The venous system of the foot: anatomy, physiology, and clinical aspects
anatomy, physiology, and clinical
aspects
Rome, Italy
Abstract
Venous return from the foot is worthy of interest for both research and clinical purposes. This review summarizes the available knowledge of venous return from the foot with a special focus on research and clinical implications. The anatomy and physiology of venous return are described with an emphasis on the differences between standing and walking and the interplay between the venous systems of both the foot and the calf. Selected conditions of clinical interest are discussed and mechanistically interpreted, including the distinctive localization of leg ulcers, the corona phlebectatica, the possible independence of dilatation of the veins of the foot from refluxing varices, and the arteriovenous fistulae of the foot. From this perspective, the practice of using a postoperative lower-leg bandage is also discussed.
Little attention has been devoted to the veins of the foot: surgeons begin the saphenectomy where the foot ends and echographists do not extend their exploration distally to the malleolus. Even anatomists have been more interested in the arteries of the foot, rather than the veins, as demonstrated by the more detailed description of arteries in anatomical tables. Finally, experts in hemodynamics focus on the calf to explain the mechanism of the limb pump, leaving the blood in the foot “undrained.” However, as shown in the present bibliography, a few well-conducted classic studies have clarified the anatomical and functional characteristics of venous circulation in the foot, although some areas of uncertainty still exist.
The main concepts concerning the anatomy and physiology of venous return from the foot will be revisited in this article, followed by observations of clinical interest and hypotheses for research and daily practice.
Anatomy
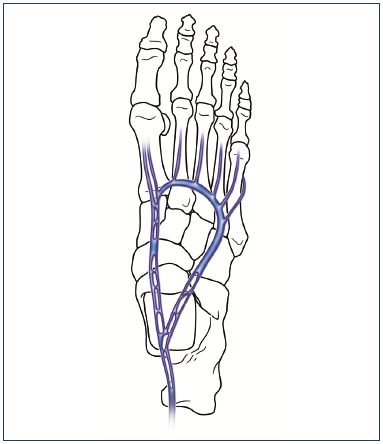
In 1968, Kuster et al provided the most complete description of the veins of the foot,1 describing five systems: (i) the superficial veins of the sole (also known as Lejars’ venous plexus)2; (ii) the deep veins of the sole; (iii) the superficial dorsal plexus; (iv) the marginal veins and dorsal venous arch; and (v) the perforating system.
i. T he superficial veins of the sole (Lejars’ venous plexus), once considered the most important impulse for venous return, is a net of tiny veins with limited clinical interest (Figure 1).3
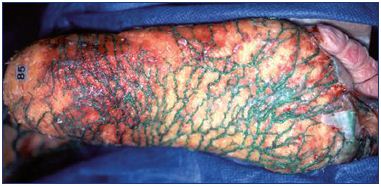
Figure 1. The superficial vein network of the sole is made by a
plexus of small veins that are 1 to 2 mm in diameter.
Image courtesy of J. F. Uhl.
ii. The deep veins of the sole are the most interesting from a functional point of view.
o The deep plantar venous arch runs from the proximal end of the first interosseous space to the base of the fifth metatarsal and accompanies the deep plantar arterial arch, which receives the deep metatarsal veins and surrounding muscular veins (Figure 2).4 The deep plantar venous arch measures ≈9 cm (range, 5 to 14 cm) according to Binns and Pho5 and ≈48 mm according to Corley,6 with an average external diameter of 5 mm.5 In 8 out of 10 foot dissections, 2 deep plantar venous arches were identified, which is often referred to as being doubled.6 A single, constant, and proximally oriented valve has been described.5
o The medial plantar vein is a thin and short vein (≈5 cm long according to Uhl et al,3 ≈12 cm according to Binns and Pho,5 and ≈38 mm according to Corley6) that is usually doubled, with a few proximally oriented valves.5 It runs along the medial border of the sole from the medial end of the plantar arch to the medial malleolus to form the posterior tibial veins after the confluence with the lateral plantar vein. The medial plantar vein receives blood from the adjacent muscles, ie, the abductor hallucis, the flexor digitorum brevis, and the plantar quadratus muscle.
o The lateral plantar vein (length between 80 mm5 and 84 mm6) is curved, constantly doubled, and large (2 mm) with fusiform dilatations (resembling the gastrocnemius sinuses),3 and it is located between the two muscle layers of the sole of the foot (ie, quadratus plantae and abductor allucis). Proximally directed valves are present in the lateral plantar vein.5 The lateral plantar artery lies between the two parts of this vein, which are interconnected an average of three times.3 The lateral plantar vein is in continuity with the lateral end of the plantar venous arch and runs back across the sole to join the medial vein at the calcaneal confluent, forming the posterior tibial veins. It receives blood from the lateral marginal vein, the calcaneal veins, and the veins in the adjacent large plantar muscles.
