VEIN STEP: Chronic VEnous dIsorders maNagement and Treatment Effectiveness Evaluation in Chronic Venous Disease, an International Observational Prospective Study. Results from Morocco
Jorge Hernando ULLOA, MD,
FACS3
1Faculty of Medicine and Pharmacy,
Mohammed V University, Rabat, Morocco;
2Clinical Hematology Department, Ibn
Sina University Hospital, Rabat, Morocco;
3Fundacion Santa Fe de Bogota, Bogota,
Colombia
Abstract
Aim: VEIN STEP is an international, observational, prospective study assessing the effectiveness of conservative treatments for the relief of chronic venous disease (CVD) symptoms and their impact on quality of life (QOL). This article presents results collected in Moroccan patients. Method: Adult outpatients consulting for symptomatic CVD were categorized via the Clinical, Etiological, Anatomical, Pathophysiological (CEAP) classification system. Intensity of symptoms and their improvement with treatment was assessed using both patient-reported (10-cm Visual Analog Scale [VAS], Patient Global Impression of Change [PGIC], and 14-item ChronIc Venous Insufficiency Questionnaire [CIVIQ-14]) and physician-reported outcomes (Venous Clinical Severity Score [VCSS]) on the first day of enrollment and after 4 weeks. Results: A total of 3425 patients were recruited by 122 general practitioners. Mean (± SD) age was 49.5 ± 13.3 years, and 83.1% were women. CEAP classifications were C0 (2.5%), C1 (23.0%), C2 (31.7%), C3 (34.8%), and C4-C6 (8%). Conservative therapy comprised: venoactive drugs (VADs) in 98.9% of patients, mainly micronized purified flavonoid fraction (MPFF) (75.7%); compression therapy (38.2%), and topical treatment (30.1%). These therapies were prescribed alone or in combination. At 4 weeks, VAS mean global severity score decreased from 4.6±2.1 to 2.2±1.5 with any VAD alone, and from 4.7±2.0 to 2.2±1.3 with MPFF alone. When used in combination with compression, the score was reduced from 5.1±1.9 to 2.7±1.6 with any VAD and from 5.0±2.0 to 2.5±1.5 with MPFF. Overall, conservative therapy reduced VAS symptom intensity by 50.3% for pain, 51.8% for heaviness, 51.0% for cramps, and 50.4% for swelling. PGIC showed an improvement for 97.8% of patients at 4 weeks. Improvements in QOL were observed across all three CIVIQ-14 dimensions (pain, physical, and psychological) (P≤0.001). Physician-reported VCSS showed conservative treatment led to a significant decrease in CVD severity at week 4 from 5.6±3.7 to 3.1±2.4 for all CEAP classes combined. Both patients and physicians reported a high level of satisfaction with conservative treatments and no adverse events. Conclusion: This observational study provides large-scale data from a real-life Moroccan setting using both patient- and physician-reported outcomes. Conservative treatment, mainly in the form of VADs, and in particular MPFF, was associated with improvements in symptoms and QOL.
Introduction
Chronic venous disease (CVD) affects a significant proportion of populations worldwide and can be associated with considerable morbidity with adverse effects on quality of life (QOL).1,2 Before the introduction of the Clinical, Etiological, Anatomical, Pathophysiological (CEAP) classification system, diagnosis of chronic CVDs lacked precision. CEAP classification provided a universally understandable description and has become an instrument to standardize diagnosis and allow better communication about CVD between health care professionals worldwide. There is a strong link between increasing CEAP clinical grades and deterioration in disease specific QOL, and demonstrable morbidity has been observed even with uncomplicated venous disease.3 The most severe CEAP grades have been associated with a level of physical impairment comparable to that seen in patients with congestive cardiac failure and/or chronic lung disease.3
The large, international VEIN Consult program used the same questionnaire and same classification method in all participating countries and reported CVD prevalence rates of 52% in Asia, 70% in Eastern Europe, 68% in Latin America, and 62% in Western Europe.2 Risk factors for the development and progression of CVD are prevalent in most populations and include advancing age, excess body weight, sedentary lifestyles, and occupations, and a family history of the disease.4 Many of these are associated with lifestyle and nutrition habits typical of industrial economies and are in common with a number of other noncommunicable diseases. Thus, the burden of CVD-related morbidity, disability, and socioeconomic costs looks set to increase in coming decades,5 including in developing countries such as Morocco.
