Treatment of iliofemoral deep vein thrombosis: challenges, opportunities, and future perspectives

Marie Josee E. van Rijn, MD, PhD
Department of Vascular and
Endovascular Surgery, Erasmus
University Medical Center,
P.O. Box 2040, 3000 CA
Rotterdam, The Netherlands
Jay M. Bakas, MD
Department of Vascular and
Endovascular Surgery, Erasmus
University Medical Center,
P.O. Box 2040, 3000 CA
Rotterdam, The Netherlands
ABSTRACT
Acute iliofemoral deep vein thrombosis (DVT) is more symptomatic than a thrombosis distal to the common femoral vein and increases the risk of postthrombotic syndrome (PTS), which reduces quality of life, and increases medical costs. To provide the best possible medical care, it all starts with identifying iliofemoral DVT. This will be achievable when visualizing the most upper thrombus part becomes standard practice. Treatment should be done by a multidisciplinary team encompassing lifestyle changes to compression therapy and adequate anticoagulation. In addition, early clot removal may lower the risk of PTS. Knowing which patients will benefit most is still unpredictable. New thrombectomy devices aim to restore patency without the use of thrombolysis. This may lower the threshold to consider a patient for early clot removal but may increase overtreatment. That is why it is equally important to focus future research on establishment of an accurate definition of PTS, a reliable prediction model to identify those who benefit from endovenous treatment, and to standardize postinterventional anticoagulation. Intensive collaboration between medical disciplines must therefore be continued during the complete pathway, from referral to long term follow-up. In an aging population with a rapid increase in obesity and sedentary lifestyle, prioritizing the need for appropriate care for iliofemoral DVT patients is key.
Introduction
Approximately a quarter of deep venous thrombosis (DVT) is localized in the iliac and/or common femoral veins (CFV), also referred to as iliofemoral DVT.1 Acute symptoms and signs of iliofemoral DVT often involve both the upper and lower part of the leg and are more severe compared with a DVT distal to the CFV. Also, the risk for developing chronic complaints related to postthrombotic syndrome (PTS) is increased.2,3 Various symptoms and signs related to deep venous obstruction and/or reflux may arise, starting from months to even years after the initial DVT.4 The incurability, chronicity, and severity of symptoms makes it one of the worst long-term complications. Therefore, adequate treatment of iliofemoral DVT involves PTS prevention, beside the acute symptom resolution. Noninvasive treatment such as compression therapy and adequate anticoagulation, together with lifestyle changes like exercise and weight loss, should always be applied immediately after acute DVT and may also successfully relieve complaints of PTS.5,6 European Society of Vascular Surgery (ESVS) guidelines recommend an endovenous treatment for symptomatic patients with acute iliofemoral DVT.7 Beside resolution of acute symptoms and signs, it lowers the risk of PTS and improves quality of life (QOL) compared with noninvasive treatment.8 However, the reality is that in most hospitals treatment is still only conservative.
The increased use of endovenous treatment has changed the field for acute iliofemoral DVT treatment over the past decades. This article provides an overview of the challenges, opportunities, and future perspectives for these patients.
Deep venous thrombosis
Incidence of acute DVT is approximately 1-2 per 1000 patients per year.9 Hypercoagulability, stasis, and endothelial damage (Virchow’s triad) are key elements in DVT pathophysiology. New insights show many more involving factors like altered flow around the valves generating hypoxia, leading to endothelial activation and triggering adhesion molecules. In turn, there is recruiting of blood cells such as monocytes, neutrophils, and platelets. Monocytes and neutrophils activate coagulation through the extrinsic and intrinsic pathways, favoring thrombus formation and growth, and trapping more cells.10
Various risk factors can provoke a DVT, such as hormone therapy, pregnancy, immobilization, thrombophilia, recent surgery, endovenous procedures or placement of central venous catheters, cancer, obesity, and advanced age.10 An “unprovoked” DVT arises without any preceding risk factors.
Distinction between “provoked” and “unprovoked” DVT is important in terms of prognostic and treatment implications.11 Stopping anticoagulants might be considered after DVT with a transient risk factor, whereas life-long prescription is considered for persistent risk factors and unprovoked DVT.
