediates.48,49 Importantly, activation of specific MMPs also occurs by the plasminogen activation system in vivo.50 Plasmin is generated from plasminogen by tissue plasminogen activator bound to fibrin and urokinase plasminogen activator bound to a specific cell surface receptor. Both plasminogen and urokinase plasminogen activator are membrane-associated, thereby creating localized proMMP activation and subsequent ECM turnover. Plasmin has been reported to activate proMMP-1, proMMP-3, proMMP-7, proMMP-9, proMMP-10, and proMMP-13.51
ProMMP-2 is not readily activated by proteinases. Activation of proMMP-2 and proMMP-13 can be initiated by a cell-bound MT-MMP-complex,52-54 which includes MT1- MMP, MT2-MMP,55 MT3-MMP,56 MT5-MMP,57,58 and MT6- MMP.33 MT4-MMP does not activate proMMP-2. The main activation of proMMP-2 takes place on the cell surface and is mediated by MT-MMPs.59 The unique aspect of this activation step is that it requires the assistance of the inhibitor TIMP-2.52,60,61 ProMMP-2 forms a tight complex with TIMP-2 through their C-terminal domains, therefore permitting the N-terminal inhibitory domain of TIMP-2 in the complex to bind to MT1-MMP on the cell surface. The cell surface–bound proMMP-2 is then activated by an MT1-MMP that is free of TIMP-2.
Inhibition of matrix metalloproteinases
Natural inhibitors of MMPs are multifunctional proteins with both MMP inhibitor activity and cell growth modulating properties. This group of related, endogenous inhibitors are known as tissue inhibitors of metalloproteinases (TIMPs). Distinct TIMP molecules have been isolated, cloned, sequenced, and characterized from several species.62-65 TIMPs are specific inhibitors that bind MMPs in a 1:1 stoichiometry, and MMPs form predominantly binary, inhibited non-covalent complexes with their TIMPs.66 At present, four TIMPs (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) have been identified in vertebrates.5 TIMPs have 12 identically conserved cysteine residues forming six disulfide bonds which confer marked stability to the molecules. TIMPs (ranging from 21 to 29 kDa) have an N-and C-terminal domain.67 The N-terminal domain folds as a separate unit and is capable of inhibiting MMPs.68 A two-domain structure of three loops each can be delineated, and it could be shown that the N-terminal three loops can fold independently and function as an efficient inhibitor of most MMPs. Under the pathological conditions associated with unbalanced MMP activities, changes in TIMP levels are considered to be important because they directly affect the level of MMP activity. TIMPs inhibit all MMPs tested so far, except that TIMP-1 fails to inhibit MT1-MMP.69 The inhibitory property of TIMP-3 is different from the rest, inasmuch as it inhibits A disintegrin and metalloproteinase (ADAM 17) (TNF-á converting enzyme, TACE),70 ADAM-10,71 ADAM-12,72 and the aggrecanases (ADAMTS-4 and ADAMTS-5).73 Another unique feature of TIMP-3 is that it binds tightly to sulfated glycosamino glycans.74 A possible role for TIMP-3 heart failure was observed with a reduction in the levels of TIMP-3, corresponding with adverse matrix remodeling in a cardiomyopathic hamster model and in the failing human heart.75
Venous ulcer formation
Lipodermatosclerosis is associated with patients suffering from venous circulatory disorders of the lower limbs. Lipodermatosclerosis is the preceding stage of venous leg ulcers, and is associated with a combination of skin changes seen in venous hypertension.76,77 Long-term inflammation results in scarring and induration of the skin, which resembles scleroderma. The woody hardening of the skin is located on the medial aspect of the leg. Within lipodermatosclerosis there can furthermore be areas of atrophie blanche, which are ivory-white sclerotic plaques. Liposclerotic lesions can surround venous ulcerations, thereby displaying a close relationship between both clinical entities. Characteristically ongoing subcutaneous fibrosis and sclerosis in lipodermatosclerosis lead to a constriction of the mid-portion of the leg. Extensive sclerosis can finally end in the affected leg resembling an inverted