The consequences of CVD are far more than cosmetic, as even those with low CEAP classifications (C0-C2) may experience associated symptoms such as aching or heaviness in their legs.6,7 At these early stages, interventions may not even be sought or prescribed, yet effective treatment may provide immediate symptom relief8 and has the potential to delay or prevent the progression of the disease and the development of severe complications.9,10 QOL and impact on productivity only worsen with more severe stages of CVD, and represents a substantial burden on health care systems.11-13
A wide range of treatment options is available for the management of CVD, both conservative and invasive.14 Conservative approaches aim mainly to restore the altered physiological functions of the venous system. They incorporate the following: advice on lifestyles changes including weight loss, exercise, and leg elevation; use of compression therapy to decrease ambulatory venous hypertension; and pharmacotherapy. The latter, in the form of venoactive drugs (VAD), has assumed an increasingly important role since the recognition of inflammation and primary venous valve failure as important pathogenetic mechanisms in CVD.15,16 Subsequent updates to international guidelines also acknowledged that the beneficial effects of VAD were not just due to their effects on venous tone.17,18
Micronized purified flavonoid fraction (MPFF), in particular, via its ability to attenuate various elements of the inflammatory cascade,19 has been shown to be a highly effective agent,20 which is associated with a series of grade A recommendations in recent guidelines in relation to beneficial effects on CVD symptoms, skin changes, and QOL.14
In order to ensure optimal treatment practices and outcomes, there is a need to assess the characteristics of CVD in representative samples of the general population and to examine the way it is managed in everyday clinical practice. The aim of the VEIN STEP program (Chronic VEnous dIsorders maNagement and Treatment Effectiveness Evaluation in Chronic Venous Disease, an International Observational Prospective Study) was to obtain up-to-date data on the conservative treatments that patients are being prescribed in routine clinical practice and the effectiveness of these treatments for the relief of CVD signs and symptoms. VEIN STEP is being conducted in a number of countries around the world. This article describes the findings from Morocco.
Methods
Study design
The VEIN STEP program in Morocco is part of an international, observational, prospective study. It was conducted between June 2020 and December 2020 to gather nationally representative data on the conservative treatment of patients with venous disorders in routine clinical practice. General Practitioners were asked to select consecutive patients, whenever possible, who met the following minimal set of inclusion criteria: aged ≥18 years old; consulting spontaneously or referred for treatment of symptomatic CVD; having a diagnosis of CVD according to physician’s judgment; and existence of written, informed patient consent. Non-inclusion criteria comprised the following: undergoing current treatment for CVD either with a VAD or compression hosiery; presence of lower-limb arterial disease; presence of concomitant disease or treatment that may interfere with lower-limb pain or edema; having any procedure or surgery planned during the study for CVD; and, if female, being pregnant or breastfeeding.
The primary objective of the study was to assess, in a real-life setting, the effectiveness of conservative treatments on CVD signs and symptoms and QOL using a combination of patient-reported outcomes and physician assessments. Data were collected from ambulatory patients who were eligible for inclusion via a standardized electronic case-report form.
