An update on operative treatments of primary superficial vein incompetence: part I.
of primary superficial vein
incompetence: part I.
of primary superficial vein incompetence.” These two chapters will be published
consecutively.
Unité de Pathologie Vasculaire
Jean Kunlin
Chassieu, France
Abstract
For more than a century, open surgery and liquid sclerotherapy were the only options used for operatively treating primary varices. In the last 20 years, management of primary varices has dramatically changed due to ultrasound investigations and innovative techniques. Development of endovenous treatments, including thermal ablation and/or chemical ablation, has provided a patient friendly option for an office-based procedure, improving both the postoperative course and convalescence duration. This article will be published in two parts. The first part will describe all the procedures used for treating varices and their possible complications. The second part, which will be published in a later issue, will analyze the outcomes of all procedures for short-, mid-, and long-term follow-up.
Preface
The term operative treatment has been intentionally chosen for this article instead of interventional treatment because interventional treatment means any kind of treatment that interferes with the natural history of the disease. For example, both compressive treatments and venoactive drugs modify the natural evolution of primary varicose veins.
Introduction
For a century, ancillary open surgery had the highest recommendation, and subsequently, was the most frequently used procedure for operatively treating varicose veins. In the past decade, the development of minimally invasive endovenous techniques for primary superficial venous reflux has provided a patient-friendly means of treating this disorder as an office-based procedure with ablation of the saphenous veins and tributary varicosities by using radiofrequency ablation, endovenous laser ablation, or sclerotherapy. Sclerotherapy regained favor for two reasons: (i) ultrasound investigation, which provided security for the procedure; and (ii) the use of foam, which enhances the efficacy of the sclerosing agent. More recently, new procedures have been used, including steam ablation, ClariVein, laser-assisted foam sclerotherapy, and glue, and these procedures will be described in the present article.
Simultaneously, surgery, including the CHIVA procedure (cure hemodynamique de l’insuffisance veineuse en ambulatoire [conservative ambulatory hemodynamic management of varicose veins]),1 and more recently, the ASVAL procedure (ablation selective des varices sous anesthésie locale [ambulatory selective vein ablation under local anesthesia]),2 were developed to preserve the great saphenous vein.
Open surgery without conservation of
saphenous trunks
Modern open surgery should be performed under local anesthesia and directed by preoperative ultrasound assessment and skin mapping. Treatment of the great saphenous vein involves flush ligation of the saphenofemoral junction, which is completed using saphenous invagination stripping. Stripping can also be done using a cryoprobe. Treatment of the incompetent small saphenous vein usually involves flush saphenopopliteal junction ligation and stripping by invagination. Nontruncal varicosities can be excised using stab avulsion–powered phlebectomy or they can be treated with sclerotherapy in the same session or later.
Stripping of both the great saphenous vein below the knee and the distal small saphenous vein may reduce varicose vein recurrence, but it is associated with an increased risk of nerve injury.3 The usefulness of flush ligation was recently called into question after a randomized controlled trial.4 In addition, there is a consensus for recommending elastic compression stockings for no more than 1 week after the operation.5,6
Complications of surgery
The early complications of surgery include discomfort (common), bruising (common), hematoma (rare), bleeding (very rare), lymphatic damage (rare), femoral vein or artery injury (extremely rare),7 wound infections (2% to 6%), and injury of the saphenous or sural nerve (10%). Symptomatic and asymptomatic deep venous thrombosis and pulmonary embolism following open surgery vary from 0.4% to 5.3% and 0% to 0.5%, respectively.8,9 The risk of complications, such as venous thromboembolisms, increase with redo surgery and surgery of the small saphenous vein.8 Modern open surgery under local anesthesia has dramatically lowered the rate of thromboembolic complications. Late complications include permanent nerve damage (5%).10
