Varicose veins during pregnancy: risk factors and impact on quality of life

Sena Dilek Aksoy, PhD, RN
Department of Midwifery,
Faculty of Health Science,
Kocaeli University, Kocaeli, Turkey
Derya Kanza Gul, MD
School of Medicine Health,
Medipol University, Istanbul,
Turkey
Ayça Solt Kirca, PhD, RM
Department of Midwifery, School
of Health, Kirklareli University,
Kirklareli, Turkey
ABSTRACT
Objective: This study was carried out to examine the risk factors for varicose veins during pregnancy and their impact on quality of life.
Material and methods: This was a prospective, cross-sectional observational study in pregnant women in their second and third trimesters. In addition to the collection of sociodemographic and lifestyle data, the presence of varicose veins was assessed using the clinical, etiological, anatomical, and pathophysiological (CEAP) questionnaire, and the quality of life was assessed using the Chronic Venous Insufficiency Questionnaire (CIVIQ-20). Logistic regression analysis was conducted to identify independent risk factors for varicose veins.
Results: A total of 658 women were included. Considering all types of varicose veins, prevalence of varicose veins was 29% (191 women). Varicose veins presence was found to be significantly associated with gestational week (odd ratio [OR], 1.047; 95% CI, 1.013-1.083; P=0.03), thyroid diseases (OR, 2.474; 95% CI, 1.109-5.522; P=0.019), smoking status during pregnancy (OR, 7.294; 95% CI, 2.408-22.093; P<0.001). As regards the quality-of-life evaluation, scores in all CIVIQ-20 dimensions—physical (mean deviation [MD], -4.30; 95% CI, -4.76 to 3.83; P<0.001), psychological (MD, -8.67; 95% CI, -9.60 to 7.73; P<0.001), social (MD, -3.13; 95% CI, -3.48 to 2.79; P<0.001), pain (MD, -3.94; 95% CI, -4.37 to 3.51; P<0.001)—and the global index score (MD, 25.06; 95% CI, 22.50 to 27.62; P<0.001) were significantly higher in patients with varicose veins than in those without (P<0.001).
Conclusions: In this prospective, observational study in pregnant women, gestational week, thyroid diseases, smoking status during pregnancy, and positive family history were identified as risk factors for varicose veins, and the presence of varicose veins was found to negatively impact quality of life in this setting.
Introduction
Approximately half of the world’s populations suffer from varicose veins (VV).1 It is thought that both valve dysfunction and venous pressure play a key role in the onset and progression of the disease.2 In addition, sexual hormones are implicated in venous pathology.3 Pregnancy related dilatation that occurs in various parts of the body triggers lower extremity VV formation.4 In pregnancy, the total blood, serum, and erythrocyte volumes show an increase. Especially the total blood volume, which is around 4000 mL, reaching 5300 mL in the 36th gestational week. Moreover, the decrease in the tension of the blood vessels leads to slowed blood flow and swollen legs. As a result of this, VV formation occurs in women who are predisposed.5 Whereas some studies have shown that the blood flow rate was significantly lower in pregnant women with venous deficiency, a significant reduction was observed in the blood flow rate especially in the last 3 months of pregnancy. This rate is especially at its lowest level in the 36th gestational week of pregnancy.5,6 A reduction in blood flow rate may lead to substantial pain, night cramps, numbness, tingling sensation, and itching.7 Although VV observed in pregnancy is a distressing experience during pregnancy, it may decrease or disappear after birth.4 Only limited data are available on the prevalence of VV and on risk factors for VV during pregnancy.4,8 This is an observational study conducted to estimate the prevalence of VV, to identify risk factors for VV, and to assess the impact on quality of life (QOL) of VV presence in pregnancy.