At the initial inclusion visit (visit 0 [V0]), patients underwent a clinical examination of the lower limbs and were assigned a CEAP clinical classification.21 Patients were asked to indicate the global intensity of their symptoms (pain, heaviness, cramps, sensation of swelling) with the use of a 10-cm visual analog scale (VAS). The intensity of paresthesia (tingling), itching, and burning sensation symptoms was assessed using a 4-point scale. Patient QOL was assessed using the validated 14-item ChronIc Venous Insufficiency Questionnaire (CIVIQ-14).22
Following examination of the patient, the physicians completed the Venous Clinical Severity Score (VCSS).23 The score includes 10 clinical parameters (pain, varicose veins, venous edema, skin hyperpigmentation, inflammation, induration, number of ulcers, durations of ulcers, size of ulcers, and adherence to compression therapy). Each item is graded from 0 to 3 depending on severity (None = 0, Mild = 1, Moderate = 2, Severe = 3). At V0, patients were prescribed conservative treatment according to the physicians’ usual practice. This could include pharmacological or nonpharmacological treatment (such as compression therapy, oral VAD, painkillers, topical treatment). Provision of lifestyle advice was optional and dependent on the physician’s usual practice.
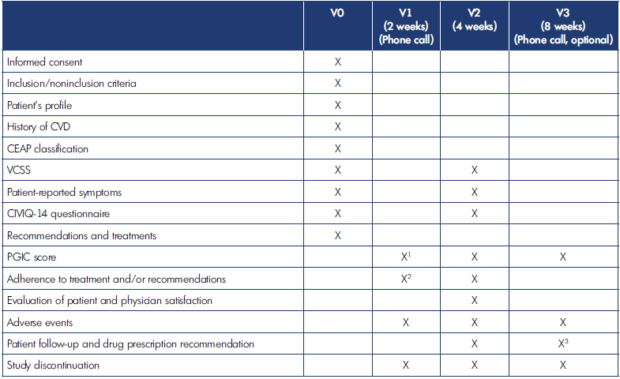
Table I. Summary of assessments performed according to visit. CEAP, Clinical, Etiological, Anatomical, Pathophysiological classification; CIVIQ-14, 14-item ChronIc Venous Insufficiency Questionnaire; CVD, chronic venous disease; PGIC, Patient Global Impression of Change; VCSS, Venous Clinical Severity Score.
1 At V1, the patient will be asked to indicate which symptoms have improved and time to improvement, if any.
2 At V1, abbreviated compliance without providing details.
3 At V3, the investigator will record treatment and follow-up without providing details.
Visit 1 (V1) took place approximately 2 weeks later by telephone, at which point global symptom improvement was determined by questioning the patient and completing the Patient Global Impression of Change (PGIC) questionnaire. PGIC is a 7-point scale depicting a patient’s rating of overall improvement. Patients rate their change as “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” or “very much worse.”
Visit 2 (V2) took place approximately 4 weeks after inclusion and assessed global and individual symptom intensity (VAS), symptom improvement (PGIC), QOL (CIVIQ-14), and physician reported symptom severity (VCSS). Patients’ satisfaction with treatment was a secondary end point and assessed at V2 using a 5-item scale (very unsatisfied, unsatisfied, neutral, satisfied, very satisfied). An additional, optional telephone follow-up (visit 3 [V3]) could be conducted around 8 weeks after inclusion. Patients were questioned about adherence to treatment and lifestyle recommendations and about adverse events at each contact with the physician. A summary of the assessments performed at each visit is provided in Table I.
VEIN STEP was a noninterventional study according to the European Regulation EU No. 536/2014. Physicians were instructed to continue management and treatment of participants according to their usual practice. No specific investigations or therapies were prescribed as part of this study, and therefore patient care was not influenced. All data were collected anonymously. As this was not an investigation of clinical outcomes with any particular intervention, neither Ethics Committee approval nor clinical trial registration was required.
Statistical analysis
Statistical analyses were mainly descriptive and performed in included patients with complete data for the main variables. Quantitative data were represented by the number of patients and expressed as the mean value and standard deviation; qualitative data were expressed as the number and percentage of patients. All statistical analyses were performed with the SAS_ software version 9.4 or higher. Statistical significance was assumed when P<0.05 (2-sided).