Open surgery with preservation of the
saphenous trunk
CHIVA
Due to the possible future use of the great saphenous vein as a vascular graft, it is necessary to preserve the vein.1 The principle of the CHIVA technique consists of redistributing refluxes from the superficial to the deep system using staged ligations on the great saphenous vein or tributaries. CHIVA is a complex procedure that requires careful mapping and understanding of the anatomy and function of the superficial system by well-trained and experienced physicians who are aware of the shunt classifications.11
ASVAL
While CHIVA is based on a descending theory, the ASVAL method is based on an ascending or multifocal approach to the primary varicose veins. In order to improve or suppress the saphenous vein reflux, a stab phlebectomy of incompetent tributaries is performed to remove the distal venous reservoir. Compared with trunk varicose vein ablation, the major advantage of ASVAL is the preservation of the great saphenous vein. After the ASVAL procedure, most patients had less advanced stages of varicose veins.2
Endovenous ablation
Endovenous thermal ablation
The term “endovenous thermal ablation” includes radiofrequency ablation, endovenous laser ablation, endovenous steam ablation, and endovenous microwave ablation. In endovenous thermal ablation procedures, ablation of the treated vein is achieved using heat, which is delivered into the vein through a percutaneously placed catheter or probe. The heat causes a direct thermal injury to the vein wall, resulting in destruction of the endothelium, denaturation of collagen in the media, and subsequently, thrombotic and fibrotic occlusion of the vein. Endovenous thermal ablation is performed under local tumescent anesthesia (except for endovenous microwave ablation) to provide anesthesia; protect the perivenous tissue from the heat created by the catheter, probe, or wire when activated; and spasm the vein to obtain the best contact with the heating device. In addition, all endovenous thermal ablation procedures are performed using ultrasound guidance and conducted as an outpatient-based procedure.
For the great saphenous vein, echo-guided vascular access occurs just below the knee (except for endovenous microwave ablation); therefore, heating is done from the groin (2 cm below the saphenofemoral junction) down to the distal part of the vein, usually just below or above the knee. For the small saphenous vein, echo-guided access occurs at the lower one-third of the lower leg, and heating is done from the popliteal fossa (2 cm below the saphenopopliteal junction) down to just above (8 to 10 cm) the tibial malleolus.
Radiofrequency ablation
Introduced in 2007, the current ClosureFAST radiofrequency catheter (VNUS Medical Technologies/Covidien) (Figures 1 and 2) is easy to use. The entire pullback time takes 3 to 4 minutes, generating heat around 120°C. Celon RFITT, another radiofrequency ablation system for bipolar radiofrequency-induced closure, is now available (Olympus Medical Systems). This system generates heat at 60 to 85°C and operates with a continuous pullback speed of 1 to 1.4 cm/second.
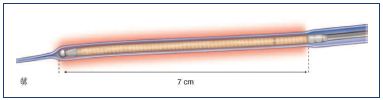
Figure 1.* ClosureFAST catheter.
The first 7 cm (left) of the coated heating element and the
thermocouple (right).
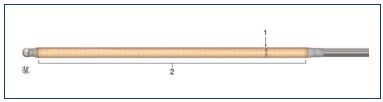
Figure 2.* ClosureFAST heater and thermocouple catheter.
The thermocouple (1) is mounted distally to the heating element
(2).
* From Perrin M. Traitement chirurgical endovasculaire des
varices des membres inférieurs. Techniques et résultats, EMC
(Elsevier Masson SAS, Paris. All rights reserved), Techniqueschirurgicales-
chirurgie vasculaire, 43-161-C, 2007
Endovenous laser ablation
Fiber lasers can provide either low wavelength beams (810, 940, and 980 nm) or high wavelength beams (1319, 1320, 1470, and 1500 nm). Theoretically, light of lower wavelengths is less specifically absorbed by the chromophores (hemoglobin, water, proteins) compared with the light of higher wavelength lasers.12 Previously, the fibers were bare tipped, but the new radial fibers are more effective and include the Radial fiber R (Biolitec) (Figure 3), Never-Touch R (Angiodynamics), and Tulip fiber R (Tobric). A continuous withdrawal technique is the current rule and it is recommended to deliver 50 to 70 J/cm of energy.