Impaired blood flow in compression syndromes may also trigger DVT. Left iliac vein compression, caused by the May-Thurner syndrome (MTS), explains why most iliofemoral DVTs are left-sided.12 The attributing risk of MTS to DVT, and whether it counts as a “provoking factor,” is unclear.
Anatomical sites of a DVT are divided into the calf veins, popliteal vein, femoral vein, CFV, and iliac veins with or without the inferior vena cava (IVC).13 An iliofemoral DVT without thrombus below the groin is uncommon. Therefore, the term “proximal” DVT can be misleading and should be avoided.13
Diagnostics
Management of suspected DVT starts with the assessment of patient history and physical examination. These factors are used to estimate the probability of acute DVT by using the Wells score. A D-dimer test and/or duplex ultrasound (DUS) can be added next.14,15 The preferred imaging modality for suspected DVT is DUS, but its accuracy depends on the technique used and the ultrasonographers’ experience. Combined color Doppler techniques have optimal sensitivity, whereas compression has optimal specificity for detecting DVT.16 Ideally, both techniques should be applied. In addition, 2 ultrasound assessments are practiced: 2- or 3-point compression scanning, and whole-leg ultrasound scanning. Both techniques may miss iliofemoral DVT when the upper thrombus part is not visualized. If thrombus is present in the femoral vein or CFV, the iliac veins should be visualized as well, with additional computed-tomography venography (CTV) or magnetic resonance venography (MRV) if DUS is inconclusive.17
Intravascular ultrasound (IVUS) is an increasingly performed imaging modality to visualize the extent of venous lesions during intervention for both acute and chronic cases.18 Because of its invasive nature, IVUS should not be used purely as a diagnostic. Peri-interventional use of IVUS provides useful complementary information, as multiplanar venography might underestimate the amount of residual thrombus during performance of a clot removal technique (Figure 1), as well as residual obstruction.19 Also, IVUS discriminates between acute and chronic clots, the latter being more echogenic, surrounded by a thickened vein wall.20 However, the recommendation for IVUS use during endovenous treatment is weak because the uncertain clinical and economic benefits require validation from randomized controlled trials (RCTs).21
Recently, a risk score was developed to distinguish iliofemoral DVT from all other suspected DVTs, with 77% sensitivity and 82% specificity.22 The model includes D-dimer levels, Wells score, age, and anticoagulation therapy, and may help to prioritize patients for immediate imaging to confirm or exclude iliofemoral DVT.
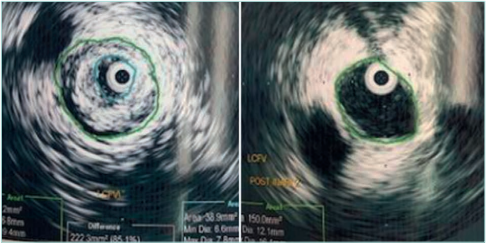
Figure 1. Peri-interventional intravascular ultrasound before and after thrombus removal at the level of the common femoral vein. Left panel (within the green circle): hyperechogenic, older age, clot. Right panel
(within the green circle): a cleared vein without thrombus.
Postthrombotic syndrome
Localization of DVT is important because thrombus extending into the CFV, iliac veins, and/or the IVC, may be considered for early clot removal.17 These DVTs are often more symptomatic than DVTs distal from the CFV and have an increased risk for PTS.2 Various symptoms and signs of impaired venous outflow may arise in PTS, as a result of deep venous obstruction and/or reflux after DVT. It occurs in 20% to 50% of DVT, of which 5% to 10% result in severe PTS. Whereas PTS frequently arises within the first year, it may develop 10 to 20 years after the initial DVT.4,23 Common symptoms include pain, cramps, heaviness, paresthesia, and pruritis of the leg; common signs are edema, skin changes, redness, and pain during calf compression, with venous ulceration as one of the worst clinical features.23,24
Preventing PTS, or reducing the severity, is important as PTS decreases QOL and imposes a burden on the health care system through high medical costs, lost workdays, and job loss.23 Optimized anticoagulation, compression therapy, lifestyle changes (eg, exercise and weight loss), and early thrombus removal may restore vessel and valve patency, thereby preventing (recurrent) DVT and worsening of PTS.