Results
Patient characteristics
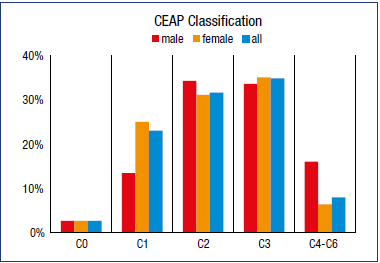
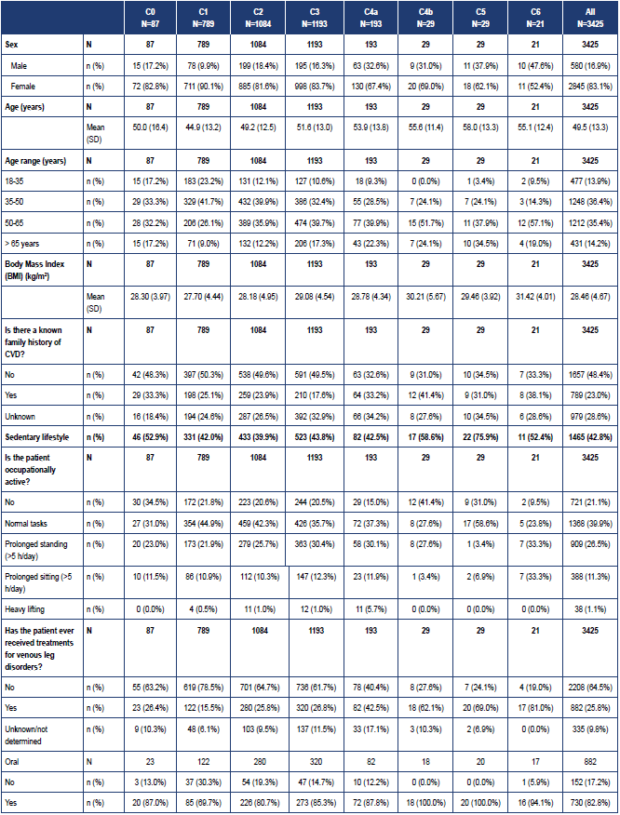
In the Moroccan arm of VEIN STEP, a total of 3425 patients were enrolled by 122 investigators. Data were available for 2929 patients at V1, 3285 at V2, and 1554 at the optional 8-week follow-up visit (V3). Mean age was 49.5 ± 13.3 years, mean body mass index was 28.5 ± 4.7 kg/m2, and the majority had never smoked (90.5%). In the overall population, there was a higher proportion of women than men (83.1% versus 16.9%), but the proportion of women to men differed for the individual CEAP classes, with a higher proportion of women than men in the C1 class, and a higher proportion of men than women in classes C4-C6 (Figure 1). Patients’ baseline characteristics by CEAP class are shown in Table II.
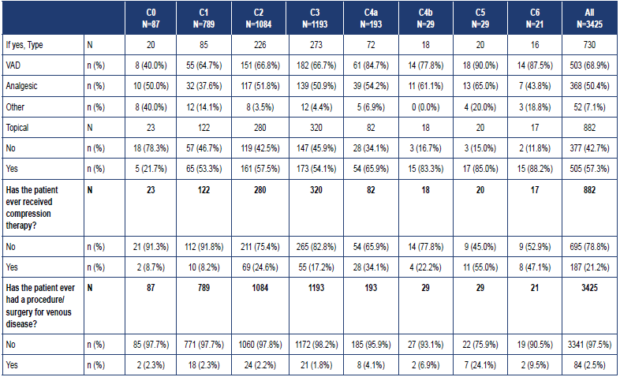
Among the women in the study, two-thirds (65.5%) had given birth at least once, and the mean number of births was 3.4 ± 1.7 with a mean time since last delivery of 17.8 ± 12.5 years. A sedentary lifestyle was reported for 42.8% of patients, just over a quarter (26.5%) reported their occupation required spending more than 5 hours standing per day, and 11.3% more than 5 hours sitting. A family history of CVD was reported in 23.0%. In 24.9%, the first signs or symptoms of CVD appeared before the age of 30 years. Among the 18.2% of patients who reported suffering from other venous disorders: 91.8% had previously had hemorrhoidal disease, 7.2% deep vein thrombosis, 1.4% pelvic congestion syndrome, and 1.0% post-thrombotic syndrome. A quarter (25.8%) had received previous treatment for venous leg disorders: 82.8% had received an oral agent (68.9% VAD, 50.4% analgesic, 7.1% other), 57.3% had received a topical agent, and 21.2% a prescription for compression therapy (38.0% bandages, 65.2% stockings). The proportion who had received previous treatment ranged from 26.4% of C0 patients to 81.0% of C6 patients. Only 2.5% of patients had previously had a procedure/surgery for venous disease.