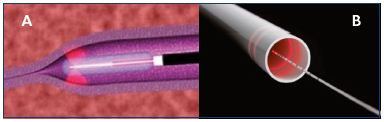
Figure 3. Fiber with radial emission.
Fiber with single radial emission (Panel A) and double radial
emission (Panel B).
Radiofrequency ablation vs endovenous laser ablation
Endovenous laser ablation and radiofrequency ablation are similar techniques that treat similar patient profiles. After percutaneous access, the radiofrequency ablation catheter or laser fiber is pushed proximally until the tip is positioned 2 cm from the saphenofemoral junction or saphenopopliteal junction (Figure 4). After tumescent anesthesia, the vein is ablated in a retrograde fashion. The postablation procedures are similar for both techniques.
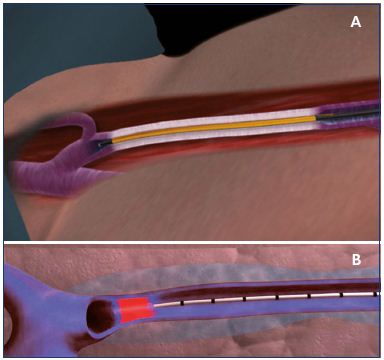
Figure 4. Positioning of the ClosureFAST catheter (A) and the
laser fiber (B).
Panel A. The ClosureFAST catheter is positioned 2 cm below
the saphenofemoral junction at the beginning of the procedure
before generator activation. Panel B. The laser fiber catheter
is positioned 2 cm below the saphenofemoral junction at the
beginning of the procedure before activation. The veins are
colored blue.
Endovenous steam ablation
In 2006, Milleret et al introduced steam as a cheaper alternative to laser and radiofrequency ablation. The principle consists of injecting pulses of water vapor at 120°C in the vein to be ablated, with each pulse delivering 60 J of energy into the lumen. Steam is injected under pressure, whereby the first pulse dislodges the blood and the subsequent ones heat the vein wall. A 5F gauge stainless steel catheter is used because it is flexible enough to navigate through the tortuosity without using a guide wire. Two lateral holes close to the tip eject the steam, avoiding the risk of heating deep veins when heating the junctions.
A comparative animal study by Thomis et al compared steam with either ClosureFAST radiofrequency or a 1470 nm TULIP fiber laser. The three methods generated comparable results regarding scores for low perivenous tissue destruction and high vein wall destruction.13
In a pilot study by van den Bos et al, 11 out of the 19 veins treated were completely obliterated at 6 months, with a partial reopening in the other veins. However, the energy delivered was too low, 1 pulse/cm instead of the 2 to 4 pulses/cm that is advised by the manufacturers of the technique.14 In a series of 75 patients, the complications included a thrombus protrusion in the femoral vein, an ecchymosis at the entry site in 1 patient, and moderate pain lasting 8 days in 6 patients.15 Subsequently, a randomized controlled trial was designed and it is still ongoing.