Various PTS scoring systems are available. The Villalta score combined with a venous disease–specific QOL score is the recommended golden standard25; however, PTS definition and classification should be further optimized. The ideal scoring system is easy to use, reliable, and objective. It should take into account the broad spectrum of PTS manifestation, such as venous claudication,26 and exercise intolerance,27 which are both absent in current PTS scores.
Endovenous treatment
The ESVS guidelines on the management of venous thrombosis (2021) recommend considering early thrombus removal for selected patients with symptomatic iliofemoral DVT, a class IIa, level A recommendation.7 The recommendation is based on reduced PTS risk after early clot removal for iliofemoral DVT. However, in most hospitals, treatment is still mainly conservative. Four randomized-controlled studies—TORPEDO (Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion), CaVenT (Catheter Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis), ATTRACT (Acute venous Thrombosis Thrombus Removal with Adjunctive Cather-directed Thrombolysis), and CAVA (Catheter Versus Anticoagulation Alone for Acute Primary Iliofemoral DVT)—are randomized between i) oral anticoagulants only; and ii) early thrombus removal with catheter-directed thrombolysis (CDT), combined with (pharmaco)mechanical thrombectomy (PMT), and/or percutaneous transluminal angioplasty and stenting.28-31 A meta-analysis showed that early thrombus removal was more effective than anticoagulation alone in preventing PTS (relative risk [RR] 0.67; 95% confidence interval [CI]: 0.45- 1.00; P=0.05), but unfortunately with an increased major bleeding risk (RR: 5.68; 95% CI: 1.27–25.33; P=0.02).17 An increased risk reduction for PTS after endovenous treatment was shown during long-term follow-up of the CaVenT and CAVA trials,29,32 indicating the added impact of early clot removal over time.
Venous stenting in addition to clot removal is successful in terms of venous patency with primary, assisted-primary, and secondary patency rates between 74% and 95%, 90% and 95%, and 84% and 100%, respectively after 12 months.33,34 Venous stenting should open up compression points or obstructed segments from earlier (asymptomatic) thrombotic events. Restoring venous patency is believed to lower the risk of re thrombosis, although no RCT has been performed to confirm this. High patency and low migration rates suggest that there is little harm in performing additional stenting.33,34 At the least, 12% of all DVTs would be eligible for early clot removal13; however, overtreatment should be avoided. Patients are frequently symptomatic in the acute phase, but only a portion develop PTS over time. A prediction system is therefore needed to optimize patient selection for endovenous treatment.
Less-invasive treatment methods, such as compression therapy and daily walking, should always be encouraged to prevent PTS, stimulate venous recanalization, and increase inflow in case venous intervention is indicated during follow-up.35,36 Thrombectomy devices that are used without thrombolysis are dealing with the bleeding risks of endovenous treatment.37 These devices are able to shorten hospitalization, as they aim to clear the veins in a single session (Figure 2). Patients can therefore be discharged the next day, preferably after confirming patency, but even same day discharge might be possible.38 The largest prospective series is the CLOUT registry (ClotTriever® Outcomes, Inari Medical, Irvine, CA). In 250 patients (82% iliofemoral and/ or caval DVT), no thrombolytics were used and 99.6% were treated in a single session. At 6 months, 24% of patients had PTS and all clinical outcomes improved, including the Revised Venous Clinical Severity Score, the numeric pain rating scale, and the EuroQoL Group 5-Dimension Self-Report Questionnaire.37 Figure 3 shows a ClotTriever® device with extracted thrombus in it.