In the deep plantar system, doubled veins have been constantly observed with the corresponding arteries.6 The vessels are surrounded by connective tissue and this arrangement facilitates venous compression by the artery, serving as a localized pumping action.7 Furthermore, while performing cadaveric dissections, Corley found a consistent presence of an evident secondary arch either located deep in the quadratus plantae or as part of a more complex network of deep interconnections.6 This could represent a potential blood reservoir explaining the interindividual differences in venous outflow recorded during muscular activity.8
iii. The superficial dorsal plexus may be clinically important because it is in continuity with the superficial veins of the leg and ankle and may be involved in varicose dilatation of the superficial veins. These veins are very superficial (limited to the fat layer), well visible (esthetically demanding), and contiguous with the cutaneous nerves (easily encountered during foot phlebectomies).
iv. The marginal veins and the dorsal arch are separated from the superficial dorsal plexus by a relatively strong connective fascia (corresponding to the fascia covering the great saphenous vein and the small saphenous vein all over the limb); thus, the superficial network of the dorsum runs separately over these veins in a distinct layer (Figure 3 and 4).4,9 The dorsal arch lies over the proximal ends of the metatarsal bones and is the origin of the marginal veins, receiving the dorsal metatarsal veins and several perforating veins. The medial marginal vein arises from the perforator of the first metatarsal interspace and is contiguous with the great saphenous vein. It receives several perforators from the plantar veins that are important from a functional point of view. The lateral marginal vein ends in the short saphenous vein, which receives important perforators from the deep plantar veins.
v. The perforating system is the most distinctive system of the foot because these veins are valveless or contain valves oriented from deep to superficial veins. According to Uhl et al:3
o The perforator of the first metatarsal interspace generally has a large diameter without valves and connects the dorsal venous arch with the deep plantar system, and as a consequence, it is the true starting point of all venous networks in the foot. It accompanies the dorsalis pedis artery.
o The medial marginal perforators, which open into the medial marginal vein, differentiate into plantar veins (ie, malleolar, navicular, and cuneiform veins) and dorsal veins (ie, anterior tibial vein).
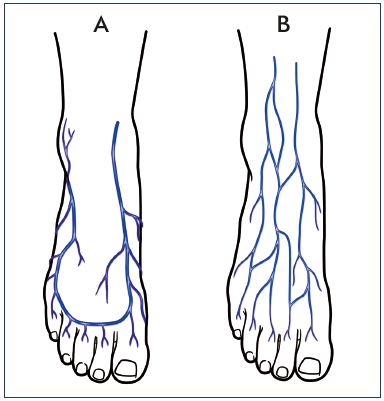
Figure 3. Marginal veins and their superficial network.
The marginal veins, connected anteriorly by the anterior arch
vein (Panel A), are the origin of the two saphenous veins, and
are similarly situated under the superficial fascia. The superficial
network of the dorsum of the foot is in continuity with the
superficial network of the anterior leg (Panel B).
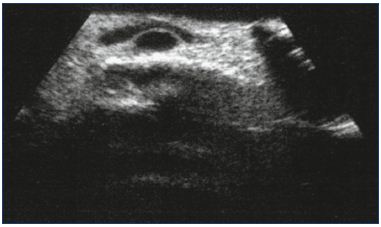
Figure 4. Superficial network of the dorsum (longitudinal) is
clearly visible with a duplex scan.
The network runs independently over an arch vein (transversal),
which is separated by the superficial fascia.
From reference 9: Ricci. Phlébologie. 2000;53:223-228. © 2000.
Reproduced with the kind permission of the publisher.
o Consistently, the lateral marginal perforators—the calcaneal and cuboidal veins—join the lateral marginal vein at the perimalleolar plexus.
Venomuscular Pumps
The energy needed for venous blood to overcome the hydrostatic pressure, which is generated by the distance between the heart and the leg in standing subjects in a dynamic state, is created by multiple myofascial compartments that are separate yet integrated, act like muscle pump units,10 and are known as the venomuscular pumps (VMP). The composition of VMPs includes the following11: (i) the venous foot pump; (ii) the distal and proximal calf pumps; (iii) the thigh pump; and (iv) the abdominal pump. The contraction and relaxation of the skeletal muscles surrounding the veins impress volume and pressure variations to the venous blood, while the flow direction is conditioned by the valvular arrangements.11
The position of each deep venous valvular tract will determine the pump output. A tract located inside the muscle creates very effective ejection systems (gastrocnemius, soleus).12 At the soleus and gastrocnemius sites, the veins are numerous and arranged in a spiral shape due to the longitudinal excursion amplitude of the muscles between contraction and relaxation (volumetric pump). Extramuscular tracts are subjected to compression over tough surfaces, ie, bone or aponeuroses, by the close muscles; therefore, they have a lower, though still satisfactory, efficiency (distal calf pump or peristaltic pump). This is the case for the posterior deep compartment veins (posterior tibial veins and peroneal veins) and the anterior external compartment veins (anterior tibial veins), which have a rectilinear organization, as the containing muscles lean against the bones and have a limited shortening during contraction.12
The superficial venous network is only indirectly affected by the VMPs through aspiration during muscle relaxation or diastole; there is an exception for the venous foot pump where the superficial veins may be directly filled, which is in contrast with the deep veins.