Endovenous microwave ablation
After ligation of the saphenofemoral junction, the microwave treating wire is inserted into the great saphenous vein until the medial aspect of the ankle and is guided by the illuminated tip of the wire. The treating wire is withdrawn from distal to proximal at 2 to 4 mm/s, delivering 80 J/cm of energy. In 16.4% of patients, the treating wire could not be passed to the ankle; therefore, it was inserted in the great saphenous vein at a puncture in the ankle and the vein ablation was conducted from groin to ankle. In the same session, all superficial varicose veins and perforators are ablated using short-wire power (10 to 15 W) under ultrasound guidance.16
Complications of endovenous thermal ablation
In a review analyzing randomized controlled trials conducted on radiofrequency ablation (317 patients), endovenous laser ablation (1057 patients), and open surgery (975 patients), the short-term complications included venous thromboembolism, wound infection, and paresthesia. There was a significantly higher rate of wound infection for open surgery (2.3%; 95% CI, 1.3%-3.1%) vs endovenous laser ablation (0.5%; 95% CI, 0.3%-1.3%; P=0.006), but not between open surgery and radiofrequency ablation (1.5%; 95% CI, 0.4%-3.0%; P=0.094). The paresthesia rate was significantly lower with endovenous laser ablation (3.8%; 95% CI, 2.4%-4.5%) compared with radiofrequency ablation (5.2%; 95% CI, 3.1%-7.9%; P<0.001) and open surgery (7.4%; 95% CI, 5.3%-8.3%; P<0.001). The rate of thrombophlebitis was significantly lower for open surgery (3.0%; 95% CI, 2.9%-4.0%) compared with both radiofrequency ablation (5.5%; 95% CI, 3.0%-7.8%; P=0.003) and endovenous laser ablation (5.6%; 95% CI, 4.2%-7.0%; P=0.003). Thermal skin burns occurred with equal frequency between radiofrequency ablation and endovenous laser ablation.17
A review of radiofrequency ablation complications has been reported and this method has been compared with those of other operative procedures. Early complications include pain, phlebitis (7% to 9.6%), arteriovenous fistula (0.15%), endovenous heat-induced thrombosis (EHIT), deep vein thrombosis (<0.01%), lidocaine toxicity, wound problems (6% to 8%), and skin burns (0.5%). Late complications are mostly transient and may include skin pigmentation (6% to 19%) and nerve damage (4% to 20%).18 Complications from endovenous laser ablation have also been compiled and include phlebitis (1.87%), skin burns (0.46%), nerve injury (3.08%), arteriovenous fistula (0.15%), endovenous heat-induced thrombosis, and deep venous thrombosis (0.27%).19
Only one multicenter trial has reported the outcomes of endovenous steam ablation (n=117). Postprocedural pain was lower in endovenous steam ablation compared with endovenous laser ablation. Other outcomes included thrombophlebitis (9.2%), nerve injury (0.9%), and hyperpigmentation (4.6%), but no deep vein thrombosis or skin burns were identified.20 Complications after endovenous microwave ablation have been reported in a single-center study, where endovenous microwave ablation was responsible for skin burns related to ablation of subcutaneous tributaries (10.2%).16
Chemical ablation
Sclerotherapy
Sclerotherapy refers to the introduction of a foreign substance into the lumen of a venous vessel to damage the venous wall and occlude the vessel. Liquid sclerotherapy has been used primarily for obliteration of spider veins. However, interest in using sclerotherapy for telangiectasia and varicose veins significantly increased in 1995 when Cabrera et al reported that foam, prepared by mixing gas with the detergent polidocanol, was effective for obstruction of larger veins.21 The use of ultrasound-guided foam sclerotherapy has rapidly spread for the treatment of primary and recurrent varicose veins, including the great saphenous vein, small saphenous vein, saphenous tributaries, and perforating veins.
Sclerosing agents
The mechanism of action for sclerosing agents includes destruction of venous endothelial cells, exposure of subendothelial collagen fibers, and ultimately, the formation of a fibrotic obstruction. Delivery of the solution as a foam prolongs the contact time and amplifies the effect of the chemical substance. For producing endothelial injury, sclerosing solutions can be classified into three categories: detergent, osmotic, or chemical irritant.
In Europe, approved agents for sclerotherapy include sodium tetradecyl sulfate, polidocanol, morrhuate sodium, hypertonic saline, and glycerin.
• Sodium tetradecyl sulfate is a detergent that destroys the endothelium by denaturation of the cell surface proteins. The solution is safe and painless when injected. When the solution is injected at higher concentrations, extravasation may result in tissue necrosis. Hyperpigmentation, matting, and allergic reactions have been described, but rarely occurred. Generating foam with a sodium tetradecyl sulfate agent is easy.
• Polidocanol is another detergent that is safe and painless when injected and has a low risk of tissue necrosis when used at low concentrations. It may cause hyperpigmentation, but has a very low rate of allergic or anaphylactic reactions. There is a consensus that polidocanol has fewer overall complications compared with sodium tetradecyl sulfate.
• Sodium morrhuate is a detergent that is used less frequently due to a relatively higher incidence of skin necrosis observed with extravasation and a higher risk of anaphylactic reactions within a few minutes after injection.