Early clot removal, using either PMT or CDT, is cost-effective and falls below the United Kingdom National institute threshold for cost-effectiveness.39 An incremental cost effective ratio of US $20 000 per quality-adjusted life year makes CDT a cost-effective alternative to conservative treatment of iliofemoral DVT.40 However, the long-term cost effectiveness is higher for PMT than CDT.41 Lower costs for PMT compared with CDT are likely to be caused by shorter length of hospitalization and less invasive surveillance.42
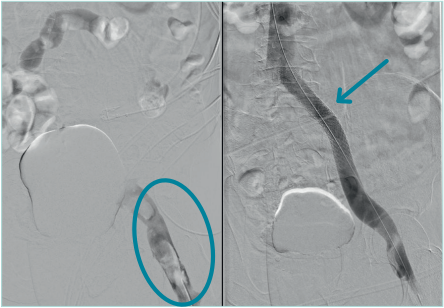
Figure 2. Venography in patient with acute deep vein thrombosis with complete occlusion of the iliac veins, before and after mechanical thrombectomy with ClotTriever® followed by venous stenting of the common iliac vein to overcome compression at the May Thurner point. Left panel: a stop of contrast/flow proximal from the common femoral vein (CFV), with visible thrombus (blue circle) in the CFV. Right panel: open common femoral and iliac veins and a stent (blue arrow) in the common iliac vein.

Figure 3. Mechanical thrombectomy
device (ClotTriever®) with extracted
thrombus.
Thrombus age
Thrombus age is critical for treatment success because older collagen-rich thrombi are less susceptible to lysis than younger fibrin-rich thrombi.43 Mean thrombolysis duration for >90% thrombus resolution was 23 hours for acute, 43 hours for subacute, and 85 hours for old clots.44 CDT was almost 11 times more successful for acute and subacute clots than for older thrombi (odds ratio [OR]: 10.7; 95% CI: 2.1- 55.5). Thrombus age was defined using MRV. Acute DVT was defined as an enlarged dilated vein with hypointense signal intensity surrounded by a thin rim of contrast; subacute DVT, as an enlarged dilated vein with heterogeneous signal intensity surrounded by a thick rim of contrast; and older DVT, as a normalized caliber vein with heterogeneous and/ or hypointense material without an evident rim of contrast and no apparent edema.45
The most reliable DVT-staging method before endovenous treatment is unknown. Both magnetic resonance imaging and ultrasound elastography were previously used to distinguish between acute and chronic DVT, but the optimal method in terms of accuracy, cost-effectiveness, and reproducibility is yet to be found.46 The CLOUT registry showed that chronicity assessed using symptom duration alone mismatched morphology-based chronicity in 55.1% of limbs (P<0.0001).38 Mechanical thrombectomy might lower the need for pre-interventional clot staging because of its favorable effectiveness of thrombus removal at various stages.37
Technical aspects
Open thrombectomy is performed by venotomy in the CFV with a Fogarty embolectomy catheter to evacuate the thrombus proximally and manual massage of the entire leg to evacuate the thrombus distally, or over-the-wire catheterization in the direction of the foot, passing the valves, and then performing a thrombectomy. Open thrombectomy improves patency and reduces PTS compared with anticoagulant therapy alone.47 Patency after 10 years was 83% compared with 41% after anticoagulant therapy only (P<0.05). However, the open technique has become less popular with percutaneous options readily available.
Percutaneous techniques can be divided into CDT, mechanical thrombectomy, and PMT. Table I provides an overview of devices used for early thrombus removal in the venous system.48 Often, the popliteal vein is punctured for access under DUS guidance. However, depending on the technique used and the thrombus extent, veins distal from the popliteal vein can be used for access too (for PMT devices with a small sheath size or for CDT), as well as the internal jugular vein (in case of deep femoral vein catheterization), or the CFV (up and over to catheterize the deep femoral vein, or if only the iliac or IVC is thrombosed). When choosing the puncture site, physicians should always anticipate venous stenting. Determining the degree of clot removal can be estimated using multiplanar venography during all different techniques, but IVUS might be more accurate. The same is true for determining whether postthrombotic obstruction and/or compression syndromes have contributed to DVT and should be considered for stenting.19 Intravenous heparin is administered during intervention. Immediately after cessation of the procedure, low-molecular-weight heparin (LMWH) or direct oral anticoagulants (DOAC) should be prescribed to prevent re-thrombosis of cleared segments.