Methods
Study design and participants
This observational study was carried out between July 21, 2020 and October 31, 2020 at the gynecology and obstetrics polyclinics of a private hospital in Istanbul, which is the largest province of Turkey. The number of pregnant women who visited the gynecology polyclinics between January 1 and December 31, 2019 was approximately 2500. In the study, the sample size was calculated by using the method of known population sampling. The minimum number of individuals to be included in the sampling was calculated with a 95% confidence interval (CI) (α=0.05), P=0.050, and N=2500 population numbers and determined to be 334. Considering the possibility of loss of participants to no follow-up and/or data loss, a design effect of 2 was accepted, and approximately 600 patients were considered sufficient for the sample size (Figure 1).
Inclusion criteria
To fulfill inclusion criteria, participants needed to be in the second or third trimester of pregnancy, having a singleton pregnancy.
Exclusion criteria
Participants were excluded if they were unable to understand the research (eg, could not speak or understand Turkish).
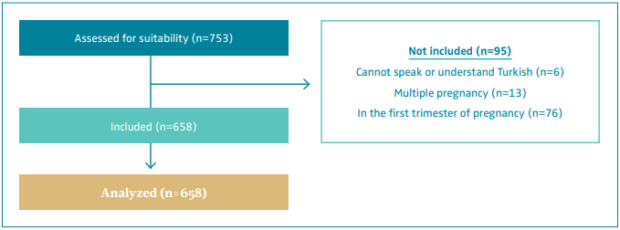
Figure 1. Diagram of the research process.
Data collection instruments and data
collection procedure
collection procedure
Information about sociodemographic and obstetric characteristics was collected and the Chronic Venous Insufficiency Questionnaire (CIVIQ-20)8-11 and clinical, etiological, anatomical, and pathophysiological (CEAP) classification questionnaire were completed by one of the investigators during a face-to-face interview with each participant. In addition, a second investigator (a gynecologist) filled out the CEAP scoring instrument by observation.
Disease diagnoses were recorded not according to the statements of the participants but within the framework of the examinations in the hospital’s pregnancy follow-up system.
The CEAP classification questionnaire is a well-established tool to aid in the diagnosis and classification of chronic venous disease. Women with CEAP >C1 are considered to have VV.9
The CIVIQ-20 is a QOL questionnaire that consists of 20 questions and covers 4 QOL fields regarding chronic venous insufficiency: physical, psychological, social disorders, and severity of pain. The total CIVIQ-20 score is obtained by summing the scores of 20 items for all subscales. The lower the score, the higher the QOL. The global index score is reached via a special calculation technique. The higher the global index score, the higher the QOL.10,11
Statistical methods
The data were analyzed by using IBM SPSS V23. Compatibility with normal distribution was examined by the Kolmogorov Smirnov test. Chi-squared and Fisher’s exact tests were used to compare the categorical variables based on the groups. The Mann-Whitney U test was used to compare the non-normally distributed data between 2 groups. Binary logistic regression analysis and the Hosmer and Lemeshow Test were used to identify independent risk factors for VV. The results of the analyses are presented as mean ± standard deviation and median (minimum – maximum) for the quantitative data and as frequencies (percentages) for the categorical dWata. P values less than 0.05 were accepted as statistically significant.
Ethics committee approval
Ethics committee approval was received for this study from the Ethics Committee of Kirklareli University (Protocol No. 69456409-199-E.10627). The study was carried out in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its subsequent amendments. The author(s) can provide copies of the appropriate documentation if requested.
Patient consent
All participants were informed about the study and signed a consent form, knowing that they could withdraw from the study whenever they wanted.
Results
The study included a total of 658 pregnant women, whose mean age was 29.14±4.59 (min:18; max:45) years. Their sociodemographic, obstetric, and CEAP classification features are shown in Table I. Considering all types of VV, prevalence of VV was 29% (191 pregnant women) (Table I).
On average, patients with VV had a higher body mass index (P=0.008) and had a more advanced pregnancy (P=0.030). They were also more frequently smokers (during pregnancy) (P<0.001) than those without VV. Additionally, patients with VV were significantly more likely to have diseases accompanying pregnancy (P=0.019) and to have a family history of varices (P<0.001). On the other hand, these 2 groups were not significantly different in terms of age, gravida, and parity.