• Glycerin is a chemical irritant that destroys the cell surface proteins by affecting chemical bonds. Chromated glycerin is frequently used as a solution of glycerin, sterile water, and benzyl alcohol. Chromated glycerin is safe and rarely leads to tissue necrosis, hyperpigmentation, or allergies, but frequently there is local pain at the injection site. This treatment is particularly suitable for treating small veins or telangiectasia.
• Hypertonic saline, an osmotic agent, is a weak sclerosing agent that causes dehydration of endothelial cells through osmosis, which leads to endothelial cell death. Burning pain is frequent during injection. Extravasation may cause skin ulcers and tissue necrosis.
Liquid sclerotherapy
Liquid sclerotherapy is currently used for treating reticular veins and telangiectasia.
Foam sclerotherapy
Due to the enhanced sclerosing properties of foam, ultrasound-guided foam sclerotherapy has been shown to be more effective than liquid sclerotherapy, Tessari et al used a three-way stopcock connected to two syringes to produce foam and they developed the most popular technique used today.22 Other techniques for producing foam involve a two-way female-to-female connector.
Experts recommend a ratio of 1 part sodium tetradecyl sulfate or polidocanol to 4 or 5 parts air. Mixing the drug with air using the two syringes and pushing the mixture from one syringe into the other 20 times results in an approximate bubble size of <100 μm. Coleridge Smith advises puncturing the veins in supine patients and then elevating the limb 30 degrees to inject the foam.23 Ultrasonography is used to monitor the movement of foam in the veins. The saphenous vein is injected first, followed by varicose and perforating veins, if indicated. A maximum of 10 mL of foam is injected during one session. The procedure is completed by placing a short-stretch bandage or a 30 to 40 mm Hg graduated compression stocking on the limb. Most experts recommend 1 to 2 weeks of compression.
Severe complications of ultrasound-guided foam sclerotherapy comprise anaphylaxis (extremely rare), large tissue necrosis (extremely rare), stroke and transient ischemic attack (extremely rare), distal deep venous thrombosis (very rare), pulmonary embolism (extremely rare), and motor nerve injury (extremely rare). Benign complications are visual disturbances (uncommon), headaches and migraines (uncommon), sensory nerve injury (rare), chest tightness (very rare), dry cough (very rare), superficial thrombophlebitis (unclear), skin reaction (very rare), matting (common), residual pigmentation (common), minimal skin necrosis (very rare), and embolia cutis medicamentosa (very rare).
The complications are listed in the European guidelines for sclerotherapy in chronic venous disorders, along with recommendations to avoid and manage these complications. Ultrasound-guided foam sclerotherapy of the saphenous vein is the least invasive of the endovenous ablation techniques. In 2008, the European Consensus Meeting on Foam Sclerotherapy reported that foam was an effective, safe, and minimally invasive endovenous treatment for varicose veins with a low rate of complications.24 The most complete book on sclerotherapy was written by a team of editors in 2007.25
Cyanoacrylate glue ablation
A new nonablative procedure that intravenously delivers a cyanoacrylate adhesive mixture has been developed to improve some of the limitations of radiofrequency ablation, endovenous laser ablation, and sclerotherapy ablation. Upon intravascular injection, the cyanoacrylate adhesive rapidly solidifies via a polymerization reaction and results in an inflammatory reaction in the vein wall.
