Update on anticoagulants: clinical support for the use of selective factor Xa inhibitors
Bioengineering
Department of Surgery, Evanston
Northwestern Healthcare, Evanston, IL, USA
Northwestern University Feinberg School of
Medicine, Chicago, IL, USA
and Robert R. McCormick School of
Engineering and Applied Sciences,
Evanston, IL, USA
ABSTRACT
Over the past decade, a number of new anticoagulant compounds have been developed, including the low-molecular-weight heparins (LMWHs) and the factor Xa inhibitors fondaparinux and idraparinux. Factor Xa inhibitors are powerful anticoagulants that act by producing a reversible conformational change in the antithrombin III molecule. Unlike unfractionated heparin and warfarin, these new compounds, which have a linear pharmacokinetic profile, do not require frequent patient monitoring. Factor Xa inhibition has been studied in the prevention and treatment of venous thromboembolic problems in orthopedic, general surgical, and medical patients, and, more recently, in the reduction of thrombotic complications associated with acute coronary syndrome. At present, most of the randomized trial data pertain to fondaparinux, the first selective factor Xa inhibitor to become available for clinical use. The purpose of this review will be to present clinical support for the use of selective factor Xa inhibitors for thrombosis prevention and treatment.
INTRODUCTION
Since the 1960s, unfractionated heparin (UFH) has been the gold standard for the prophylaxis and treatment of venous thromboembolism (VTE). Although this drug has an immediate onset of action, short half-life, and can easily be measured and reversed, there are a number of problems associated with its use. The effects of UFH on the clotting system are often unpredictable. In addition, quickly establishing a therapeutic level (within 12 to 24 hours) in patients with acute thrombosis can be challenging due to significant binding of nonclotting proteins. Finally, the incidence of heparin-induced thrombocytopenia (HIT) may be as high as 1% to 3% in patients receiving the drug. The emergence of the low-molecular-weight heparins (LMWHs) in the 1970s and 1980s represented a major breakthrough in the approach to thrombosis prophylaxis and treatment. These drugs have a greater bioavailability, longer half-lives, more predictable dose response, and are associated with a lower incidence of HIT and heparininduced osteoporosis. They also have a greater survival benefit in the patient with cancer.1
Until recently, warfarin was the only available oral anticoagulant. It has been widely used for the prevention of secondary thrombosis, as well as for the treatment of acute VTE. This agent is relatively inexpensive and is reversible with vitamin K. Problems associated with this drug include a delayed onset and offset of action (36 to 72 hours to achieve an appropriate therapeutic effect), frequent food and drug interactions, and the need for careful monitoring and possible dose adjustment. In addition, warfarin actually suppresses the body’s natural anticoagulants before achieving a full circulating anticoagulant effect in the patient. This is due to the order in which the clotting factor levels decline following warfarin administration. Factor VII is the first to decline and produces a prolonged prothrombin time and an increased international normalized ratio (INR). The patient is not therapeutically anticoagulated since the other vitamin K-dependent factors (factor II, factor IX, and factor X) have not yet been depleted. The intrinsic clotting pathway is intact, as can be measured by a normal activated partial thromboplastin time (aPTT) about 48 to 72 hours following the initiation of warfarin. At the same time, protein C and protein S, which are naturally occurring anticoagulants in the circulation, begin to decline because they are vitamin K-dependent. This results in hypercoagulability during the next 24 hours until levels of coagulation factors II, IX, and X in the plasma decline. These effects can sometimes produce skin necrosis or paradoxical thrombotic complications in patients with borderline or low levels of protein C or protein S. Finally, after a period of about 5 days, the patient is adequately anticoagulated with warfarin. If the patient is being treated for VTE with UFH or LMWH in an overlapping fashion, these drugs can be stopped after the 5-day period.2 The practice of using only warfarin for postoperative thrombosis prophylaxis can be risky in some cases, and can precipitate thrombosis as mentioned above.