Proximal calf muscle pump
The most active pump of the lower limb is the one due to the sural and gastrocnemius muscles: these muscles are rich in venous sinuses that are strongly squeezed during the impulse phase of the step, when pressure exceeds 200 mm Hg and calf volume decreases by 80%.2
When the calf muscles contract, the pressure rises in all veins of the lower limb, and the increase is three times greater in muscle than in superficial veins. During muscle contraction (systole), the strong pressure gradient between the deep calf veins and the popliteal vein causes a rapid efflux of blood from the calf to the thigh (Figure 5). Venous pressure exceeds the intramuscular pressure in calf compartments in most of the step phases, but competent venous valves prevent retrograde flow. On subsequent muscle relaxation (diastole), venous pressure falls below the pressure at rest. The fall is greater in the deep veins, less in the superficial veins, and negligible in the popliteal vein. In this phase, perforator veins allow blood to flow from the superficial to the deep veins, while competent valves prevent backflow from the popliteal to the deep calf veins.3
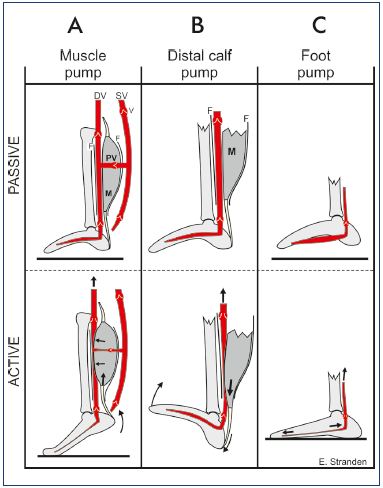
Figure 5. Mechanism of action for the distal calf pump.
Panel A. Muscles (M) are unsheathed by common fascia (F)
and veins within the same compartment. Contraction of the calf
muscles, as in plantar flexion of the ankle joint during walking
(bottom), expels blood into the proximal collecting vein. During
relaxation (top), the blood is drained from the superficial veins
(SV) into the deep veins (DV) in addition to the arterial inflow;
thereby, preparing for the subsequent ejection. V, venous valve.
Panel B. Distal calf pump: upon dorsiflexion of the ankle (passive
or active), the bulk of the calf muscle (M) descends within the
fascial sheath (F) and expels blood in the distal veins like a
piston. Panel C. The venous foot pump: upon weight bearing,
the tarso-metatarsal joints are extended and the tarsal arch is
flattened. Thus, the veins are stretched, causing them to eject
their content of blood.
Image courtesy of E. Stranden.
Distal calf “piston” pump
In contrast with conventional descriptions, there are two pumping systems in the calf, a proximal (gastrocnemius/ soleus) and a distal system.4 The distal one is activated by dorsiflexion of the ankle (Figure 5), ie, when the calf muscles are stretched and their distal parts descend within the fascial sheath. This movement acts like a piston, which expels venous blood in a proximal direction. The pump mechanism has been documented by ultrasound Doppler measurements of venous blood flow4 and is supported by compartment pressure measurements.5
Venous foot pump
According to Browse et al,13 the force required to overcome the pressure of the blood column within the venous system of the lower legs exceeds that generated within the muscular compartments of the calf during motion. For Gardner and Fox,14 the plantar venous plexus could overcome this pressure. Located within the plantar surface of the foot, the plantar venous plexus is submitted to high-pressure compression during ambulation, possibly constituting a mechanism for driving the venous outflow from the leg (Figure 6).15 During the gait process, the plantar plexus is able to overcome the pressure of the blood column within the deep venous system of the calf.16 It squeezes a small volume (20 to 30 mL),17 but the pumping mechanism is very effective; it works by voiding chambers distal to their axis and without a proximal valve, but closed distally in a cul-de-sac formation (C. Franceschi, unpublished data).
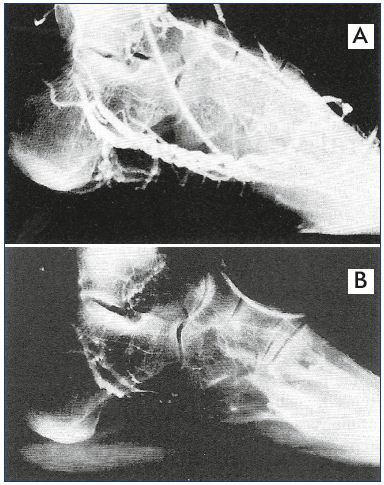
Figure 6. Phlebogram of the lateral plantar veins with a dilated
middle portion that acts like a reservoir.
Panel A. Nonweight bearing. Panel B. Weight bearing, which
empties the venous foot pump into the calf.
From reference 14: Gardner and Fox. IOS Press. 2001. © 2001.