The disposable Sapheon Closure System includes 4 mL of Sapheon Cyanoacrylate Adhesive and a Sapheon delivery system (Figure 5). The Sapheon delivery system consists of a 7F-introducer sheath/dilator, a 5F-delivery catheter, a 3 mL syringe, and a dispenser gun. The hydrophobic 5F-delivery catheter has a novel configuration with air-filled microchannels to enhance sonographic visibility. The dispenser gun will deliver 0.08 to 0.16 mL of Sapheon Cyanoacrylate Adhesive with each trigger pull. Access to the great saphenous vein is achieved by applying the Seldinger technique, which uses a standard micropuncture kit under ultrasound localization. The Sapheon introducer sheath and dilator is advanced to the saphenofemoral junction over a 0.035 J guide wire.26 The cyanoacrylate adhesive is extracted from its glass vial and loaded into a syringe, which is then attached to the 5F delivery catheter. The combined syringe and catheter are connected to a dispenser gun. The catheter is then primed by advancing the glue with the dispenser gun to within 3 cm of the catheter tip. To prevent thrombus extension through the saphenofemoral junction, the hydrophobic delivery catheter is placed approximately 5 cm below the saphenofemoral junction. The saphenofemoral junction is manually compressed with the ultrasound transducer and the proprietary adhesive is delivered using the Sapheon delivery system using two injections at 1 cm intervals. Compression of the saphenofemoral junction and the delivery site is maintained for 3 minutes. The adhesive is delivered at 3 cm intervals through the remainder of the target vein using 30 seconds of compression for each subsequent delivery of adhesive (Figure 6). The last injection site is 2 to 4 cm from the entrance site to prevent the glue from migrating outside the vein. After venous closure is confirmed by ultrasound imaging, the catheter is removed and compression is applied to the catheter entry site until hemostasis is achieved. A single adhesive bandage is applied; neither compression stockings nor compression bandages are used. This protocol has been described in details in two articles.26,27 Postoperative complications were minimal.
Almeida et al reported a series of 38 patients treated for great saphenous vein incompetence. Postoperative side effects included a thread-like thrombus or glue extension across the saphenofemoral junction (21.1%), which resolved at 3 months, transient thrombophlebitis (16%), and hyperpigmentation (2.4%).28 In another series including 43 great saphenous veins and 22 small saphenous veins, thrombophlebitis of the great saphenous vein occurred 4 times.6 The primary potential advantage with this new technique is that it does not require tumescent anesthesia and patients do not need postoperative compression stockings.
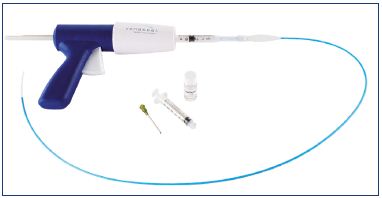
Figure 5. Sapheon kit that includes the Sapheon delivery system
and the Sapheon cyanoacrylate adhesive flask.
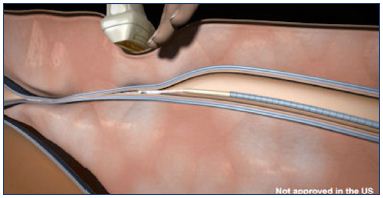
Figure 6. Compression of the treated vein using an ultrasound
transducer above the catheter and injected glue.
Mechanochemical ablation
Recently, a new hybrid mechanochemical device (ClariVein) has been developed. Mechanochemical endovenous ablation (MOCA) achieves venous occlusion by utilizing a wire within the lumen of the vein that rotates at 3500 rpm, which abrades the intima and causes venospasms, thereby increasing the efficacy of the sclerosant (Figures 7 and 8). A liquid sclerosant (sodium tetradecyl sulfate or polidocanol) is concomitantly infused through an opening close to the distal end of the catheter near the rotating wire. These two modalities–mechanical and chemical–achieve venous occlusion results equal to endothermal methods. The system includes an infusion catheter, motor drive, stopcock, and syringe. The dispersion wire extends through the catheter lumen and it is connected to an interface cartridge unit for connection to the 9V DC battery of the motorized handle unit on the proximal end, which controls wire rotation. The handle unit also provides a grip and syringe holder to facilitate physician-controlled infusion. The wire and the catheter sheath are inserted percutaneously into the vein under site anesthesia while the patient is in a reversed Trendelenburg position. The catheter sheath is retracted to expose the wire tip, which is positioned 2 cm from the saphenofemoral junction. The patient is then rotated into a flat position for the remainder of the procedure. The catheter motor is turned on and the catheter is pulled down the vein at a rate of approximately 1 to 2 mm/second, while the wire rotates and the sclerosing agent is infused. After removal of the catheter, occlusion of the great saphenous vein and patency of the common femoral vein is checked by duplex ultrasound.