bottle, in contrast to lipodermatosclerosis, which shows specific histologic alterations of the epidermal and dermal layer. The main histological features of lipodermatosclerosis are loss of papillary structures in the dermalepidermal junction zone, dermal pericapillary fibrin cuffs, and fibrosis of the reticular epidermal and partly cellular and matrix tissue components.6 Venous leg ulcers, in contrast, are characterized by total loss of epidermal and partly dermal cellular matrix tissue components. In spite of many attempts to understand the underlying pathophysiology of venous ulceration, there has been no clear explanation for these major and drastic progressive tissue changes. Analysis of venous ulcer exudates revealed elevated expression and activation of different subtypes of MMPs.17,78,79 Through their ability to degrade important matrix proteins, MMPS have been considered having a major implication in maintaining venous ulceration by impairing wound healing.78,46 We showed that different subtypes of MMPs are expressed and activated in lesional skin of lipodermatosclerosis.80 Our investigations provide evidence of these enzymes having important pathogenic implications in forming venous leg ulcers. Despite collagen bundles being packed densely in lipodermatosclerosis, there is evidence of ongoing collagen digestion by proteolytic active MMP-1 and gelatinase A (MMP-2). Elevated expression on mRNA- and protein levels of MMP-1, MMP-2, and TIMP-1 can be proven in liposclerotic tissue. TIMP, which inhibits preferentially MMP-2 does not show elevated expression in comparison with controls. Currently, the mechanisms initiating the imbalance between MMP-2 and TIMP-2 in lesional skin of lipodermatosclerosis are not known. Enhanced proteolytic MMP-2 activity can be demonstrated in liposclerotic lesions. Obviously, the clinical impression and the histological findings of skin hardening with elevated collagen deposition as described previously stand in sharp contrast to the dynamic picture presented in the fibrotic stage of lipodermatosclerosis.
Consistently ongoing proteolytic processes in lipodermatosclerosis can be demonstrated by activated components of the plasminogen activation system. Earlier there had already been evidence of lipodermatosclerosis being linked to fibrinolytic abnormalities. Jarret et al showed, that patients with lipodermatosclerosis revealed increased plasma levels of D-dimer, D-monomer, and fibrin monomer.81 Apart from fibrosis of the reticular dermis, important histopathological characteristics of lipodermatosclerosis are dermal pericapillary fibrin cuffs. In spite of their well-organized structures containing (apart from fibrin) fibronectin type I and type III collagens,6 they had often been accused of being responsible for impaired gas and nutrient exchange between capillary vessels and dermis. Recently, we found that the urokinase-type plasminogen activator (uPA) and its receptor (uPAR) were highly expressed in fibrin cuffed vessels of patients with lipodermatosclerosis and with venous ulcerations. Binding of the uPA to its receptor (uPAR) accelerates plasminogen activation.82 The fibrinolysin plasmin itself degrades a variety of ECM components, which constitute perivascular fibrin cuffs. In addition, plasmin is known to be an important activator of several MMPs. Mazzieri and coworkers provided evidence of MMP-2 activity being controlled by components of the plasminogen activation system.50 Therefore, it is tempting to speculate that enhanced proteolytic MMP-2 expression is based on plasminogen activation in lipodermatosclerosis. Plasminogen activation could be interpreted as beneficial, since its target seems to consist of restoring nutrient and oxygen exchange, which is heavily impaired by pericapillary cuffs built up of matrix substrates for fibrinolysin plasmin.6 Uncontrolled activation of MMPs due to preceding activation of the plasminogen activation system could have implications on the dynamic balance of matrix synthesis and breakdown. Ulcer formation, therefore may be favored by enhanced turnover of the ECM mediated by unrestrained activity of specific MMPs.