As regards the QOL evaluation, the physical disorder scores, psychological disorder scores, social disorder scores, pain scores, and global index score median values were significantly higher in patients with VV than in those without (P<0.001) (Table II).
The independent risk factors affecting VV presence were examined by binary logistic regression analysis. According to our analysis, gestational week, disease accompanying pregnancy (thyroid diseases), smoking status (during pregnancy), and family history of VV are significantly associated with VV presence. Each gestational week led to a 1.047-increased risk for VV (OR, 1.047; 95% CI, 1.013- 1.083; P=0.006). The presence of thyroid disease increased by approximately 2.5 the chances of having VV (OR, 2.474; 95% CI, 1.109-5.522; P=0.027). We found a 7-fold increased risk for VV in women smoking during pregnancy (OR, 7.294; 95% CI, 2.408-22.093; P<0.001) and a 213-fold increased risk in those with a family history of VV (OR, 213.437; 95% CI, 87.248-522.138; P<0.001) (Table III).
Discussion
Pregnancy plays an important role in the onset and progression of VV in women. Changes that occur in the venous system during pregnancy are associated with not only hormonal secretions but also compression of the iliac veins by the uterus.1 In this study, the independent risk factors affecting VV presence were examined by a binary logistic regression analysis, and the effect of VV on QOL in pregnant women was investigated.
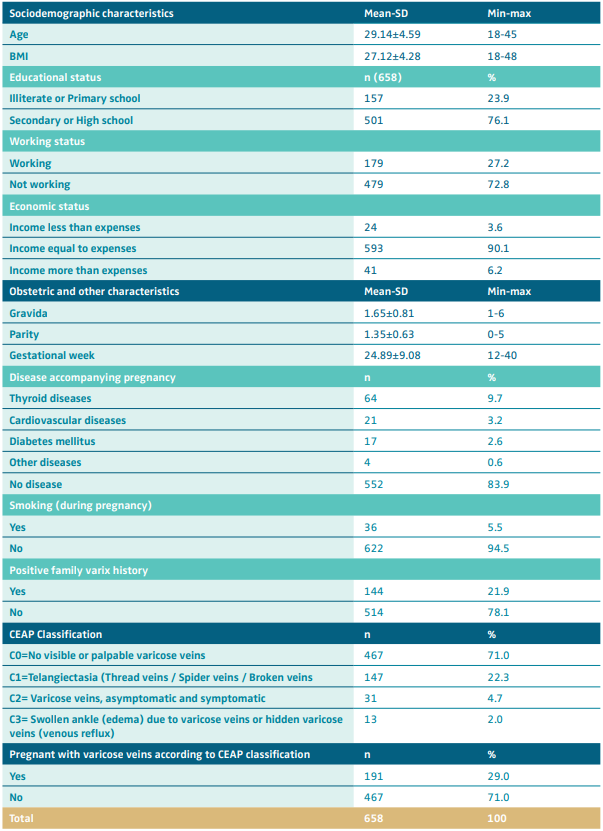
Table I. Sociodemographic and clinical characteristics of included women (n=658).
Abbreviations: BMI, body mass index; CEAP, clinical, etiological, anatomical and pathophysiological classification; SD, standard deviation.