Figure 8. The ClariVein rotating wire abrades the vein wall, while
the sclerosing agent is infused through the catheter opening.
The advantages of this hybrid system are claimed to be standard percutaneous access, endovenous treatment, local anesthesia only (without the need for tumescent anesthesia), and short procedure time. Since the system does not use thermal energy, the potential for nerve damage is minimized. Compression is applied for 2 weeks without restricting the patient’s activity.29
In a small series of 25 patients presenting with great saphenous vein incompetence, minor postoperative complications were identified, including localized ecchymosis at the puncture site in 9 patients and transient thrombophlebitis of distal tributaries in 4 patients.30 In a series of 50 patients presenting with small saphenous vein incompetence, minor postoperative complications were identified, including localized ecchymosis induration around the puncture site (12%) and transient thrombophlebitis of the treated vein (14%).31
Pelvic and ovarian vein embolization
When varicose veins are fed by incompetent pelvic and ovarian veins through the pelvic floor, which may or may not be related to left renal or iliac vein compression, embolization of the refluxive veins by coils and sclerosing agents is a minimally invasive method. Nevertheless, when reflux is related to iliac vein compression iliac stenting, another noninvasive technique, is the first-line treatment.32,33
Conclusions
Currently, there are a number of surgical options for treating varicose veins, but there is no definitive system for identifying which people will benefit the most from interventional treatment and no established framework for the diagnosis and management of varicose veins. Conversely, perioperative investigations are well stated and described. In a review of the randomized controlled trials on the treatment of varicose veins, the authors concluded that there are many treatment options available for the ablation of varicose veins, not solely thermal ablation.34,35
Part II of the present article will describe the outcomes of the various procedures for varicose vein ablation, the guidelines that have been recently established, and the tentative recommendations for the use of endovenous techniques.

1. Franceschi C. Theorie et Pratique de la Cure Conservatrice et Hémodynamique de l’ Insuffisance Veineuse en Ambulatoire. Precy-sous-Thil, France: Editions de l’Armancon; 1988.
2. Pittaluga P, Chastanet S, Rea B, Barbe R. Midterm results of the surgical treatment of varices by phlebectomy with conservation of a refluxing saphenous vein. J Vasc Surg. 2009;50:107-118.
3. Morrison C, Dalsing MC. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: prevalence, severity, and relevance for modern practice. J Vasc Surg. 2003;38:886-890.
4. Casoni P, Lefebvre-Vilardebo M, Villa F, Corona P. Great saphenous vein surgery without high ligation of the saphenofemoral junction. J Vasc Surg. 2013;58:173-178.
5. Huang TW, Chen SL, Bai CH, Wu CH, Tam KW. The optimal duration of compression therapy following varicose vein surgery: a meta-analysis of randomized controlled trials. Eur J Vasc Endovasc Surg. 2013;45:397-402.
6. Mariani F, Marone EM, Gasbarro V, et al. Multicenter randomized trial comparing compression with elastic stocking versus bandage after surgery for varicose veins. J Vasc Surg. 2011;53:115-122.
7. Rudström H, Björck M, Bergqvist D. Iatrogenic vascular injuries in varicose vein surgery: a systematic review. World J Surg. 2007;31:228-233.
8. Sutton PA, El-Duhwaib Y, Dyer J, Guy AJ. The incidence of post operative venous thromboembolism in patients undergoing varicose vein surgery recorded in Hospital Episode Statistics. Ann R Coll Surg Engl. 2012;94:481-483.
9. Van Rij AM, Chai J, Hill GB, Christie RA. Incidence of deep vein thrombosis after varicose vein surgery. Br J Surg. 2004;91:1582-1585.
10. Sam RC, Silverman SH, Bradbury AW. Nerve injuries and varicose vein surgery. Eur J Vasc Endovasc Surg. 2004;27:113- 120.