CEAP, Clinical, Etiological, Anatomical, Pathophysiological classification; CVD, chronic venous disease; VAD, venoactive drug.
Table II. Patient demographics and baseline characteristics according to Clinical, Etiological, Anatomical, Pathophysiological (CEAP) class.
CEAP clinical classification
Following a clinical examination of the lower legs by the physician, 2.5% of subjects were classed as C0, 23% were C1 (telangiectasies or reticular veins), 31.6% were C2 (varicose veins), 34.8% were C3 (edema), and 7.9% were C4-C6 (skin and subcutaneous tissue changes and/or healed or active ulcers) (Figure 1). At baseline, the mean number of symptoms across CEAP classes was 5.5 ± 1.8. Across CEAP classes C0 to C3, symptoms were more likely to be experienced after prolonged standing or at the end of the day (reported by 27% and 37% of patients). However, in classes C4 and above, symptoms were more likely to be reported all the time (reported by 22% [C4a] to 71% [C6] of patients).
Lifestyle advice and conservative treatments prescribed
At V0, 95.4% of patients received lifestyle advice. This included recommendations to exercise regularly and lose weight if necessary, avoid periods of prolonged standing or sitting, wear comfortable footwear, improve venous return by leg elevation, regular leg movement, and massage, and also addressed the importance of skin hygiene. The majority of patients were prescribed a conservative treatment that included a VAD (98.9%). Among the VADs, MPFF was the most widely prescribed agent (75.7%); other VADs included diosmin (21.5%), ruscus extract (1.2%), and proanthocyanidins (1.1%). Analgesics, either oral or topical, were prescribed in 44.5% of patients. Conservative treatments prescribed at V0 were: oral VAD alone (31.7%), oral VAD and analgesic (16.0%), oral VAD and compression (12.9%), oral VAD and compression and topical treatment and analgesic (11.7%), oral VAD and compression and analgesic (8.8%), oral VAD and topical treatment and analgesic (6.5%), oral VAD and topical treatment (6.1%), oral VAD and compression and topical treatment (4.1%), other treatment (1.6%), topical treatment alone (0.3%), compression alone (0.1%), and analgesics alone (0.1%) (Table III).
At week 4, adherence to lifestyle advice was 97.3%. Among the patients prescribed conservative treatment, 97.7% of patients were described as adherent to VAD and 85% to compression therapy. The main reasons for nonadherence to compression were described as discomfort (55.2%), skin irritation (33.3%), and sweating (32.2%).
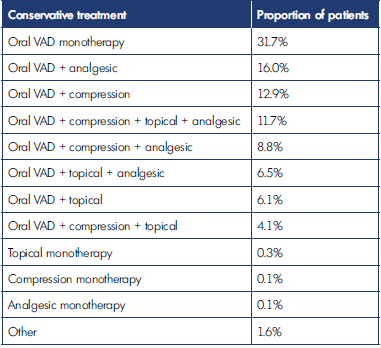
VAD, venoactive drugTable III. Conservative treatments for chronic venous disease prescribed at baseline.