Matrixins – repair and prolonged venous ulcer healing
Healing of venous leg ulcers requires properly controlled reepithelialization, angiogenesis, and matrix deposition. Delayed reepithelialization and persistant epithelial defects are typical features of chronic venous ulcers, which barely heal. Failure of reepithelialization appears to be due not to inadequate cell proliferation, but to the impaired capacity of keratinocytes to migrate across the wound bed and form stable attachments to the underlying stromal layer to tissue.46 There is mounting evidence that misregulation of MMP activity and TIMP-mediated counterregulation contributes to impaired and prolonged wound healing of venous ulcers. However, the pattern of expression of MMPs during venous ulcer healing has to be observed in a more detailed manner. Spatially and temporally controlled expression of several distinct MMPs appears to be associated with different repair phases of venous ulcers. Interstitial collagenase (MMP-1), MMP-2, and MMP-10 are consistently expressed by migrating keratinocytes that move off the basement membrane (Figure 2). Synthesis of these MMPs by migrating keratinocytes may help in dissociating the cell from the collagenous dermal matrix and promote efficient locomotion over dermal and provisional matrices. Keratinocytes use MMP-1 to cleave collagen to gelatin, thereby providing a substrate that is more conducive to migration.83 Several investigations have demonstrated a key role for altered cell-matrix interactions, particularly contact with type I collagen, in initiating keratinocyte MMP-1 synthesis.84 The expression of MMP-1 is diminished if reepithelialization is completed. By modulating the amount of intracellular calcium, the MMP-1 expression can be blocked in keratinocytes which have migrated away from the basal membrane.85
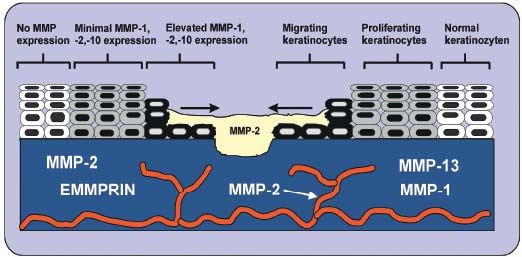
Figure 2. Pattern of matrix
metalloproteinase (MMP)
expression in wounded dermis
of venous leg ulcers. Interstitial
collagenase-1 (MMP-1),
MMP-2, and MMP-10 are
prominently expressed by
migrating basal keratinocytes.
Furthermore, MMP-3 is
expressed at sites of disrupted
basement membrane of basal
keratinocytes at a distance
from the wound edge. Fibroblasts
in dermis also
secrete different subtypes of
MMPs: MMP-1,
MMP-2, and MMP-13.
Furthermore, MMP-3 and MMP-10 are secreted by basal keratinocytes in chronic venous ulcers.17 However, only a distinct population of cells is able to express these specific metalloproteinases. MMP-3 is expressed at a distance from the wound edge and is probably not required for the process of reepithelialization, but for restructuring the newly formed basement membrane. Since MMP-3- positive keratinocytes reside on an intact basement membrane, the primary stimulus for production may be soluble mediators such as IL-1, TNF-á, EGF, PDGF, or TGF-â.16 MMP-10 displays a coexpressional pattern with MMP-2 at the epithelial tip bordering venous leg ulcers. This endopeptidase may be involved in the activation step of the interstitial collagenase (MMP-1). MMP-2 may hereby facilitate keratinocyte migration by degrading noncollagenous matrix molecules.17 During wound repair, both MMP-1 and MMP-3, but not MMP-10, are expressed in dermal fibroblasts and participate in the formation and removal of granulation tissue and resolution of scar tissue. The number of cells expressing MMP-1 and -3 in the stromal tissue is greater than the number of positive cells in normally healing wounds. Collagenase-3 (MMP-13) is not expressed by keratinocytes bordering normally healing wounds. In fact, MMP-2 and MMP-13 are abundantly expressed by stromal cells in chronic wounds distinct from areas of stromal MMP-1 expression. Human skin fibroblasts cultured in a three-dimensional collagen gel also express MMP-13. In contrast, dermal fibroblasts cultured on tissue culture plastic do not, indicating an important role for cell-matrix interactions in the control of MMP-13 expression in fibroblasts. While MMP-1 is critical for reepithelialization, MMP-13 is involved in the degradation of type I and III collagens and their cleavage products in the chronic venous leg ulcer bed may therefore play a pivotal role in the pathogenesis of chronic ulcers.46 Recently, we provided evidence of intense staining for EMMPRIN, MT1-MMP and MT2-MMP in dermal structures of venous leg ulcers, whereas only EMMPRIN is expressed in perivascular regions. Our findings indicate that venous leg ulcers are characterized by elevated expression of EMMPRIN, MT1-MMP, and MT2-MMP. The immunohistological findings of skin alterations also indirectly reflects the dynamic process of activation of soluble and membrane-bound MMPs, which may be highly induced by EMMPRIN in venous leg ulcers.86