11. Zamboni P, Franceschi C. Principles of Venous Hemodynamics. Hauppauge, NY. Nova Science Publishers; 2009.
12. Vuylsteke ME, Thomis S, Mahieu P, Mordon S, Fourneau I. Endovenous laser ablation of the great saphenous vein using a bare fibre versus a tulip fibre: a randomised clinical trial. Eur J Vasc Endovasc Surg. 2012;44:587-592.
13. Thomis S, Verbrugghe P, Milleret R, Verbeken E, Fourneau I, Herijgers P. Steam ablation versus radiofrequency and laser ablation: an in vivo histological comparative trial. Eur J Vasc Endovasc Surg. 2013;46:378-382.
14. van den Bos RR, Milleret R, Neumann M, Nijsten T. Proof-of-principle study of steam ablation as novel thermal therapy for saphenous varicose veins. J Vasc Surg. 2011;53:181-186.
15. Milleret R, Huot L, Nicolini P, et al. Great saphenous vein ablation with steam injection: results of a multicentre study. Eur J Vasc Endovasc Surg. 2013;45:391-396.
16. Yang L, Wang XP, Su WJ, Zhang Y, Wang Y. Randomized clinical trial of endovenous microwave ablation combined with high ligation versus conventional surgery for varicose veins. Eur J Vasc Endovasc Surg. 2013;46:473- 479.
17. D ermody M, O’Donnel TF, Balk EM. Complications of endovenous ablation in randomized controlled trials. J Vasc Surg Venous Lymphat Disord. 2013;1:427-436.
18. Anwar MA, Lane TR, Davies AH, Franklin IJ. Complications of radiofrequency ablation of varicose veins. Phlebology. 2012;47(suppl 1):34-39.
19. D exter D, Kabnick L, Berland T, et al. Complications of endovenous lasers. Phlebology. 2012;47(suppl 1):40-45.
20. van der Bos RR, Malskat WS, De Maeseneer MG, et al. Randomized clinical trial of endovenous laser ablation versus steam ablation (LAST trial) for great saphenous varicose veins. Br J Surg. 2014;101:1077-1083.
21. Cabrera J, Cabrera García-Olmedo JR. Nuevo método de esclerosis en las varices tronculares. Patologia Vascular. 1995;4:55-73.
22. Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27:58-60.
23. Coleridge Smith P. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32:577-583.
24. Rabe E, Breu FX, Cavezzi A, et al; Guideline Group. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29:338-354.
25. Goldman MP, Bergan JJ, Guex JJ. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. 4th edition. Philadelphia, PA. Mosby Elsevier; 2007.
26. Lawson JK, Gauw S, van Vlijmen C, et al. Sapheon: the solution? Phlebology. 2013;28(suppl 1):2-9.
27. Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. 2013;1:174- 180.
28. Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Twoyear follow-up of first human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. Phlebology. 2015;30:397-404.
29. Elias S, Lam YL, Wittens CH. Mechanochemical ablation: status and results. Phlebology. 2013;28(suppl 1):10- 14.
30. van Eekeren RR, Boersma D, Elias S, et al. Endovenous mechanochemical ablation of great saphenous vein incompetence using the ClariVein_ device: a safety study. J Endovasc Ther. 2011;18:328-334.
31. Boersma D, van Eekeren RR, Werson DA, van der Waal RI, Reijnen MM, de Vries JP. Mechanochemical endovenous ablation of small saphenous vein insufficiency using the ClariVein_ device: one-year results of a prospective series. Eur J Vasc Endovasc Surg. 2013;45:299-303.
32. Bora A, Avcu S, Arslan H, Adali E, Bulut MD. The relation between pelvic varicose veins and lower extremity venous insufficiency in women with chronic pelvic pain. JBR-BTR. 2012;95:215-221.
33. Monedero JL, Ezpeleta SZ, Perrin M. Pelvic congestion syndrome can be treated operatively with good long-term results. Phlebology. 2012;27(suppl 1):65- 73.
34. Eklöf B, Perrin M. Randomized controlled trials in the treatment of varicose veins. I. Phlebolymphology. 2011;18:196-208.
35. Perrin M, Eklöf B. Randomized controlled trials in the treatment of varicose veins. II. Phlebolymphology. 2012;19:92-99.