Patient-reported outcomes
Global symptom intensity assessed with Visual Analog Scale
At V0, mean VAS global symptom intensity across the CEAP classes was 5.2 overall, but ranged from 4.2 to 7.7, increasing in intensity with CEAP class. Following 4 weeks of conservative treatment, mean overall score was 2.6 and had decreased significantly in each CEAP class (P<0.001). VAD-based treatments led to significant reductions in global symptom intensity at week 4. For VAD treatment alone, VAS scores decreased from 4.6 ± 2.1 at V0 to 2.2 ± 1.5 at V2 (P<0.01) (Figure 2). Statistically significant reductions in symptom intensity were also observed when VAD treatment was combined with compression therapy or topical treatment, with slightly greater reductions in intensity for MPFF versus other VAD combinations (Figure 2). When analyzed individually, the greatest reductions in intensity were observed for the symptoms of pain, heaviness, cramps, and swelling with reductions in intensity of 46% to 57% (Figure 3). Reductions were slightly less (27%-39%), but still significant for the symptoms of paresthesia, itching, and burning (Figure 3). These reductions in symptom intensity were achieved whether treatment was with VAD alone or combined with compression or topical therapy. For all symptoms, a slightly greater reduction in intensity was observed with MPFF compared with all VADs combined (Figure 3).
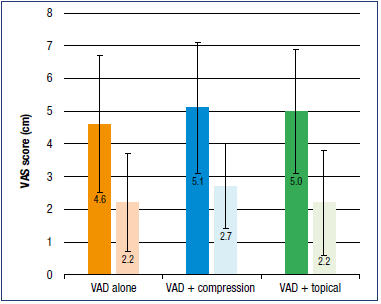
Figure 2. Mean global symptom intensity score at week 4 (light colors) vs week 0 (dark colors).
Abbreviations: VAD, venoactive drug, VAS, Visual Analog Scale.
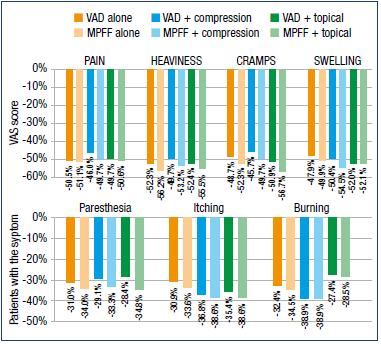
Figure 3. Mean individual symptom intensity score at week 4.
Abbreviations: MPFF, micronized purified flavonoid fraction; VAD, venoactive drug, VAS, Visual Analog Scale.
Global symptom improvement assessed with Patient Global Impression of Change
For all patients combined, regardless of conservative treatment prescribed, improvement was already noted in 92.8% of patients at V1 (week 2) and had increased to 97.8% by V2 (week 4); only 6.8% and 2.1% of patients reported no change at V1 and V2, respectively. Improvement was noted for 98.6% of patients at the optional 8-week follow-up visit (V3).
Similar results were observed for VAD treatment, either alone or in combination with other conservative therapies. Across all CEAP classes, the symptoms improved the most by conservative therapy were pain (improved in 88.6% patients), heaviness (69.3%), cramps (52.1%), and sensation of swelling (38.4%). At baseline, the mean number of symptoms across CEAP classes was 5.5 ± 1.8. At visit V2, this had decreased to 3.8 ± 2.0 (P<0.001). Improvements in these symptoms were observed within a mean of 8.4 ± 2.8 days.
Quality of life assessed with 14-item ChronIc Venous Insufficiency Questionnaire
Conservative treatment was also associated with a significant improvement in patients’ QOL after only 4 weeks, assessed using CIVIQ-14 (14-item ChronIc Venous Insufficiency Questionnaire). This was observed across all 3 CIVIQ-14 dimensions (pain, physical and psychological) (P<0.001), in the overall population and in the patients who received treatment with VAD, either alone or in combination with compression or topical therapy (Figure 4).