Unlike warfarin, UFH, or LMWH, factor Xa inhibitors act on a single point in the coagulation schema. Factor Xa inhibitors are powerful anticoagulants that block activated factor Xa by producing a reversible conformational change in the antithrombin III molecule. Fondaparinux, a synthetic pentasaccharide, is a nonbiological compound that is administered subcutaneously once daily. It possesses a high bioavailability with a 17-hour half-life. Neither HIT nor heparin-induced osteoporosis has been an observed side effect. Idraparinux, a new, long-acting, synthetic pentasaccharide, has a half-life of approximately 4 days and can be administered subcutaneously once a week. Like other pentasaccharide formulations, it has a linear pharmacokinetic profile and does not require frequent patient monitoring. Although new data continue to emerge, at present, the bulk of the randomized trial data available for this review pertain to fondaparinux, which was the first factor Xa inhibitor to be approved for clinical use.
ORTHOPEDIC SURGERY
From a clinical perspective, some of the lowest VTE rates ever observed following total hip or knee replacement or hip fracture involved prophylaxis with the factor Xa inhibitor fondaparinux. Several studies have reported that this drug may be equal to, or better than, LMWH in hip replacement, knee replacement, and following hip fracture surgical repairs.3-6 More than 7300 patients were studied in four clinical trials, resulting in approval in the United States for these three indications. Table I outlines the results of the four studies, which use the LMWH enoxaparin as the comparator. No difference in clinically relevant bleeding was observed between factor Xa inhibitor and LMWH in any of these trials. In addition, no differences existed in any of the bleeding parameters between the two drugs in either of the hip replacement trials.4,5 However, minor bleeding was increased in the hip fracture study compared with LMWH.3 An increased incidence of major bleeding was observed in the knee trial, along with a positive bleeding index compared with LMWH, although 6 of the 9 patients with a positive bleeding index had the study drug continued by their surgeon.6
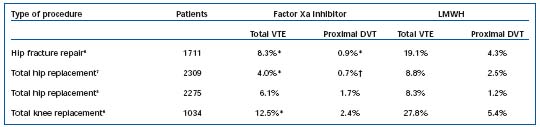
*P<0.001; †P=0.0021. Table I. Prophylaxis of venous thromboembolism following orthopedic procedures.5-7 Abbreviations: DVT, deep venous thromboembolism; VTE, venous thromboembolism; LMWH, low-molecular-weight heparin.
A post hoc analysis of these 4 studies revealed a statistically significant relationship between the timing of the first dose of factor Xa inhibitor and the incidence of major bleeding (P=0.0075), but not efficacy (P=0.67).7 An additional analysis found that major bleeding was significantly lower (P=0.045) in patients who received the first dose of factor Xa inhibitor _6 hours after surgery (as directed in the prescribing information), compared with those who received the first dose <6 hours after surgery. There were no differences between factor Xa inhibitor and LMWH in pulmonary embolism (PE) and overall safety, which included wound infections and surgical site complications.3-6 The most successful prophylaxis trial to date involved extended VTE prophylaxis following hip fracture surgery. Patients who had previously received fondaparinux for 7±1 days received fondaparinux (2.5 mg once daily) or placebo for an additional 3 weeks (±2 days).8 Factor Xa inhibitor reduced the incidence of VTE from 35% (placebo) to 1.4% (P<0.001), demonstrating a relative risk reduction of 96%. There were no differences between the groups in clinically relevant bleeding, although there was a trend toward more major bleeding in the factor Xa inhibitor group. One may conclude from these results that 30 days of therapy with the factor Xa inhibitor fondaparinux practically eliminates thromboembolism in patients with surgically repaired hip fractures. Another important observation can be made: in patients receiving factor Xa inhibitor for 7±2 days, the incidence of VTE was 8.3% when the venogram was done at 11 days,3 but jumped to 35% when the venogram was done at 30 days.8 This would reinforce the concept that the majority of VTE cases following hip operations occur following hospital discharge. Another important trial addressed the timing of the first dose of fondaparinux administered to patients undergoing major orthopedic surgery. The first dose of fondaparinux (2.5 mg) was administered either 8±2 hours postoperatively or the following morning.9 No significant difference in the incidence of symptomatic VTE was observed between the groups (1.9% and 1.8%, respectively, P=0.89). Bleeding events were also similar between the groups. Together, this evidence suggests that delaying administration of factor Xa inhibitor could provide additional treatment flexibility without compromising safety or efficacy.