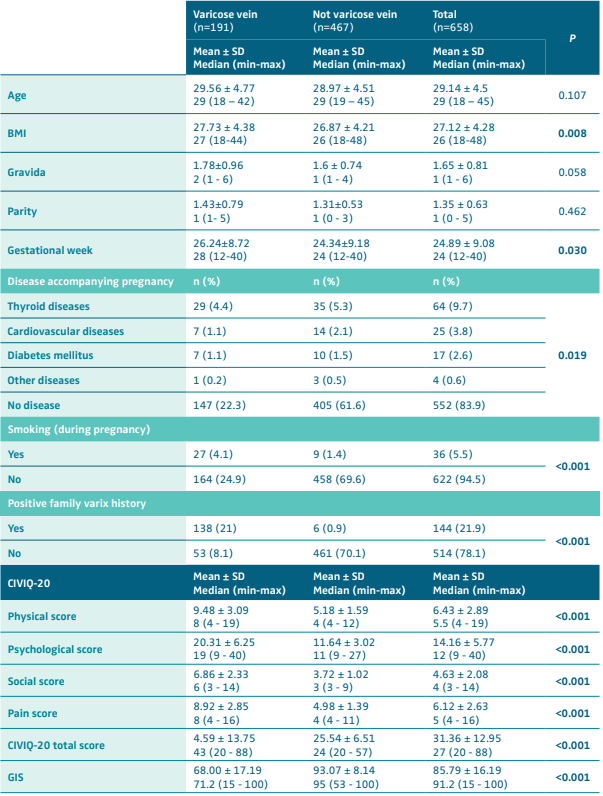
Table II. Comparaison of variables based on varicose vein presence (n+658). Abbreviations: BMI: BMI, body mass index; CIVIQ-20, Chornic Venous Insufficiency Questionnaire; GIS, global index socre; SD, standard deviation. VV, varicose veins. P<0.05, bold values are statistically significant; mean ± standard deviation, median (min-max).
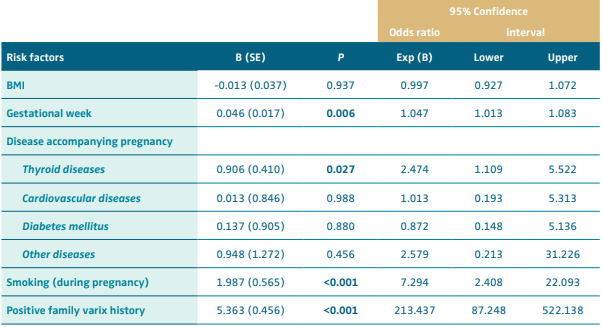
Table III. Risk factors associated with varicose vein presence (n=658). Abbreviation: B, regression coefficient; BMI, body mass index; SE, standart error. Classification table: Overall percentage: 71.0; Model Chi-Squared: 424.977; df:8; P<0.001; Hosmer-Lemeshow Test: 4.605; df: 8; P=0.799; P<0.05, bold values are statistically significant.
The prevalence of VV in this study was 29% (191 women). In a study conducted in Brazil in 2010, VV prevalence was found to be 72%.4 In the study conducted in Iran, the prevalence was 18%.8 There is a very substantial heterogeneity in the prevalence results.
Pregnancy is a significant risk factor in VV formation.8 It has been reported in the literature that hormones such as estrogen and progesterone have an important role in the emergence of varices in most women in pregnancy.3,12 In our study, it was found that the risk of VV increased as the gestational week increased. On the contrary, an epidemiological study conducted on 566 adults in Budapest determined that hormonal factors did not have an effect on VV formation.13 Our finding that the risk of VV increased with the gestational week could be due to the fact that we only included women in their second and third trimesters of pregnancy. Considering the effect of progesterone hormone on smooth muscles during pregnancy, such a result is likely. In the literature, it was reported that varix complaints in pregnancy emerge at the beginning of the second trimester.14
In our study, it was determined that thyroid diseases can be a risk factor for VV during pregnancy. Dominguez et al (2018) determined that those with venous disease had a higher prevalence of hypothyroidism (17%) than the general population (2%-5%).15 Kılınç et al (2021) emphasized that hypothyroidism may be a risk factor for the development of chronic venous insufficiency by impairing endothelial function.16 The possibility of thyroid disease to be associated with negative pregnancy and birth outcomes is a significant result that has emerged especially in recent years. For this reason, the American Thyroid Association drew attention to the guidelines on the diagnosis and management of thyroid diseases during pregnancy and in the postpartum period.17 VV can also be evaluated within this framework when thyroid screening is performed in pregnant women.