Inhibitors of metalloproteinases play a crucial role in the remodeling process of venous ulceration. A sudden disruption of the balance between proteinase activity and inhibitor level may deteriorate wound healing. TIMP-1 is spatially and temporally regulated during venous ulcer healing. In comparison with acute wounds, nonhealing venous ulcers contain high levels of activated gelatinases and low levels of TIMP-1.78,79 Lack of TIMP-1 expression in keratinocytes of chronic ulcers in comparison with normally healing wounds suggests that excessive proteolysis retards the healing of venous ulcers.65 Epidermal TIMP-1 may inhibit proteolytic active metalloproteinases from degrading the epidermal basement membrane, thereby representing a protecting role.
Among other proteases, there had been evidence of the neutrophil elastase being upregulated during acute wound healing and that an abnormality in downregulation of this protease could be partially responsible for the transition to chronic wound healing states in the aged.87 In light of these observations of elevated protease activity of chronic wound fluid, there had been also evaluations with regard to the stability of growth factors and their receptors in chronic wound fluids.88 Specific growth factors had been shown to be destroyed by fluid samples from chronic wounds. Furthermore, there had been accumulating evidence that in wound fluids of venous leg ulcers MMP-1 and MMP-2 are strongly activated. These proteases are also expressed and activated in stromal tissue of the wound bed in leg ulcers. These data indicated that MMPs play a dual role in chronic venous leg ulcers. On the one hand, they are essential components during the phase of reepithelialization, and on the other hand elevated degradation due to activated matrixins in the wound fluid, as well as in the wound bed, significantly prolong wound healing in venous leg ulcers. Therefore, antagonizing matrixins in venous leg ulcers must take into account the fact that these proteinases are distinctly spatially and temporally expressed and activated.
Future treatment modalities
Pharmaceutical research into antagonizing MMPs progressed in the past few years. Inhibition analysis of MMPs using MMP inhibitors has been performed in different diseases such as neurodegenerative and cardiovascular diseases, corneal ulcers, osteoporosis, rheumatoid arthritis, dysfunctional uterine bleeding, T-cell-mediated tissue injuries, inflammatory bowel diseases, Crohn´s disease, and host-versus-graft disease.47 In spite of efforts in the development of MMP inhibitors, limited bioavailability and a lack of enzyme selectivity have hindered a quicker progression. Earlier inhibitors were designed relying upon sites of substrate cleavage. They were peptidomimetic, with the hydroxamic acid moiety replacing the terminal carboxylic acid of the corresponding peptide cleavage product. Unfortunately, these inhibitors lacked good bioavailability, and displayed little specificity for individual MMPs. Hydroxamic acid inhibitors are rapidly metabolized by the liver and require frequent dosing to maintain therapeutic plasma drug levels. In the mean time, progression in pharmacokinetic profiles has allowed the design of nonpeptidic inhibitors.89-94
Application of TIMPs as a therapeutic tool for cardiovascular disease and cancer through gene therapy or direct protein application is still in the early phase of development.95 There is a clear potential for the application of TIMPs as endogenous inhibitors, especially because the results of clinical trials with small molecule inhibitors have been disappointing.96 Adenoviral overexpression of TIMP-1 in a mouse model of atherosclerosis showed a reduction in the lesion.97 Local expression of TIMP-1 in a rat model of aneurysm prevented aneurysm degradation and rupture.98 However, expressing wild-type TIMPs could have drawbacks because multiple MMPs may be inhibited, and in the case of TIMP-3, ADAMs and ADAMTSs may be inhibited as well. Probably the best way to achieve success will be the development of engineered TIMPs with altered specificity, to allow targeting of specific proteinases. To date, there have been no studies analyzing the effect of specific inhibitors of metalloproteinases in venous leg ulcers. Reasons limiting the general application may rely upon its poor bioavailability. It is conceivable that local application of selective compounds of MMP inhibitors may have a beneficial effect on the early phase of wound healing in venous ulcers. Success in the clinical application of MMP inhibitors in the near future might also challenge clinical investigations in the field of wound healing.