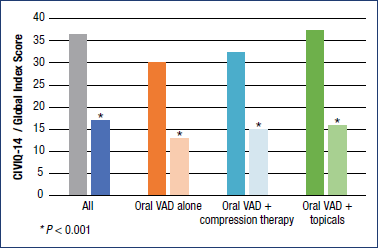
Figure 4. CIVIQ-14 assessed patient quality of life at week 4.
Abbreviations: CIVIQ-14, 14-item ChronIc Venous Insufficiency
Questionnaire; VAD, venoactive drug.
Physician-assessed CVD severity
Physician-assessed VCSS data at V0 and V2 were available for approximately two-thirds of patients (67.3% at V0 and 64.9% at V2). There was a strong association between the severity of CVD measured by the VCSS and the CEAP clinical classification, with baseline scores ranging from 3.2 ± 3.0 for C1 to 16.8 ± 3.6 for C6. The only exception was for patients in the C0 class in whom baseline VCSS score was 6.2 ± 6.0, which was similar to the score for C3 patients (6.0 ± 2.7). Nevertheless, regardless of CEAP clinical class at V0, conservative treatment led to a significant decrease in CVD severity at V2 (week 4) (P<0.001) (Figure 5).
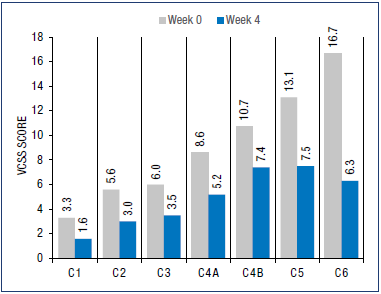
Figure 5. Venous clinical severity score at week 4.
Abbreviations: VCSS, Venous Clinical Severity Score.
Patient and physician satisfaction with treatment prescribed
Overall, 87.8% of the subjects and 89.6% of physicians were satisfied or very satisfied with the treatment prescribed. The proportions of patients and physicians satisfied or very satisfied with VAD treatment alone were 89.2% and 92.6%, respectively. Rates of patient satisfaction were similar when VAD was combined with compression (88.9% satisfied or very satisfied). High rates of satisfaction were observed whether VAD treatment, including MPFF, was prescribed alone or in combination with other conservative therapy.
Safety and tolerability
No adverse events were reported.
Discussion
The results of this observational research describe the patterns of CEAP classification of patients presenting with CVD and how they are managed in routine Moroccan clinical practice. In agreement with findings from other large, international, observational studies, including the VEIN Consult1 and VEIN ACT (Chronic VEnous dIsorders maNagement and evaluAtion of Chronic venous disease treatment effecTiveness) programs,7 the majority of patients (75%) were already classed with a CEAP grade of C2 or higher, despite three-quarters of them never having received a treatment for CVD. In VEIN STEP, patients were enrolled by general practitioners. The fact that patients were presenting for treatment only at these later CEAP stages suggests that venous disorders are not being recognized or considered serious enough for treatment in their early stages, and therefore not referred to specialists, and/or that patients do not consult until visible signs are present.
As in most CVD observational studies of this type, there was a greater proportion of women than men overall. However, when analyzed by CEAP class, the female majority was apparent in CEAP C1, sex distribution was approximately equal in C2 and C3, but thereafter in C4-C6, there was a greater prevalence in men than women. A greater predominance of trophic changes in men has also been observed in other studies.24
Current guidelines recognize the importance of VADs as part of conservative treatment for CVD.14 This was reflected in the treatments received with almost all patients (98.9%) being prescribed a VAD, alone or in combination with compression and topical treatment, as part of their conservative treatment. MPFF made up the largest proportion of VAD treatment (75.7%), and while it shares some of its benefits on CVD symptoms with other VADs, it is the only agent in the class to also receive an A grade recommendation for improvements in skin changes and QOL.14 VAD treatments including MPFF were associated with a high level of adherence with 97.7% of patients taking their medication as prescribed. Compression therapy using bandaging or graduated compression hosiery is an integral part of the management of CVD, but poor adherence to compression is well documented in the literature.7,25,26 In the VEIN ACT program, 65.2% of patients were adherent to the required duration of therapy for VADs, but only 29.1% wore their compression therapy as prescribed,7 with patients often citing discomfort as the main reason for nonadherence. Even when there is a strong indication for compression therapy, such as patients with venous leg ulcers, complete adherence has been reported to be as low as 40%.27 In the current report, compression adherence was lower than for VAD, but still higher than in other studies with a rate of 85% at 4 weeks.