Smoking is an important risk factor in many chronic venous diseases including VV.18 In their study in 1806 patients, Gourgou et al (2002) reported that smoking 10 to 20 cigarettes a day increased the risk of venous disease 1.7 times, whereas smoking more than 20 cigarettes a day increased this risk 2.4 times.19 In our study, the VV risk of those who smoked was 7 times higher than those who did not smoke during their pregnancy.
It has been proven in various studies that family history plays a significant role in the emergence of VV.4,20 In contrast, a population-based cohort study of 4903 people in Finland found that results regarding the effects of family history on VV were biased, reducing the credibility of reports suggesting a strong genetic component.21 In this study, it was determined that family history increased the risk of having VV by 213 times.
Studies have shown that the QOL of those with VV is negatively affected.22,23 In the study by Wik et al (2011), which used the VEINES (Venous Insufficiency Epidemiological and Economic Study)-QOL/Sym questionnaire, it was seen that disruption of venous circulation in pregnancy affected QOL negatively.23 In our study, we found that the presence of VV had a negative impact on pregnant women’s physical disorder, psychological disorder, social disorder, and pain scores, as well as their CIVIQ-20 total scores and global index score. Overall, it has been determined that VV may negatively affect the QOL in pregnant women. Vuylsteke et al (2015) demonstrated that factors like being a woman, advanced age, and family history of varices were associated with higher CEAP scores, and this was correlated with poorer QOL and global index score.22 In a recent study conducted with people with cardiovascular diseases in Romania, it was observed that the physical, psychological, and social functionality components of QOL were negatively affected in people with VV.24
Limitations
The strengths of the study are that it focuses on a topic that has been ignored in recent years and that it was performed in a large population. As the data were collected from a single center, the results of the study may not be generalized to the entire population. As the presence of varices was assessed visually based on CEAP scores, this may not provide as strong evidence as data obtained by measurement instruments such as Doppler ultrasonography.
Conclusions
This study we conducted regarding venous disease in pregnancy helped identify various risk factors and impact on QOL.
In the study, gestational week, disease accompanying pregnancy (thyroid diseases), smoking status (during pregnancy), and family varix history were determined as significant risk factors in VV presence in pregnancy. It was also found that the QOL of the pregnant women experiencing varix problems was negatively affected. The data we obtained may provide humble support for prevention of VV formation during pregnancy and for controlling the progression of the disease in the presence of risk factors. There is a need for broad-scoped prevalence and incidence studies that examine VV formation and effects in pregnancy.
References
1. Cornu-thenard A, Boivin P. Chronic venous disease during pregnancy. Phlebolymphology. 2014;21(3):138-144.
2. Lim CS, Kiriakidis S, Paleolog EM, Davies AH. Increased activation of the hypoxia inducible factor pathway in varicose veins. J Vasc Surg. 2012;55(5):1427-1439. doi:10.1016/j.jvs.2011.10.111.
3. Ortega MA, Asúnsolo Á, Álvarez-rocha MJ, et al. Remodelling of collagen fibres in the placentas of women with venous insufficiency during pregnancy. Histol Histopathol. 2018;33(6):567-576. doi:10.14670/HH-11-948.
4. JE de, Junior FM. Pregnancy and lower limb varicose veins: prevalence and risk factors. J Vasc Bras. 2010;9(2):29-35.
5. Gimunová M, Zvonař M, Kolářová K, et al. Changes in lower extremity blood flow during advancing phases of pregnancy and the effects of special footwear. J Vasc Bras. 2017;16(3):214-219. doi:10.1590/1677-5449.002617.
6. Ropacka-Lesiak M, Jarosław K, Bręborowicz G. Pregnancy-dependent blood flow velocity changes in lower extremities veins in venous insufficiency. Ginekol Pol. 2015;86(9):659-665. doi:10.17772/ gp/59224.