The venous foot pump, in fact, is activated by the compression caused by either body weight or plantar muscle contractions during each step. According to the anatomical disposition, the site of the pump may be identified in the lateral plantar veins, whose middle portion is dilated and acts as a reservoir with a volume of 20 to 30 mL (Figure 6).3,17 The ratio of the diameter of the lateral plantar veins when compared with the diameter of the posterior tibial veins is 1.91:1, which creates a bellows type effect to rapidly increase the velocity of flow within the posterior tibial veins.15 The distal part of the pump is a sort of “suction pole” coming from the highly vascularized toes and the large metatarsal perforator vein that drains the superficial network of the medial marginal vein.3 The posterior part, at the calcaneal confluent, corresponds to an “ejection pole,” which empties directly into the posterior tibial veins.3
During a walking exercise, the foot is in contact with the ground 60% of the time and remains off the ground 40% of the time.18 The foot architecture is designed so that weight bearing takes place almost entirely on the ball of the foot, the heel, and the lateral part of the plantar surface of the foot. The medial part remains pressure free; thus, the plantar veins, which are located here, are protected from direct pressure, except in subjects with flat feet.18
The pumping mechanism has been explained as follows. The plantar veins are connected like a bowstring between the base of the fourth metatarsal and the medial malleolus. Upon weight bearing, the tarso-metatarsal joints are extended and the tarsal arch is flattened. Thus, stretching makes the veins eject their content of blood. Successively, upon heel strike, weight bearing on the forefoot with dorsiflexion of the toes makes the muscles of the sole contract, resulting in compression of the pump in the musculotendinous plane (Figure 7).19 There is no difference between the venous volume elicited by weight bearing and by toe curls.8 It is still not clear why both of these mechanisms produce the same effect or which mechanism is dominant; however, these two different venous foot pumps would be active at slightly different points in the phase of the gait cycle and both are likely active during the stance phase.6 Probably the muscle contraction pump is a memory of the preplantigrade phase in ontogenesis (ie, suspended or immersed life) in the absence of plantar support. Finally, in the suspension phase, pump filling is allowed.
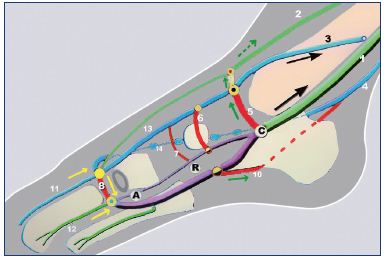
Figure 7. Anatomical structure of the foot pump.
Abbreviations. 1, posterior tibial veins; 2, anterior tibial veins; 3, great
saphenous vein; 4, short saphenous vein; 5, malleolar perforator vein; 6,
navicular vein; 7, cuneiform perforator vein; 8, perforator vein of the first
metatarsal interspace; 9, dorsal perforator vein; 10, calcaneal perforator
vein; 11, dorsal vein of the hallux; 12, intermetatarsal vein; 13, medial
marginal vein; 14, lateral marginal vein; A, suction pole; R, body of the
pump or reservoir; C, ejection pole at the calcaneal confluent.
Image courtesy of C. Gillot.
During normal walking, the three vein-pumping systems (foot, proximal calf, and distal calf pumps) are synchronized to form a complete network both in series and in parallel, which promote venous return. Even moderate muscular movements of the legs, while in a seated position, may activate the pumping mechanism, and significantly reduce mean distal vein pressure.20
Although synchronized with the calf pumps, the outflow from the foot plexus is independent of the proximal calf muscle contraction. This is possible because the proximal calf pump and the venous foot pump work “in parallel,” ie, voiding their volumes separately into the main duct (popliteal vein) and not “in series,” ie, emptying in the same longitudinal duct in succession. This arrangement allows for an independent behavior of the two stronger pumps.
Venous foot pump voiding
Considering the three main deep veins of the leg, (ie, anterior, posterior, and peroneal veins, which are all doubled, with frequent interduplication connections), during venous foot pump activity (weight bearing and flexion of the first toe) the prevalent flow is directed into the posterior tibial veins.14,15 The posterior tibial vein is doubled with a deeper vein that is parallel to a more superficial vein. The more superficial vein originates from the medial plantar vein, whereas the deeper vein originates from the lateral plantar vein.21
The peroneal vein is doubled and drains the lateral aspect of the foot surrounding the calcaneal confluent and the ankle, passing upward and posteriorly through the calf, as well as passing posteriorly and medially to the fibula.21 The peroneal veins may receive the soleus veins at midcalf, creating an independent pump.
The anterior tibial vein is doubled and drains the blood from the dorsum of the foot starting from the perforator of the first metatarsal interspace and running up the anterior compartment, lateral to the tibia, and close to the interosseous membrane that connects the tibia and fibula. At the knee, the junction of the tibia and fibula, the vessels penetrate the interosseous membrane and enter the posterior compartment of the leg. Just below the knee, the four anterior and posterior tibial veins join with the two peroneal veins to become the large popliteal vein.21
Video phlebography investigations confirmed that the preferential outflow of the pump is the posterior tibial vein, which is in direct continuity with the venous foot pump.10 The peroneal and anterior tibial veins share the same alternative outflow paths as the saphenous vein (through the malleolar perforators).