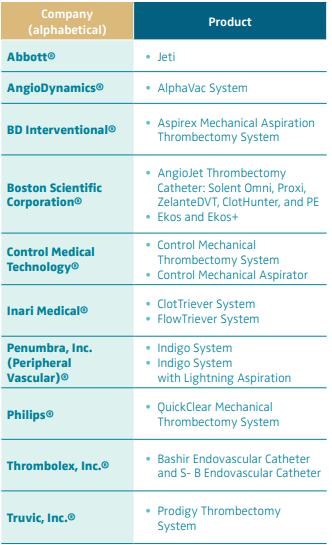
Table I. Devices for early thrombus removal in the venous system.
Based on reference 48: Endovasc Today. https://evtoday.com/
device-guide/us/mechanical-thrombectomythrombolysis
Vitamin-K-antagonists (VKA) may be started as well, after bridging with LMWH. As soon as possible, patients should be encouraged to walk. Pneumatic compression devices stimulate venous return if patients are not mobilizing after the procedure. As long as the leg is swollen, compression therapy should be applied. After clots are completely removed and leg circumference is decreased, continuing compression therapy mainly depends on patient preference.
Surveillance
Iliofemoral DVT may cause valve destruction (reflux) or scarring of the vein (obstruction), leading to PTS. Monitoring symptoms and signs of PTS during surveillance is important because PTS might develop within months to even years.4 Treatment of PTS focuses on improving symptoms and signs, in the absence of a curative treatment.5 The incurability of PTS and severity of symptoms and signs emphasize the importance of preventing it from the start.
Optimal anticoagulation, immediate mobilization, and compression therapy are first-line strategies for PTS prevention, but evidence for its efficacy is weak.6 Wearing elastic compression stockings (ECS) for at least 2 years was found to lower PTS incidence compared with patients who stopped wearing ECS.49 However, wearing ECS for at least 6 months followed by individualized extension of treatment duration is noninferior to the standard 2 years in terms of PTS prevention, as shown by the IDEAL trial (Ideal Deep Venous Thrombosis Study).50
Venous (stent) patency, residual obstruction, and venous reflux are evaluated using DUS. Peak flow velocity is combined with flow pattern analysis to diagnose, or rule out, stent occlusion and stenosis.51 If DUS is inconclusive, additional CTV should be considered, or even more invasive imaging like venography or IVUS with immediate intervention, when the suspicion of loss of patency is high. Stent patency could be referred to as primary patency, secondary patency, or permanent stent occlusion. Primary patency is the percentage of open stents without reintervention. Secondary patency is the percentage of open stents after reintervention. Permanent stent occlusions are occluded stents without further options to restore stent patency. Anticoagulant therapy compliance, thrombus burden, and poor venous flow are risk factors for reintervention.52 Reinterventions should ideally be prevented, or performed before vessel occlusion.52 Usually, follow-up is intensified in the first weeks after intervention, gradually diminishing (after uncomplicated procedures) over time until once a year or once every other year.
Anticoagulation therapy
Evidence-based guidelines providing optimal anticoagulant therapy after venous stenting are lacking.53 Patients usually receive a DOAC, or LMWH switched to a VKA. Adjunctive use of antiplatelet agents does not seem useful, based on the underlying pathophysiology and clinical experience.54 It did not affect outcomes after venous stenting for acute DVT, including recurrence of DVT, patency, incidence of PTS, and restenosis.55
Effectiveness and safety of postinterventional treatment with DOAC and VKA is similar for acute DVT patients56; however, treatment with VKA provides time within therapeutic range, providing information about compliance. Anticoagulation therapy is considered for at least 3 to 6 months after acute DVT in general. Extension of treatment duration with anticoagulants depends on the presence or absence of provoking DVT factors.57 Anticoagulants might be stopped if a transient provoking factor is present. Hypothetically, the introduction of venous stenting has transformed MTS into a “transient” factor. The ESVS guidelines recommend that duration of anticoagulant therapy after early thrombus removal (with or without venous stenting) should be based on the primary indication for anticoagulation and judged by the treating physician.17 Evidence supporting the optimal length of anticoagulant treatment after successful venous stenting is lacking.