ACUTE TREATMENT OF DVT AND PE
Fondaparinux was also evaluated in a treatment trial conducted among 2200 patients with deep venous thrombosis (DVT) randomized to receive either enoxaparin (1 mg/kg) twice daily, or 7.5 mg fondaparinux once daily with transition to oral anticoagulation for 6 months in both groups.10 A second treatment trial compared UFH with 7.5 mg fondaparinux once daily in 2200 patients with PE with or without DVT.11 Both trials showed factor Xa inhibitor to be as safe and effective as UFH or LMWH in treating these diseases. Rates of recurrent DVT, death from PE, and bleeding were not statistically significantly different among any of the groups studied.10,11 These studies have led to approval of fondaparinux in the United States for the treatment of acute DVT, and for the treatment of PE with or without DVT. The use of this drug in these trials was dose–adjusted; patients weighing <50 kg received 5 mg daily, those weighing 50 to 100 kg received 7.5 mg daily, and those weighing >100 kg received 10 mg daily. HIT has not been observed in any patients treated with factor Xa inhibitor, which is an advantage when patients have had or have been suspected of having HIT in the past. An important consideration when using this renally excreted drug is to know the creatinine clearance of the patient. Fondaparinux should not be used when the patient’s creatinine clearance is less than 30 mL/min and should be used with caution when the creatinine clearance is between 30 and 50 mL/min.12
A phase 2 clinical trial, the PERSIST study, compared four different dose regimens of the factor Xa inhibitor idraparinux with warfarin for secondary prevention of DVT.13 This new drug has an 80-hour half-life and was given once a week by subcutaneous injection. This preliminary study demonstrated that weekly administration of 2.5 mg of factor Xa inhibitor for 3 months was as safe and effective as warfarin administration when given for the same period of time.13 An analysis of liver enzymes in participants from this study also showed that factor Xa inhibitor administration did not increase plasma liver enzymes significantly.14
General surgery and medically ill in-patient therapy
Two important trials have been conducted to evaluate fondaparinux for VTE prophylaxis in general surgery patients. In the European trial, fondaparinux (2.5 mg), given once daily starting 6 hours after surgery, was compared with the LMWH dalteparin (2500 IU), given 2 hours before surgery and the evening of the same day, and once daily (5000 IU) thereafter.15 Both agents were given subcutaneously for 7±2 days. The incidence of any VTE event was 6.1% in the LMWH group and 4.6% in the factor Xa inhibitor group; these results were not significantly different. There were no differences of statistical significance in the clinical incidence of DVT and nonfatal or fatal PE. The VTE rates for patients with a median duration of surgery longer than 2.5 hours were 5.5% and 9.1% in the factor Xa inhibitor and LMWH groups, respectively. The VTE rates in patients with a median duration of surgery less than 2.5 hours were 3.7% and 2.8% in the factor Xa inhibitor and LMWH groups, respectively. There were no statistically significant differences in major or minor bleeding in either treatment group.