CONCLUSIONS
MMPs are important enzymes in many biological and pathological processes, due to their ability to degrade ECM components. This review provides new insight into the interplay between cells, the ECM, and its catabolism in venous leg ulcers. Considerable progress has been made in the understanding of biochemical aspects of MMPs in the last decade, including their activation and catalytic mechanisms, substrate specificity, and the mechanism of inhibition by TIMPs. However, there are important questions that remain unresolved. In spite of the fact that collagenase was the first member of the family to be discovered, the mechanism by which collagenases cleave triple-helical collagens is not fully understood. An explanation as to how TIMP-3 inhibits metalloproteinases of the ADAM family awaits future experimental studies. Progress in structural analyses has led to the design of synthetic metalloproteinase inhibitors, some of which have exhibited efficacy in animal models. In contrast, clinical trials have unfortunately often shown no significant benefit in humans. Such discrepancies may be due to the fact that those trials were conducted in patients at advanced stages of the disease. Another possibility is that the inhibitor concentration reached in vivo was insufficient to inhibit target enzymes in the tissue, or that nontarget enzymes were inhibited. However, it is conceivable that these trials did not take into account the fact that matrixins are distinctly spatially and temporally expressed and activated. The design of specific inhibitors of matrixins is an important future challenge. Such inhibitors are useful not only for gaining insights into the biological roles of MMPs, but also for the development of therapeutic interventions for diseases associated with unbalanced ECM degradation. An outstanding example of such a disease is venous leg ulceration. In this review, different investigations, including our own experiments, were discussed, which suggest a pivotal role of matrixins in the continuum of processes linking ulceration and repair. What remains to be learned is the exact role of these proteinases in different compartments of tissue. It might be that genetics will help to provide answers to these questions – specifically transgenic mouse technology. Mice that inappropriately express MMPs which have been engineered to eliminate matrixins both help in this regard. Transgenic mice are complex systems; however, it can be difficult to fully understand the molecular mechanism leading to a phenotype, despite the fact that alteration was made in only a single gene. Therefore, it will be necessary to combine different genetic studies. A number of pharmaceutical companies are working in the field of inhibitor development, the inhibitor being administered systemically. Approaches to inhibit MMP activity, besides direct targeting of the enzyme´s active site, still seem important to consider for the healing of venous leg ulcers. One likely possibility could be inhibiting MMP activity indirectly by targeting MMP synthesis. Understanding the molecular pathway of matrix degradation by proteolytic enzymes of MMPs may facilitate finding potential therapeutic strategies in managing patients with advanced complications of chronic venous insufficiency.
ACKNOWLEDGMENT
This manuscript is dedicated to the memory of our dear friend and colleague Prof Dr José Antonio Jiménez Cossío.

REFERENCES
2. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516.
3. Woessner JF. The matrix metalloproteinase family. In: Parks WC, Mecham RP, eds. Matrix Metalloproteinases. San Diego, Calif: Academic Press;1998:1-13.
4. Gomez DE, Alonso DF, Yoshiji H, et al. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111-122.
5. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267-283.
6. Herrick SE, Sloan P, McGurk M, et al. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol. 1992;141:1085-1095.
7. Browse NL, Burnand KG. The cause of venous ulceration. Lancet. 1982;2:243-245.
8. Coleridge Smith PD, Thomas P, Acurr JH et al. Causes of venous ulceration: A new hypothesis. BMJ. 1988;296:1726-1727.
9. Neumann HA, Van den Broek MJ. Increased collagen IV layer in the basal membrane area of the capillaries in severe chronic venous insufficiency. Vasa. 1991;20:26-29.
10. Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol. 1999;11:614-621.
11. Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823-831.
12. Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10: 602-608.
13. Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728-735.
14. Powell WC, Matrisan LM. Complex roles of matrix metalloproteinases in tumor progression. Attempts to understand metastasis formation. In: Gunther U, Schlag PM, Birchmeier W, eds. Metastasis Related Molecules. Heidelberg, Germany:Springer-Verlag;1996:1-21.
15. Seltzer JL, Jeffrey JJ, Eisen AZ. Evidence for mammalian collagenase as zinc ion metalloenzymes. Biochim Biophys Acta. 1977;485:179-187.
16. Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996;156:1-4.
17. Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, et al. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79-88.
18. Lepage T, Gache C. Early expression of a collagenase-like hatching enzyme gene in the sea urchin embryo. EMBO J. 1990;9:3003-3012.
19. Leontovich AA, Zhang J, Shimokawa K, et al. A novel hydra matrix metalloproteinase (HMMP) functions in extracellular matrix degradation, morphogenesis and the maintenance of differentiated cells in the foot process. Development. 2000;127:907-920.
20. Maidment JM, Moore D, Murphy GP, et al. Matrix metalloproteinase homologues from Arabidopsis thaliana: expression and activity. J Biol Chem. 1999;274:34706-34710.
21. Allan JA, Docherty AJ, Barker PJ, et al. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J. 1995;309:299-306.
22. Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase: inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the length fragments. J Biol Chem. 1995;270:5872-5876.
23. Patterson ML, Atkinson SJ, Knäuper V, et al. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503:158-162.
24. Itoh T, Ikeda T, Gomi H, et al. Unaltered secretion of â-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)- deficient mice. J Biol Chem. 1997;272: 22389-22392.
25. Martignetti JA, Aqeel AA, Sewairi WA, et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001;28:261-265.
26. Suzuki K, Enghild JJ, Morodomi T, et al. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990;29:10261- 10270.
27. Uria JA, López-Otín C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60:4745-4751.
28. Park HI, Ni J, Gerkema FE, et al. Identification and characterization of human endometase (matrix metalloproteinase-26) from endometrial tumor. J Biol Chem. 2000;275:20540-20544.
29. Ohuchi E, Imai K, Fujii Y, et al. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446-2451.
30. Holmbeck K, Bianco P, Caterina J, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81-92.
31. Pepper MS. Extracellular proteolysis and angiogenesis. Thromb Haemost. 2001;86:346-355.
32. Sekine-Aizawa Y, Hama E, Watanabe K, et al. Matrix metalloproteinase (MMP) system in brain: identification and characterization of brain-specific MMP highly expressed in cerebellum. Eur J Neurosci. 2001;13:935-948.
33. Velasco G, Cal S, Merlos-Suárez A, et al. Human MT6-matrix metalloproteinase: identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000;60:877-882.
34. Pei D. Leukolysin/MMP25/MT6-MMP: a novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999;9:291-303.
35. Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268:23824-23829.
36. Shipley JM, Wesselschmidt RL, Kobayashi DK, et al. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996;93:3942-3946.
37. Péndas AM, Knäuper V, Puente XS, et al. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J Biol Chem. 1997;272:4281-4286.
38. Kolb C, Mauch S, Peter HH, et al. The matrix metalloproteinase RASI-1 is expressed in synovial blood vessels of a rheumatoid arthritis patient. Immunol Lett. 1997;57:83-88.
39. Yang M, Kurkinen M. Cloning and characterization of a novel matrix metalloproteinase (MMP), CMMP, from chicken embryo fibroblasts: CMMP, Xenopus XMMP, and human MMP19 have a conserved unique cysteine in the catalytic domain. J Biol Chem. 1998;273:7893-17900.
40. Velasco G, Pendas AM, Fueyo A, et al. Cloning and characterization of human MMP- 23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274:4570-4576.
41. Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265:87-93.
42. Lohi J, Wilson CL, Roby JD, et al. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001; 276:10134-10144.
43. Saarialho-Kere U, Kerkela E, Jahkola T, et al. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J Invest Dermatol. 2002;119:14-21.
44. Esteve PO, Tremblay P, Houde M, et al. In vitro expression of MMP-2 and MMP-9 in glioma cells following exposure to inflammatory mediators. Biochim Biophys Acta. 1998;1403:85-96.
45. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378: 151-160
46. Vaalomo M, Mattila L, Johansson N, et al. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109:96-101.
47. Johnson LL, Dyer R, Hupe DJ. Matrix metalloproteinases. Curr Opin Chem Biol. 1998;466-471.
48. Okada Y, Harris ED, Jr, Nagase H. The precursor of a metalloendopeptidase from human rheumatoid synovial fibroblasts: purification and mechanisms of activation by endopeptidases and 4-aminophenylmercuric acetate. Biochem J. 1988;254:731-741.
49. Nagase H, Enghild JJ, Suzuki K, et al. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4- aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783-5789.
50. Mazzieri R, Masiero L, Zanetta L, et al. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 1997;16:2319-2332.
51. Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324-333.
52. Strongin AY, Collier I, Bannikov G, et al. Mechanism of cell surface activation of 72 kDa type IV collagenase. Isolation of the activated form of the membrane metalloproteinase. J Biol Chem. 1995;270:5331-5338.
53. Cao J, Sato H, Takino T, Seiki M. The Cterminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for progelatinase A activation. J Biol Chem. 1995;270:801-805.
54. Murphy G, Stanton H, Cowell S, et al. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38-44.
55. Butler GS, Will H, Atkinson SJ, et al. Membrane-type-2 matrix metalloproteinase can initiate the processing of progelatinase A and is regulated by the tissue inhibitors of metalloproteinases. Eur J Biochem. 1997;244:653-657.
56. Takino T, Sato H, Shinagawa A, et al. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library: MTMMPs form a unique membrane-type subclass in the MMP family. J Biol Chem. 1995;270:23013-23020.
57. Llano E, Pendas AM, Freije JP, et al. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase A overexpressed in brain tumors. Cancer Res. 1999;59:2570-2576.
58. Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999;274:8925-8932.
59. English WR, Puente XS, Freije JM, et al. Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor convertase activity but does not activate pro- MMP2. J Biol Chem. 2000;275:14046-14055.
60. Butler GS, Butler MJ, Atkinson SJ, et al. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A: a kinetic study. J Biol Chem. 1998;273:871-880.
61. Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000; 275:26411-26415.
62. Ward RV, Hembry RM, Reynolds JJ, et al. The purification of tissue inhibitor of metalloproteinase-2 form ist 72 kDa progelatinase complex. Demonstration of the biochemical similarities of tissue inhibitor of metalloproteinase-2 and tissue inhibitor of metalloproteinse-1. Biochem J. 1991;278:179- 187.
63. Wilde CG, Hawkins PR, Coleman RT, et al. Cloning and characterization of human tissue inhibitor of metalloproteinases-3. DNA Cell Biol. 1994;13:711-718.
64. Leco KJ, Apte SS, Taniguchi GT, et al. Murine tissue inhibitor of metalloproteinases- 4 (TIMP-4) cDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997;401:213- 217.
65. Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2,-3 and -4) in normal and aberrant wound healing. Hum Pathol. 1999;30:795-802.
66. Howard EW, Bullen EC, Banda MJ. Preferential inhibition of 72- and 92-kDa gelatinases by tissue inhibitors of metalloproteinase-2. J Biol Chem. 1991;266:13070-13075.
67. Williamson RA, Martorell G, Carr MD, et al. Solution structure of the active domain of tissue inhibitor of metalloproteinases-2: a new member of the OB fold protein family. Biochemistry. 1994;33:11745-11759.