Challenges, opportunities, and future perspectives
Iliofemoral DVT increases the risk for PTS, a devastating, lifelong syndrome that lowers QOL and burdens the health care system.23 Optimal treatment is key in preventing PTS, but several hurdles still need to be overcome. First of all, patients with an iliofemoral DVT should be identified. Physicians involved in the diagnostic phase of DVT should therefore be trained to always visualize the upper thrombus part. Next, these patients need to get referred to expert centers to discuss all treatment options, including early clot removal. This requires better, more intensive collaboration between vascular surgeons, interventionalists, phlebologists, dermatologists, internal medicine doctors, and general practitioners. The reality, however, is that most hospitals still only consider a conservative treatment for these patients.
Fortunately, (deep) venous disease is getting increased interest at conferences around the world. Increased awareness is also achieved through social media, at the level of physicians and by patients themselves. Interests from different medical specialists brings the opportunity for optimized care in a multidisciplinary approach.
Next, an objective, reliable, and easy to use scoring system for definition and classification of PTS should be developed, taking into account “typical” signs and symptoms, but also complaints such as exercise intolerance and venous claudication.17 Studies aiming to prove benefits of early clot removal should have a much longer follow-up than the typical first year, as PTS may take years to develop.4 New studies should also overcome the flaws of previous RCTs like low technical success, low rate of stenting, the use of nondedicated venous stents, and the absence of DUS follow-up.
Another challenge is motivating healthy lifestyles in an aging population with a rapid increase in obesity and a sedentary lifestyle. Lifestyle education is a huge part of patient care. With increased popularity of activity-tracking devices, motivation for exercise may become more easily accessible, as well as more playful and fun.
Opportunities arise from the evolution of new devices, presumably lowering the threshold for considering endovenous treatment. Aspiration devices without thrombolysis should, ideally, shift endovenous treatment to a low-risk technique, which also seems feasible for management of older thrombi.37,38 The DEFIANCE trial randomizes between mechanical thrombectomy with the ClotTriever® and anticoagulation only and will hopefully further reveal the impact of early clot removal on PTS prevention. Another exciting discovery is the involvement of inflammation and microRNAs in the pathophysiology of DVT, which might lead to therapeutic targets for drug development.10
Clinicians must aim to give (iliofemoral) DVT patients a clot- and PTS-free future, with joined forces from all medical specialties involved. Venous thrombosis patients deserve just as much attention as those with arterial occlusions. With so many new and exciting developments in the field of acute iliofemoral DVT, it is our strong belief that this can and will happen.
References
1. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698-707.
2. Tick LW, Kramer MH, Rosendaal FR, Faber WR, Doggen CJ. Risk factors for post thrombotic syndrome in patients with a first deep venous thrombosis. J Thromb Haemost. 2008;6(12):2075-2081.
3. Bikdeli B, Caraballo C, Trujillo-Santos J, et al. Clinical presentation and short- and long-term outcomes in patients with isolated distal deep vein thrombosis vs proximal deep vein thrombosis in the RIETE registry. JAMA Cardiol. 2022;7(8):857-865.
4. Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4(4):734-742.
5. Schleimer K, Barbati ME, Gombert A, Wienert V, Grommes J, Jalaie H. The treatment of post-thrombotic syndrome. Dtsch Arztebl Int. 2016;113(50):863-870.
6. Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164(1):17-26.
7. De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267.
8. Kahn SR, Julian JA, Kearon C, et al. Quality of life after pharmacomechanical catheter directed thrombolysis for proximal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8(1):8-23.e18.
9. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363-2371.
10. Navarrete S, Solar C, Tapia R, Pereira J, Fuentes E, Palomo I. Pathophysiology of deep vein thrombosis. Clin Exp Med. 2022 Apr 26. Epub ahead of print. doi:10.1007/ s10238-022-00829-w.
11. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480-1483.
12. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(5):419-427.
13. De Maeseneer MG, Bochanen N, van Rooijen G, Neglén P. Analysis of 1,338 patients with acute lower limb deep venous thrombosis (DVT) supports the inadequacy of the term “proximal DVT.” Eur J Vasc Endovasc Surg. 2016;51(3):415-420.
14. Oudega R, Moons KG, Hoes AW. Ruling out deep venous thrombosis in primary care. A simple diagnostic algorithm including D-dimer testing. Thromb Haemost. 2005;94(1):200-205.
15. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
16. Goodacre S, Sampson F, Thomas S, van Beek E, Sutton A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6.
17. Kakkos SK, Gohel M, Baekgaard N, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61(1):9-82.
18. Woods MA, Knavel Koepsel EM, et al. Intravascular US: applications in interventional radiology. Radiographics. 2022;42(6):1742-1757.
19. Murphy EH, Broker HS, Johnson EJ, Modrall JG, Valentine RJ, Arko FR 3rd. Device and imaging-specific volumetric analysis of clot lysis after percutaneous mechanical thrombectomy for iliofemoral DVT. J Endovasc Ther. 2010;17(3):423-433.
20. Anne S, Margarita VR, Jeffrey P, et al. Deep vein thrombosis: update on mechanical thrombectomy and intravascular US. Radiographics. 2022;42(6):E184-E185.
21. Vedantham S, Desai KR, Weinberg I, et al. Society of Interventional Radiology position statement on the endovascular management of acute iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2023;34(2):284-299.e7.
22. Shekarchian S, Notten P, Barbati ME, et al. A risk score for iliofemoral patients with deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10(1):33-41.e2.
23. Kahn SR, Comerota AJ, Cushman M, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130(18):1636-1661.
24. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7(5):879-883.
25. Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post-thrombotic syndrome. J Vasc Surg. 2013;57(1):254-261.
26. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239(1):118-126.
27. Morris RI, Sobotka PA, Balmforth PK, et al. Iliocaval venous obstruction, cardiac preload reserve and exercise limitation. J Cardiovasc Transl Res. 2020;13(4):531- 539.
28. Sharifi M, Bay C, Mehdipour M, Sharifi J, Investigators T. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. J Endovasc Ther. 2012;19(2):273-280.
29. Haig Y, Enden T, Grøtta O, et al. Post thrombotic syndrome after catheter directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3(2):e64-e71.
30. Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252.
31. Notten P, Ten Cate-Hoek AJ, Arnoldussen C, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post thrombotic syndrome (CAVA): a single blind, multicentre, randomised trial. Lancet Haematol. 2020;7(1):e40-e49.
32. Notten P, de Smet A, Tick LW, et al. CAVA (Ultrasound-Accelerated Catheter Directed Thrombolysis on Preventing Post-Thrombotic Syndrome) Trial: long term follow-up results. J Am Heart Assoc. 2021;10(11):e018973.
33. Taha MA, Busuttil A, Bootun R, Davies AH. A systematic review on the use of deep venous stenting for acute venous thrombosis of the lower limb. Phlebology. 2019;34(2):115-127.
34. Badesha AS, Siddiqui MM, Bains BRS, Bains PRS, Khan T. A systematic review on the incidence of stent migration in the treatment of acute and chronic iliofemoral disease using dedicated venous stents. Ann Vasc Surg. 2022;83:328-348.
35. Amin EE, Bistervels IM, Meijer K, et al. Reduced incidence of vein occlusion and postthrombotic syndrome after immediate compression for deep vein thrombosis. Blood. 2018;132(21):2298-2304.
36. Labas P, Ohrádka B, Vladimír J, Cambal M. The home treatment of deep vein thrombosis with low molecular weight heparin, forced mobilisation and compression. Int Angiol. 2000;19(4):303- 307.
37. Dexter DJ, Kado H, Schor J, et al. Interim outcomes of mechanical thrombectomy for deep vein thrombosis from the All-Comer CLOUT Registry. J Vasc Surg Venous Lymphat Disord. 2022;10(4):832-840.e2.
38. Maldonado TS, Dexter DJ, Kado H, et al. Outcomes from the ClotTriever Outcomes Registry show symptom duration may underestimate deep vein thrombus chronicity. J Vasc Surg Venous Lymphat Disord. 2022;10(6):1251-1259.
39. Pouncey A, Babigumira J, Johnson O, Black S. Cost utility analysis of treatment modalities for iliofemoral deep venous thrombosis: oral anticoagulation vs. catheter directed thrombolysis and pharmacomechanical thrombectomy. Eur J Vasc Endovasc Surg. 2019;58(6):e700.