Mechanical compression of the plantar venous plexus produced a mean peak velocity of 123±71 cm/sec in the posterior tibial veins, 29±26 cm/sec in the peroneal veins, and 24±14 cm/sec in the anterior tibial veins.15
Consequently, each volumetric pump is anatomically independent (although with multiple connections) and connected in parallel with the final formation of the popliteal vein. However, the venous foot pump has a supplementary way of emptying through the saphenous vein, possibly by being fed from the medial marginal vein (ie, the dorsal perforator that communicates with the anterior tibial vein) and the malleolar perforator vein, which is connected to the calcaneal confluent.3
This event is indirectly demonstrated by the studies of Stranden et al that showed greater ambulatory pressure reduction in the dorsal foot vein (behaving like deep foot veins) than in the saphenous vein of the calf (mean, 25 mm Hg) during exercise,22 suggesting that a part of the ejected volume goes via the great saphenous vein. In another more recent study by Neglén and Raju, the drop in venous pressure in the dorsal foot is significantly more marked compared with both the popliteal vein and great saphenous vein at all levels (Figure 8).23 The recovery time is significantly increased in the long saphenous vein compared with the deep vein, and it is then further prolonged in the dorsal foot vein, proving that the three veins hydraulically behave as separate compartments. As the measurements are made distally to the calf pump, this indicates that the venous foot pump is the “engine” of the distal blood return. This may explain why signs and symptoms of chronic venous insufficiency occur with normal ambulatory venous pressures in the dorsal foot. The channel with the lower gradient will be favored in any occasion, depending on the muscular activity, temperature, position, overflow, and/or obstruction.
Gait
At the beginning of a step, the distal calf pump is activated. This process is initiated by dorsiflexion of the foot as the leg is lifted to take a step. The anterior compartment muscles contract, dorsiflect the foot, and empty its veins (ie, the anterior tibial veins). Dorsiflexion passively stretches the Achilles tendon and empties blood from the lower portions of the peroneal and posterior tibial veins. As the foot strikes the ground, weight bearing activates the second phase: the above-described venous foot pumps. Plantar flexion initiates the third phase as the foot comes up on its toes: the muscles of the posterior compartments, particularly the gastrocnemius and soleus muscles, contract, and then empty the proximal venous reservoir. Plantar flexion also tenses and shortens the Achilles tendon, which maintains pressure on the distal portion of the calf muscle pump.14
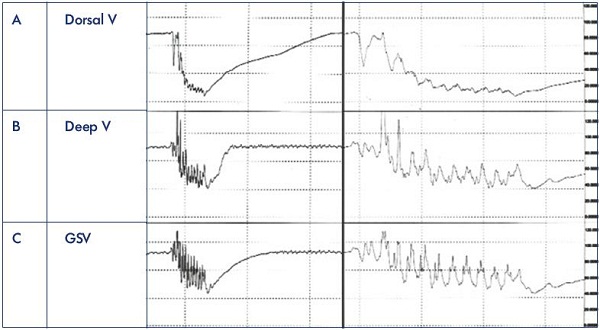
Figure 8. Lower leg venous pressure tracings.
The dorsal foot (Panel A), popliteal (Panel B), and great saphenous (Panel C) venous pressure tracings simultaneously recorded 5
to 7 cm above the ankles during 10 toe stands in a patient with no reflux or obstruction. The right set of curves is a magnification
of the left set.
Abbreviations: GSV, great saphenous vein; V, vein.
More detail regarding dorsiflexion of the ankle (passive or active) is provided in Figure 9. The bulk of the calf muscle descends within the fascial sheath and expels blood in the distal veins like a piston, which provides space to the blood coming from the venous foot pump (due to weight bearing), which will prevalently feed the posterior tibial veins. The proximal calf pump and venous foot pump act “in series,” ie, in succession on the same axis. If the deep veins are not emptied regularly, the venous foot pump finds resistance to expel the blood in the deep veins; thereby, creating a favorable gradient. The proximal pump, on the other hand, is strong and can empty a high (although variable) volume of blood in the popliteal vein, even in the absence of a favorable gradient, and it works “in parallel” with the more distal complexes (ie, the systems working separately on variable volumes at independent pressures).
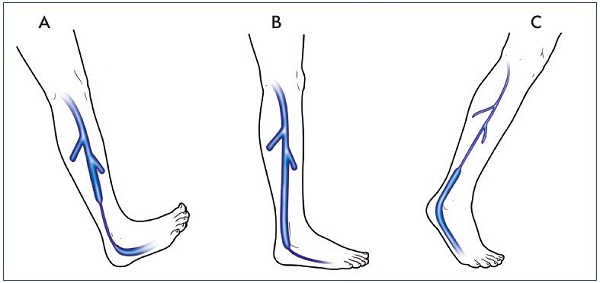
Figure 9. Synchronization of the leg pumps.
Dorsiflexion, weight bearing, and plantar flexion lead to distal calf pump emptying (Panel A), foot emptying (Panel B), and upper
calf emptying (Panel C), respectively.
The distal leg pump may become insufficient, ie, unable to void the blood volume coming from the perforators during diastole, due to a reflux or functional overflow (excess of volume) or an organic or functional obstruction (excess of pressure). In this instance, the venous foot pump will redirect the blood in the alternative direction, that is, from the saphenous veins (marginal veins at the foot) through the perforators, which are normally valveless.
Many of the records by Pegum and Fegan were generated by cannulation of deep and superficial veins through an incision at the first interosseous space and show that when the pressure in the deep veins rose to exceed the superficial pressure, the superficial pressure also began to rise, although to a lesser extent.24 This suggests that pressure in the deep veins of the sole was being transmitted to the superficial veins (dorsal venous arch) by the perforating veins along the pressure gradient. This finding is consistent with the deep layer of the superficial fascia of the foot, which also supports the dorsal venous arch against this pressure (similar to, but stronger than, the saphenous fascia).