The second study in general surgery patients was done in the USA and compared the combined efficacy of fondaparinux and intermittent pneumatic compression (IPC) versus IPC alone for VTE prevention after major abdominal surgery.16 Patients in this trial were treated with IPC and either fondaparinux (2.5 mg) or placebo subcutaneously 6 to 8 hours after surgery, and then once daily for 7±2 days. Combined therapy significantly reduced the VTE rate from 5.3% (placebo + IPC) to 1.7% (factor Xa inhibitor + IPC; P=0.004). Rates of proximal DVT were also reduced in the factor Xa inhibitor + IPC group (0.2%) compared with IPC alone (1.7%; P=0.037). The incidence of major bleeding was 1.6% in the factor Xa inhibitor + IPC group versus 0.2% in the placebo + IPC group (P=0.006); however, no bleeding was fatal or involved critical organs. Although the bleeding risk was higher in patients treated with factor Xa inhibitor, this risk was low and consistent with that reported in other similar trials. Results from this trial demonstrated that factor Xa inhibitor and IPC combination therapy was significantly more effective than IPC alone for VTE prevention after major abdominal surgery.
An additional trial investigated fondaparinux VTE prophylaxis in acutely ill medical patients. In this trial, factor Xa inhibitor reduced the incidence of VTE to 5.6%, compared with 10.5% in the placebo group (relative risk reduction, 46.7%, P=0.029).17 No significant difference in bleeding risk was observed between the two groups. In addition, a reduction in mortality up to day 32 was observed in older medical patients (_60 years) receiving factor Xa inhibitor compared with patients receiving placebo (3.3% versus 6%; P=0.06).
ACUTE CORONARY SYNDROME
Acute coronary syndrome (ACS) refers to the full spectrum of coronary artery disease (CAD), including STsegment elevation myocardial infarction (STEMI), non- ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA).18,19 Atherosclerotic plaque formation, and disruption, and subsequent thrombus formation are characteristic of the pathophysiology that underlies ACS.18,19 Antithrombotic therapies, including anticoagulants and platelet inhibitors, are therefore mainstays of treatment for preventing thrombosis in these patients. Two recent, large, multicenter trials have evaluated the effects of fondaparinux in patients with ACS. The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-5) and Sixth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-6) studies compared fondaparinux with standard approaches in patients with UA/NSTEMI or STEMI, respectively.20,21
OASIS-5 compared the anti-ischemic benefits and bleeding risk of factor Xa inhibitor versus the LMWH enoxaparin in patients with UA/NSTEMI.20 Ischemic events (a composite of death, myocardial infarction, or refractory ischemia) occurred in 5.8% of patients assigned to factor Xa inhibitor, as compared with 5.7% of patients receiving LMWH, thus satisfying the prespecified criteria for noninferiority. The primary safety endpoint, the rate of major bleeding at 9 days, was reduced by about half with factor Xa inhibitor (2.2%) compared with LMWH (4.1%) (P<0.001), a difference that persisted over time. In addition, factor Xa inhibitor was associated with significantly reduced mortality at 30 days (2.9% versus 3.5%; P=0.02) and at 6 months (5.8% versus 6.5%; P=0.05), and a significant reduction in the long-term risk of stroke (factor Xa inhibitor, 1.3% versus LMWH, 1.7%; P=0.04). In patients with UA/STEMI, factor Xa inhibitor provided similar short-term antiischemic benefits to LMWH, and improved long-term mortality and morbidity, thus demonstrating that factor Xa inhibitor is an attractive option for the treatment of patients with ACS. OASIS-6 compared the effect of fondaparinux versus usual care (UFH or placebo in patients for whom UFH was not indicated) in patients with STEMI.21 The incidence of death or reinfarction at 30 days was significantly reduced from 11.2% in the control group to 9.7% in the factor Xa inhibitor group (hazard ratio [HR], 0.86; P=0.008). There was a consistent and significant reduction in death throughout the study with use of factor Xa inhibitor (7.8% versus 8.9% in controls; P=0.03); this reduction in mortality was due entirely to a reduction in cardiac deaths. A nonsignificant trend toward fewer severe bleeding episodes was observed with factor Xa inhibitor compared with the control group at day 9 (61 versus 79; P=0.13). In patients undergoing interventional procedures (percutaneous catheter intervention), treatment with factor Xa inhibitor was associated with a significantly higher incidence of guiding catheter thrombosis and coronary complications. Overall, OASIS-6 showed a significant reduction in mortality and reinfarction with use of factor Xa inhibitor, when compared with usual care. Notably, this reduction occurred without the increase in bleeding or hemorrhagic stroke observed with other antithrombotic and antiplatelet agents,22-24 and OASIS-6 is the only STEMI trial to date to demonstrate a mortality benefit without an increased risk of bleeding.