40. Enden T, Resch S, White C, Wik HS, Kløw NE, Sandset PM. Cost-effectiveness of additional catheter-directed thrombolysis for deep vein thrombosis. J Thromb Haemost. 2013;11(6):1032-1042.
41. Li G, Xu M, Xu Z, Sun Y, Zhang J, Zhang X. Cost-effectiveness analysis of AngioJet and CDT for lower extremity deep vein thrombosis among Chinese population. Clin Appl Thromb Hemost. 2021;27:10760296211061147.
42. Mahmoud O, Vikatmaa P, Räsänen J, et al. Catheter-directed thrombolysis versus pharmacomechanical thrombectomy for upper extremity deep venous thrombosis: a cost-effectiveness analysis. Ann Vasc Surg. 2018;51:246-253.
43. Saha P, Andia ME, Modarai B, et al. Magnetic resonance T1 relaxation time of venous thrombus is determined by iron processing and predicts susceptibility to lysis. Circulation. 2013;128(7):729-736.
44. Arnoldussen CWKP, Notten P, Brans R, et al. Clinical impact of assessing thrombus age using magnetic resonance venography prior to catheter-directed thrombolysis. Eur Radiol. 2022;32(7):4555-4564.
45. Arnoldussen C, Strijkers RHW, Lambregts DMJ, Lahaye MJ, de Graaf R, Wittens CHA. Feasibility of identifying deep vein thrombosis characteristics with contrast enhanced MR-Venography. Phlebology. 2014;29(1 suppl):119-124.
46. Dharmarajah B, Sounderajah V, Rowland SP, Leen EL, Davies AH. Aging techniques for deep vein thrombosis: a systematic review. Phlebology. 2015;30(2):77-84.
47. Plate G, Eklöf B, Norgren L, Ohlin P, Dahlström JA. Venous thrombectomy for lliofemoral vein thrombosis — 10- year results of a prospective randomised study. Eur J Vasc Endovasc Surg. 1997;14(5):367-374.
48. Endovasc Today. US device guide/other devices: mechanical thrombectomy/ thrombolysis (peripheral/venous). Accessed March 16, 2023. https://evtoday.com/device-guide/us/ mechanical-thrombectomythrombolysis
49. Mol GC, van de Ree MA, Klok FA, et al. One versus two years of elastic compression stockings for prevention of post-thrombotic syndrome (OCTAVIA study): randomised controlled trial. BMJ. 2016;353:i2691.
50. Ten Cate-Hoek AJ, Amin EE, Bouman AC, et al. Individualised versus standard duration of elastic compression therapy for prevention of post-thrombotic syndrome (IDEAL DVT): a multicentre, randomised, single-blind, allocation-concealed, non-inferiority trial. Lancet Haematol. 2018;5(1):e25-e33.
51. Sebastian T, Barco S, Engelberger RP, et al. Duplex ultrasound investigation for the detection of obstructed iliocaval venous stents. Eur J Vasc Endovasc Surg. 2020;60(3):443-450.
52. Pouncey AL, Kahn T, Morris RI, Saha P, Thulasidasan N, Black SA. Risk factors and classification of reintervention following deep venous stenting for acute iliofemoral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1051-1058. e3.
53. Notten P, van Laanen JHH, Eijgenraam P, et al. Quality of anticoagulant therapy and the incidence of in-stent thrombosis after venous stenting. Res Pract Thromb Haemost. 2020;4(4):594-603.
54. Meissner MH. Indications for platelet aggregation inhibitors after venous stents. Phlebology. 2013;28(1 suppl):91-98.
55. Eijgenraam P, ten Cate H, Arina J. Venous stenting after deep venous thrombosis and antithrombotic therapy: a systematic review. Rev Vasc Med. 2014;2(3):88-97.
56. Sebastian T, Hakki LO, Spirk D, et al. Rivaroxaban or vitamin-K antagonists following early endovascular thrombus removal and stent placement for acute iliofemoral deep vein thrombosis. Thromb Res. 2018;172:86-93.
57. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693-4738.