Blood from the lateral side of the foot and ankle is not drained in an upward direction by the saphenous veins, but downward through the intermetatarsal veins that feed the lateral plantar vein, ie, the venous foot pump “reservoir.” These veins are all interconnected by the rich net of vessels around the ankle joint. The high number of perforators in the foot and the absence of an oriented valvular system are the most interesting aspects and the anatomical basis of the venous foot pump. They allow a rapid filling of the reservoir, draining of the deep and superficial network, and they make possible, in alternative outflow channels, the ejection of a volume of blood greater than could be achieved in a closed system.4 The saphenous veins of the distal calf are able to transfer the blood received from the venous foot pump in the deep veins via the valvular perforating veins during the diastole (relaxation) of surrounding muscles, when the gradient is favorable.
Clinical Considerations
As a consequence of these observations, some clinical events could be related to the venous foot pump’s work against venous overload of the more proximal sections of the limb, as in most chronic venous insufficiency (CVI) situations.25 The pressure increase, due to the proximal obstacle (functional or organic) on the main duct, charges the collateral veins and activates shunts with a subsequently possible dilatation. This mechanism may overcome the increased flow resistance and be fully compensatory. However, even if full compensation is achieved, the valvular system may become insufficient, thereby causing an inefficient action of the VMPs, and consequently, a functional obstruction, generating a short circuit.
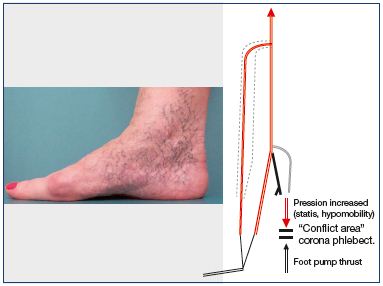
Corona phlebectatica
Corona phlebectatica has been described as a fan-shaped cluster of small, dilated veins radiating down from the soleus perforator area over the medial side of the ankle and foot corresponding to C1 of the clinical, etiological, anatomical, pathophysiological (CEAP) classification (Figure 10).26 The Society for Vascular Surgery and the American Venous Forum consider it an early sign of advanced venous disease.27
In the Phlebolymphological Guidelines of the Italian College of Phlebology, the corona phlebectatica paraplantaris (also known as malleolar or ankle flares) is, by definition, due to the telangiectatic dilatation of intradermic veins at the medial (more frequent) and lateral sides of the foot. This is sometimes considered a sign of venous hypertension at an advanced stage of CVI; however, it may also be present independently, ie, in the case of diffuse telangiectasias. Either definition or significance is uncertain. The dilatation of the superficial network of the skin of the foot could be explained as a hypertensive state due to an initially normal activity of the venous foot pump associated with events leading to a slowing of the blood flow in the deep veins, such as for chronic hypomobility (long periods of sitting, obesity, laziness), to the use of the wrong shoes, to agerelated progressive inactivity, which is not necessarily in association with CVI. The association with CVI could be casual, or alternatively, the venous hypertension could enhance an already present tendency.
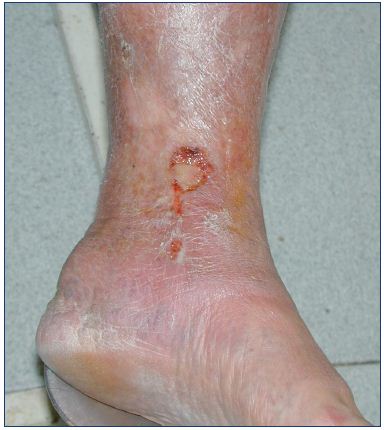
Skin lesions
Venous ulcers, lipodermatosclerosis, and pigmentations are typical skin manifestations of venous hypertension during CVI (Figure 11). Such signs typically appear in the medial supramalleolar area, regularly sparing the foot skin, with limited exceptions. An explanation may be that many patients with CVI have normal venous value pressure in the foot, as shown in 1986 by Stranden et al.22
This lesion’s location is probably due to the relative “hemodynamic weakness” of the supramalleolar area, containing perforators that act as reentry ways for superficial reflux, but missing a strong pumping mechanism.
Indeed, the distal calf pump, generates a pressure that is much lower than the pumping energy of the venous foot pump (distally) and the sural-gastrocnemius pump (proximally).
When submitted to reentry flow due to saphenous incompetence, hypertension may be aggravated by pressure peaks generated at every step by the venous foot pump that radiates upward. Skin lesions could then be the result of the conflict between the venous overflow due to the refluxing volume reentering the distal calf perforators and the active “kicks” coming from below. Similar effects could take place when venous hypertension is due to impairment of the deep veins (post-thrombotic syndrome).