68. Murphy G, Houbrechts A, Cockett MI, et al. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30: 8097-8102
69. Will H, Atkinson SJ, Butler GS, et al. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation: regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–17123.
70. Amour A, Slocombe PM, Webster A, et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435: 39-44.
71. Amour A, Knight CG, Webster A, et al. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473:275-279.
72. Loechel F, Fox JW, Murphy G, et al. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem Biophys Res Commun. 2000;278:511-515.
73. Kashiwagi M, Tortorella M, Nagase H, et al. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAMTS5). J Biol Chem. 2001;276:12501-12504.
74. Yu WH, Yu S, Meng Q, et al. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226-31232.
75. Fedak PW, Altamentova SM, Weisel RD, et al. Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP-3. Am J Physiol. 2003;284: H626-H634.
76. Vowden K. Lipodermatosclerosis and atrophie blanche. J Wound Care. 1998;7: 441-443.
77. Herouy Y, Schöpf E, Vanscheidt W. Lipodermatosclerosis. Phleb Digest. 1998;3:6-8.
78. Weckroth M, Vaheri A, Lauharanta J, et al. Matrix metalloproteinases, gelatinase and collagenase in chronic leg ulcers. J Invest Dermatol 1996;106:1119-1124.
79. Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64-68.
80. Herouy Y, May AE, Pornschlegel G, et al. Lipodermatosclerosis is characterized by elevated expression and activation of matrix metalloproteinases. Implications for venous ulcer formation. J Invest Dermatol. 1998;111:822-827.
81. Jarret PE, Burnand KG, Morland M, et al. Proceedings: Fibrinolysis and fat necrosis in the lower leg. Br J Surg. 1976;63:157-160.
82. Herouy Y, Nockowski P, Schöpf E, et al. Lipodermatosclerosis and the significance of proteolytic remodeling in the pathogenesis of venous ulceration. Int J Mol Med. 1999;3: 511-515.
83. Pilcher BK, Dumin JA, Sudbeck BD, et al. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. Cell Biol. 1997;137:1445-1457.
84. Sudbeck BS, Parks WC, Welgus HG, et al. Collagen-stimulated induction of keratinocyte collagenase is mediated via tyrosine kinase and protein kinase C activities. J Biol Chem. 1994;269:30022-30029.
85. Sudbeck BD, Pilcher BK, Pentland AP, et al. Modulation of intracellular calcium levels inhibits secretion of collagenase-1 by migrating keratinocytes. Mol Biol Cell. 1997;8:811-824.
86. Norgauer J, Hildenbrand T, Idzko M, et al. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane-type matrix metalloproteinases in venous leg ulcers. Br J Dermatol. 2002;147:1180-1186.
87. Herrick S, Ashcroft G, Ireland G, et al. Upregulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest. 1997;77:281-288.
88. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environement. Wound Rep Reg. 1996;4:321-325.
89. Levy D, Lapierre F, Liang W, et al. Matrix metalloproteinase inhibitors: a structure activity study. J Med Chem. 1998;41:199-223.
90. Gomis-Ruth F, Maskos K, Betz M, et al. Mechanism of inhibition of human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77-81.
91. Dhanaraj V, Ye QZ, Johnson L, et al. X-ray structure of a hydroxamate inhibitor complex of stromelysin catalytic domain and its comparison with members of the zinc metalloproteinase superfamily. Structure. 1996;4:375-386.
92. Ye QZ, Hupe D, Johnson L. Catalytic domains of matrix metalloproteinases: a molecular biology approach to drug discovery. Curr Med Chem. 1996,3:407-418.
93. Beckett R, Whittaker M. Matrix metalloproteinase inhibitors. Exp Opin Ther Patents. 1998;8:259-282.
94. Levy D, Ezrin A. Matrix metalloproteinase inhibitor drugs. Emerg Drugs. 1997;2:205-230.
95. Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719-3727.
96. Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642-6650.
97. Rouis M, Adamy C, Duverger N, et al. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein Edeficient mice. Circulation. 1999;100:533-540.
98. Allaire E, Forough R, Clowes M, et al. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413-1420.