7. Smyth RM, Aflaifel N, Bamigboye AA. Interventions for varicose veins and leg oedema in pregnancy. Cochrane Database Syst Rev. 2015;2015(10):CD001066. doi:10.1002/14651858.CD001066.pub3.
8. Dara A, Mahrokh A, Mehdi KG. Prevalence of pregnancy varicose and its effective factors in women referred to gynecology hospitals in Tabriz. Iran J Obstet Gynecol Infertil. 2019;22(9):1-7.
9. Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342-352. doi:10.1016/j. jvsv.2019.12.075.
10. Launois R, Mansilha A, Jantet G. International Psychometric Validation of the Chronic Venous Disease Quality of Life Questionnaire (CIVIQ-20). Eur J Vasc Endovasc Surg. 2010;40(6):783-789. doi:10.1016/j.ejvs.2010.03.034.
11. Launois R. A quality of life tool kit in chronic venous disorders. Phlebolymphology. 2014;21(3):152-160.
12. Lenković M, Cabrijan L, Gruber F, et al. Effect of progesterone and pregnancy on the development of varicose veins. Acta Dermatovenerol Croat. 2009;17(4):263- 267.
13. Bihari I, Tornoci L, Bihari P. Epidemiological study on varicose veins in Budapest. Phlebology. 2012;22(2):77-81. doi:10.1258/phleb.2011.010063.
14. Rabe E, Breu FX, Cavezzi A, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2013;29(6):338-354. doi:10.1177/0268355513483280.
15. Dominguez JU, Lopez PF, Ulloa JH, Salazar J, Penagos A. Prevalence of hypothyroidism in adults with chronic venous insufficiency. Acta Phlebol. 2018;19(3):112-114. doi:10.23736/S1593-232X.18.00430-7.
16. Kılınç F, Akbaş A, Şener S, Hayran Y, Aktaş A. Cutaneous findings in patients with chronic venous insufficiency. J Cosmet Dermatol. 2022;21(5):2106-2112. doi:10.1111/jocd.14337.
17. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315-389. doi:10.1089/ thy.2016.0457.
18. Piazza G. Varicose Veins. Circulation. 2014;130(7):582-587. doi:10.1161/ CIRCULATIONAHA.113.008331.
19. Gourgou S, Dedieu F, Sancho-Garnier H. Lower limb venous insufficiency and tobacco smoking: a case-control study. Am J Epidemiol. 2002;155(11):1007- 1015. doi:10.1093/aje/155.11.1007.
20. Sharma S, Vashist M, Vashist MG. Family history as major predisposing factor in varicose veins disorder. Eur J Biomed Pharm Sci. 2017;4(12):392-396.
21. Ahti TM, Makivaara LA, Luukkaala T, Hakama M, Laurikka JO. Effect of family history on the risk of varicose veins is affected by differential misclassification. J Clin Epidemiol. 2010;63:686-690. doi:10.1016/j.jclinepi.2009.10.003.
22. Vuylsteke ME, Thomis S, Guillaume G, Modliszewski ML, Weides N, Staelens I. Epidemiological study on chronic venous disease in Belgium and Luxembourg: prevalence, risk factors, and symptomatology. Eur J Vasc Endovasc Surg. 2015;49(4):432-439. doi:10.1016/j.ejvs.2014.12.031.
23. Wik HS, Enden TR, Jacobsen AF, Sandset PM. Long-term quality of life after pregnancy-related deep vein thrombosis and the influence of socioeconomic factors and comorbidity. J Thromb Haemost. 2011;9(10):1931-1936. doi:10.1111/ j.1538-7836.2011.04468.x.
24. Branisteanu D-E, Feodor T, Baila S, Mitea I-A, Vittos O. Impact of chronic venous disease on quality of life: results of vein alarm study. Exp Ther Med. 2019;17:1091-1096. doi:10.3892/ etm.2018.7054.