Postexercise pressure, percentage of pressure drop, and recovery times are widely different in the deep veins, long saphenous veins, and dorsal foot veins, indicating that the three veins hydraulically behave as separate compartments. This may explain why signs and symptoms of chronic venous insufficiency occur with normal ambulatory venous pressures in the dorsal foot.23
Varices of the foot
Varices of the foot, often present in patients with varicose veins, have a peculiar behavior (Figure 12). Although the most distal part of the leg undergoes the strongest hydrostatic pressure, usually these varices appear late in the progression of the disease, and interestingly, they often do not seem to be directly involved in the reflux pathways, as if they were “suspended varices.” Indeed, the most important reentry perforators are located more proximally; the dilated veins of the foot are connected to foot perforators and may sometimes, but not always, communicate with the reflux pathway.
These varices might result from the action of the plantar pump “against” a system submitted to hypertension, particularly the deep veins, which are involved in the reentry of the refluxing volume. The strong venous foot pump would empty into the more compliant superficial network, developing varicose dilatations of the foot, which are connected with, but functionally unrelated to, the shunt circuit. Interestingly, dilatations of the foot veins almost exclusively involve the superficial network, which is unprotected by the superficial fascia, and spares the saphenous-type veins (ie, the marginal veins), which are sheltered by the superficial fascia (Figure 4) and are particularly robust in the foot.4,9
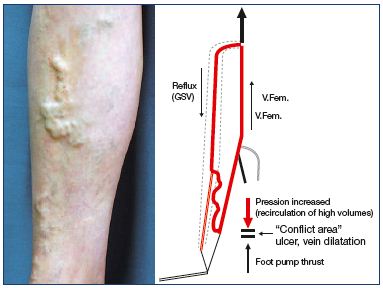
Figure 12. Varices of the calf fed by saphenous incompetence
have the main reentry point at the distal calf.
At the foot, varicose dilatation exists, which is separated from
venous incompetence.
Abbreviations: GSV, great saphenous vein; V.Fem., femoral vein.
Is compression necessary for the foot?
Postsurgery compression of the limb is mandatory, especially when treatment is partly based on phlebectomy of varicosities (under local anesthesia). The compression by selective pads provides hemostasis and allows the patient to walk immediately after the procedure. It also improves venous return and provides an analgesic effect.
With 40 years of experience in this practice, one of the authors observed that excluding the foot from the compression does not result in distal swelling and congestion. Naturally, this is possible only in C2 (of the CEAP classification) patients with a normal deep venous system, without edema, and who are actively walking (like most of our patients), and when the foot and distal limb are not involved in varices. Furthermore, the compression must be inelastic, which only acts during muscle activity and not at rest, and must involve the gastrocnemius area. It works like a “Perthes test.” Limited swelling may be present in the morning when rising from bed and disappears soon after the subject begins to walk. Inelastic compression of the leg allows the patient to wear normal shoes, conceal the surgical procedure, wash the foot, and is more comfortable.
Of the 183 phlebectomies (associated with or without great saphenous vein exclusion) performed from September 2010 to June 2011 at our institution, about half (90) of the feet were not given compression and did not show any notable inconveniences.28 According to this concept, a similar “suspended compression” may be used in cases of knee joint inflammatory swelling for decongestive and analgesic purposes, provided that the patient is able to actively walk. The same experience is made by athletes and sportsmen wearing compression sleeves in limited areas of the limb. Furthermore, a new stocking has been commercialized, which provides more compression at the upper calf than at the ankle, producing a “progressive” compression as opposed to the traditional “regressive” one.29
According to Gardner, “If function can be maintained, encircling bandages limited to the proximal limb are permissible–as long they are not excessively tight–since the venomuscular pump are able to overcome resistances in excess of 150 mm Hg.”14
Arteriovenous fistulae
Yoshida et al30 suggests, using a potential method to identify spontaneous arteriovenous fistulae, that arteriovenous fistulae occur between the plantar artery and the dorsal venous arch. These fistulae are activated when the foot is heated, eg, using a 38°C to 40°C footbath for 5 minutes, and can be visualized by duplex ultrasonography employing a 12 MHz transducer. Arteriovenous shunts (Sucquet-Hoyer canals) are present in the skin of toes, fingers, ears, and nose with thermoregulating functions. In fact, during vasodilation induced by heat, even vigorous walking movements fail to reduce the mean venous pressure below 0 mm Hg. However, when in the comfort zone, far less activity is required to effect such a reduction. Finally, when cool, even normal involuntary postural movements will reduce venous pressure to 50 mm Hg.31
While arteriovenous fistulae have been reported as a potential contributor to the development of thigh and calf varices,32 no information is available on the clinical relevance of arteriovenous fistulae of the feet. The hemodynamic role of these fistulae would be worthy of clarification, especially in the presence of varices of the lower leg or foot.
Conclusion
The pathophysiological and clinical conditions summarized here show that venous return from the foot is more than a gait-activated sponge and could be hemodynamically more important than has been previously considered. However, the incomplete knowledge of the physiopathology of venous return from the foot univocally hinders defining the relationship between dysfunction and clinical consequences. Some clinical aspects of venous pathology (eg, ulcer localization, corona phlebectatica, and venous dilatations) could be explained by a conflicting mechanism between the venous foot pump and the more proximal leg pumps, rather than a generic venous pump insufficiency. More thorough anatomy/physiology knowledge of the venous system of the foot could even enhance new methods of compression treatment (ie, “progressive” stockings).3

Part of this paper has been published in Dermatol Surg (Ricci S, Moro L, Antonelli Incalzi R. The foot venous system: anatomy,
physiology and relevance to clinical practice. Dermatol Surg. 2014;40:225-233).