STROKE
Patients with atrial fibrillation are at risk for the formation of thrombi within the heart and subsequent vascular occlusive events as a result of embolization of these thrombi. A phase 3 study, AMADEUS (Atrial fibrillation trial of Monitored, Adjusted Dose vitamin k antagonist, comparing Efficacy and safety with Unadjusted SanOrg 34006/idraparinux), was initiated to evaluate idraparinux for the prevention of stroke in patients with atrial fibrillation.25 The results of this study are not yet available.
CONCLUSION
The ideal thrombosis prophylaxis agent should be efficacious, inexpensive, easy to administer and monitor, and have no complications or side effects. Although we are still searching for the perfect thrombosis prophylaxis agent, the emergence of newer anticoagulant therapies such as LMWHs and the selective factor Xa inhibitors fondaparinux and idraparinux have brought us closer to this goal.

REFERENCES
2. Wittkowsky AK. Why warfarin and heparin need to overlap when treating acute venous thromboembolism. Dis Mon. 2005;51:112-115.
3. Eriksson BI, Bauer KA, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345:1298- 1304.
4. Lassen MR, Bauer KA, Eriksson BI, Turpie AG. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hipreplacement surgery: a randomised double-blind comparison. Lancet. 2002;359:1715-1720.
5. Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002;359:1721- 1726.
6. Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305-1310.
7. Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833-1840.
8. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, doubleblind study. Arch Intern Med. 2003;163:1337-1342.
9. Colwell CW Jr, Kwong LM, Turpie AG, Davidson BL. Flexibility in administration of fondaparinux for prevention of symptomatic venous thromboembolism in orthopaedic surgery. J Arthroplasty. 2006;21:36-45.
10. Buller HR, Davidson BL, Decousus H, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867-873.
11. Buller HR, Davidson BL, Decousus H, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695-1702.
12. Fondaparinux sodium (Arixtra®) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2005.
13. PERSIST Investigators. A novel long-acting synthetic factor Xa inhibitor (SanOrg34006) to replace warfarin for secondary prevention in deep vein thrombosis. A Phase II evaluation. J Thromb Haemost. 2004;2:47-53.
14. Reiter M, Bucek RA, Koca N, Heger J, Minar E. Idraparinux and liver enzymes: observations from the PERSIST trial. Blood Coagul Fibrinolysis. 2003;14:61-65.
15. Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92:1212-1220.
16. Turpie AG, Bauer KA, Caprini J, et al. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of VTE after major abdominal surgery: results of the APOLLO study [abstract]. J Thromb Haemost. 2005;3(suppl 1):P1046.
17. Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325-329.
18. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). Circulation. 2002;106:1893-1900.
19. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1-E211.
20. Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464-1476.
21. Yusuf S, Mehta SR, Chrolavicius S, et al. Effects of fondaparinux on mortality and reinfarction in patients with acute STsegment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295:1519-1530.
22. Topol EJ; GUSTO V Investigators. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet. 2001;357:1905-1914.
23. Yusuf S, Mehta SR, Xie C, et al. Effects of reviparin, a low-molecular-weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST-segment elevation. JAMA. 2005;293:427-435.
24. The ASSENT-3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605-613.
25. ClinicalTrials.gov [database online]. Bethesda, Md: U.S. National Library of Medicine; 2006. Updated April 4, 2006.
