Variants of functional venous disease
Anita CARLIZZA
San Giovanni Hospital,
Rome, Italy
SUMMARY
In the continuing absence of a consensus definition, functional venous disease (FVD) can be described as encompassing any situation in which, over time, hemodynamic changes in venous drainage transform the risk factors for varicose veins into overt disease. The condition includes patients with primary varicose veins. This description is consistent with epidemiological evidence of incipient reflux, and encompasses functional chronic venous insufficiency (CVI) due to extravenous causes, also described as the functional phase of the prevaricose syndrome. In addition, it includes the syndrome of constitutional functional venous disease (CFVD) in which decreased vein wall tone and stasis microangiopathy are associated with the symptoms (heaviness, tension, and pain) and certain signs (lower-limb edema, cellulite, and permanent acral hypothermia) of venolymphatic hypertension, but without reflux, organic macrovascular disease or progression to overt varicose disease.
INTRODUCTION
In chronic venous insufficiency (CVI), blood flow is impaired by acquired or congenital venous dilatation or obstruction. The hemodynamic common denominator in all venous disease is reflux due to valvular incompetence, obstruction (thrombosis), or congenital venous or extravenous abnormalities.1 Reflux can be long or short, depending on the junction (saphenofemoral or saphenopopliteal) or perforator involved.
In functional venous disease (FVD), the symptoms of CVI occur in the absence of organic superficial and/or deep venous disease. Classic-variant FVD is due to extravenous impairment of the push-and-pull mechanisms of venous drainage. The push mechanism consists of compression of the venous plexus in the sole of the foot, the calf muscle pump, and the pulsation of the contiguous arterial system. Pull is exerted by the diaphragm and negative mediastinal pressure.
Multiple extravenous factors can impair these mechanisms: sedentary lifestyle, obesity, respiratory and cardiac disease, work in the standing position, repeated pregnancy (inferior vena caval compression, altered endocrine environment, inadequate diaphragmatic excursion), peripheral arterial disease (where low perfusion pressure is combined with low or absent pulsatility), lower-limb disease (nerve root pathology, foot arch deformities), and anatomically inappropriate shoes.
The consequences range from transient edema to venolymphedema and trophic lesions, without organic venous disease. Significant reflux, whether long or short, is absent. Venous drainage from the periphery is simply impaired, resulting in stasis or slow flow. The relative contributions to the cause of varicose veins by these extrinsic factors and a still putative inherited predisposition remain unknown.2 However, the combination of flat feet, obesity, sedentary lifestyle and/or respiratory failure is almost inevitably associated with venolymphedema, even in the absent of a positive family history.
Cloarec et al defined a prevaricose syndrome encompassing intermediate phases between a normal venous system and overt varicose disease.3,4 Careful investigation for reflux in symptomatic but apparently healthy subjects revealed incipient hypotonicity of the vein wall, altered venous reactivity, hyperdistensibility, and wall thickening. Cloarec et al argued that subjects at risk required routine screening and prophylactic intervention to avoid progression to overt disease triggered by coexistent extravenous risk factors.
A subsequent prevalence study, the Italian Acireale project, found a prevaricose syndrome (family history, altered venous compliance) in only 22.6% of its population; it was absent in 15.9% of symptomatic subjects, whom the authors classified as having hypotonic venous disease. They concluded that this hypotonic variant of FVD was unlikely to be a prodromic phase of varicose disease, ie, prevaricose syndrome, since their controlled 10-year follow-up showed progression to overt disease only in the ‘valvular incompetence without varicose veins’ group.5 These results were consistent with those of Cloarec et al, who found no progression to overt varicose disease in 75% of their symptomatic population.
In addition to prevaricose syndrome, these studies thus identified a group of patients with the symptoms of CVI (peripheral edema, and heavy, restless, and painful legs), but with no specific risk factors, no evidence of reflux, and no progression to varicose disease.
CONSTITUTIONAL FVD
In 1997 we published our 10-year data on 300 controls and 820 patients with sporadic diffuse and/or regional edema, heavy restless legs, lipodermatosclerosis (cellulite), permanent objective acral hypothermia, subjective diffuse hypothermia, an allergic diathesis, and low-normal systolic blood pressure (100 ± 5 mm Hg; World Health Organization: 90–130 mm Hg). The women in this population also reported menstrual abnormalities (frequency, quantity, and duration) and benign breast disease.6 Investigations included endocrine screening (testosterone, dehydroepiandrosterone, 17â estradiol, progesterone, and prolactin on day 21 of the menstrual cycle) and traditional nailfold capillaroscopy (Wild capillaroscope, magnification x 10; parameters: loop count, morphology, distribution, dimension); 78 consecutive patients underwent dynamic capillaroscopy (capillary red blood cell velocity, relative microhematocrit), microlymphography (microlymphatic loop count, microlymphatic diameter, endolymphatic pressure), laser Doppler (resting flux, venoarterial reflex), and venous echotomography (femoral and popliteal venous diameter ratios between the supine and upright positions). The data (Tables I–V) were analyzed using Student’s t test.
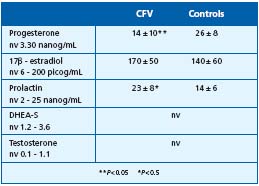
Table I. Results

Table II. Results
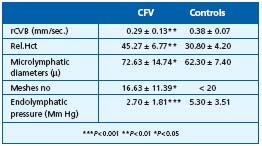
Table III. Constitutional and functional venopathy Dynamic
capillaroscopy and microlymphography.

Table IV. Constitutional functional venopathy Laser – Doppler.

Table V. Changes in large vein diameter during Valsalva manoeuver
and in orthostatic position.
COMMENTS
In women, endocrine changes account for many dysfunctional complaints typical of the childbearing years, including idiopathic cyclic edema, menstrual migraine, and premenstrual mastalgia. In women with FVD, they also account for diffuse edema, specific adipose tissue distribution, a menstrual symptom pattern, and cellulite.
The demonstration of venular and endolymphatic hypertension is consistent with postural edema and could be considered the microvascular pathogenesis of cellulite. Our study identified a constitutional FVD syndrome – CFVD – defined by symptoms of venolymphatic hypertension (heavy, restless legs, tension, cramps, pain), decreased venous tone, cellulite, and permanent acral hypothermia, and by the absence of reflux or any evidence of structural macrovascular pathology on clinical examination or standard methods of investigation. The signs differentiating it from the prevaricose patients described by Cloarec et al
CFVD is a syndrome lying midway between an endocrine disorder and the stasis microangiopathy possibly responsible for cellulite. In terms of the Clinic-Etiologic-Anatomic- -Pathophysiologic (CEAP) classification, it may be difficult to differentiate from the prevaricose syndrome (C: 0-4; E: Ep; A: 0; P: unclassified; clinical score: 1-2; anatomic score: 0; disability score: 1). However, diagnosis is important for several reasons: CFVD is common, accounting for 30% of our referrals (Table VI); the CFVD entity is more than a cosmetic complaint of cellulite; and correct diagnosis avoids inappropriate therapy, whether with compression (which is poorly tolerated) or, worse, surgery. Patients can instead be offered effective venotonics (flavonoids and dihydroergotamine), lifestyle advice (high-protein diet, avoidance of sport involving high sustained mediastinal and/or abdominal pressure), and physiotherapy (heat therapy in iodinated salt water, manual lymph drainage, etc).

Table VI. Constitutional functional venopathy.