1. Kuster G, Lofgren EP, Hollinshead WH. Anatomy of the veins of the foot. Surg Gynecol Obstet. 1968;127:817-826.
2. Lejars F. Les Veines de la Plante du Pied. Archives de Physiologie. 5ème série, 1890.
3. Uhl JF, Bertier C, Prevoteau C, Gillot C. La pompe veineuse plantaire: anatomie et hypothèses physiologiques [in French]. Phlébologie. 2009;62:9-18.
4. Fegan G. Varicose Veins: Compression Therapy. London, UK; Heinemann Med. 1967.
5. Binns M, Pho RW. Anatomy of the ‘venous foot pump.’ Injury. 1988;19:443-445.
6. Corley GJ. The anatomy and physiology of the venous foot pump. Anat Rec. 2010;293:370-378.
7. Benninghoff A, Drenckhahn D. Anatomie. 16th ed. Munich, Germany: Elsevier GmbH; 2004.
8. Broderick BJ, Corley GJ, Quondamatteo F, Breen PP, Serrador J, Ólaighin G. Venous emptying from the foot. Influences of weight bearing, toe curls, electrical stimulation, passive compression and posture. J Appl Physiol. 2010;109:1045- 1052.
9. Ricci S. Phlébectomie des varices du pied [in French]. Phlébologie. 2000;53:223- 228.
10. G ardner AMN, Fox RH. The return of blood to the heart against the force of gravity. In: Negus D, Jantet G, eds. Phlebology ‘85. London, UK: Libbey; 1986:68-71.
11. Franceschi C, Zamboni P, eds. Principles of venous hemodynamics. New York, NY: Nova Science Publishers; 2009.
12. Pieri A, Gatti, M, Santini M, Marcelli, F, Carnemolla A. Ultrasonographic anatomy of the deep veins of the lower limb. J Vasc Tech. 2002;26:201-211.
13. Browse NR, Burnand KG, Thomas ML. Physiology and functional anatomy. In: Arnold E, ed. Diseases of the Veins, Diagnosis and Treatment. London, UK: Hodder & Stoughton; 1988:53-69.
14. G ardner AMN, Fox H. The venous system in health and disease. Amsterdam, the Netherlands: IOS Press. 2001. ISBN 9051994338.
15. White JV, Katz ML, Cisek P, Kreithen J. Venous outflow of the leg: anatomy and physiologic mechanism of the plantar venous plexus. J Vasc Surg. 1996;24: 819-824.
16. Ludbrook J. The musculovenous pumps of the human lower limb. Am Heart J. 1996;7:635-641.
17. Scurr JH, Coleridge Smith PC. La pompe musculaire du pied importance physiologique et Clinique [in French]. Phlébologie. 1993;46:209-216.
18. Murray MP, Drought AB, Kory RC. Walking patterns in normal men. J Bone Joint Surg. 1964;46A:335-360.
19. G ardner AMN, Fox RH. The return of blood to the heart. London, UK: John Libbey; 1993:63-65.
20. Stranden E. Dynamic leg volume changes when sitting in a locked and free-floating tilt office chair. Ergonomics. 2000;43:421- 433.
21. Corley GJ, Broderick BJ, Nestor SM, et al. The anatomy and physiology of the venous foot pump. Anat Rec. 2010;293: 370-378.
22. Stranden E, Ogreid P, Seem E. Venous pressure gradients in patients with chronic venous disease. Phlebology. 1986;1:47-50.
23. Neglén P, Raju S. Differences in pressures of the popliteal, long saphenous, and dorsal foot veins. J Vasc Surg. 2000;32: 894-901.
24. Pegum JM, Fegan WG. Anatomy of venous return from the foot. Cardiovasc Res. 1967;1:241-248.
25. Tibbs DJ Varicose Veins and Related Disorders. Oxford, UK: Butterworth- Heinemann Ltd; 1992:204-232.
26. Ruckley CV, Evans CJ, Allan PL, Lee AJ, Fowkes GR. Chronic venous insufficiency: clinical and duplex correlations. The Edinburgh Vein Study of venous disorders in the general population. J Vasc Surg. 2002;36:520-525.
27. G loviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53:2S-48S.
28. Ricci S, Moro L, Trillo L, Incalzi RA. Foot sparing postoperative compression bandage: a possible alternative to traditional bandage. Phlebology. 2013;28:47-50.
29. Mosti G, Partsch H. Compression stockings with a negative pressure gradient have a more pronounced effect on venous pumping function than graduated elastic compression stockings. Eur J Vasc Endovasc Surg. 2011;42:261- 266.
30. Yoshida Y, Fujita M. Shunt flow of arteriovenous fistulas from plantar artery. Phlebology. 2011;26:32-34.
31. Henry JP, Gauer OH. The influence of temperature upon venous pressure in the foot. J Clin Invest. 1950;29:855-861.
32. Haimovici H. Role of precapillary arteriovenous shunting in the pathogenesis of varicose veins and its therapeutic implications. Surgery. 1987;101:515-522.