Conservative treatment, whether it comprised VAD alone or in combination with compression or topical therapy, was effective at reducing the global intensity of CVD symptoms across all CEAP classes after only 4 weeks of treatment, with VAS scores approximately halved compared with baseline. Significant reductions in symptom intensity were also observed when the CVD symptoms were considered individually. Whereas reductions were greatest for the symptoms of pain, heaviness, cramps, and swelling (in the region of 46%-57%), important reductions in intensity for paresthesia, itching, and burning were also observed (in the region of 27%-39%). All of these symptoms can be present from the outset, before any clinical signs of the disease are evident. Previous data have shown that QOL can be affected by even “mild-” or “moderate intensity” CVD symptoms,28 further highlighting the importance of an early CVD diagnosis. The current study assessed QOL via CIVIQ-14, a validated international scale specific to CVD. At 4 weeks, conservative treatment had resulted in a significant improvement in the CIVIQ-14 Global Index Score (which includes physical, psychological, and social functioning components of QOL) whether VADs were prescribed alone or in combination with other conservative treatments.
The self-reported PGIC measure, which reflects a patient’s belief about the efficacy of treatment, was performed at 2 and 4 weeks. It requires patients to calculate the difference between their current and previous health state based on a Likert scale. Patients experienced the benefits of conservative treatment rapidly with 92.7% already reporting they were improved at 2 weeks, and almost all patients noting a change for the better at 4 weeks (97.8%). The benefits of treatment were also reflected in the secondary end point of patient and physician satisfaction, with around 90% reporting they were satisfied or very satisfied with treatment at 4 weeks, regardless of whether VAD was administered alone or in combination with compression or topical therapy.
To supplement the patient-reported outcome data, the effects of treatment were also assessed by physicians using the VCSS. The VCSS was designed to complement the CEAP classification by providing a method for physicians to assess changes over time and in response to an intervention and thereby provide a standard clinical language to report and compare differing approaches to CVD management.23 As expected, baseline VCSS was mostly correlated with baseline CEAP class. The exception was for patients classified as C0, who had a similar mean VCSS score to C3 patients. A potential explanation is that a proportion of C0 patients may be suffering from valvular incompetence at the microvascular level, which while not sufficient to cause visible venous signs can nevertheless trigger the release of inflammatory mediators and stimulate nociceptive fibers.29,30 Significant reductions in VCSS were observed for patients in each CEAP class after 4 weeks of conservative treatment.
The results from VEIN STEP confirm that conservative therapy, which mainly comprised VAD, was effective at reducing the intensity of CVD symptoms and improving patient QOL at all stages of the disease. Nevertheless, there are a number of advantages to early diagnosis and treatment. First, lifestyle changes and avoidance measures can be introduced, for example by reducing excess body mass, implementing exercise routines, and raising the legs at rest. Second, appropriate management of patients with early stage venous disease can relieve symptoms and improve QOL, which can already be seriously affected even in patients in low CEAP classes.20,31 Third, by targeting the causes of CVD, namely inflammation and retrograde venous flow, early treatment can help limit the number of patients who progress to more severe CVD stages and avoid the need for expensive and invasive treatment. This not only improves the QOL of patients, but will also provide economic advantages for a disease that has significant costs, particularly in its later stages.14
This observational study is potentially limited by the possibility of sample bias, incomplete survey response data, as well as the inaccuracy of self-reported behavior. To minimize variability and ensure results were representative of the general Moroccan population with CVD, efforts were taken to recruit as wide a range of participants as possible from practices distributed throughout the country in both urban and rural areas and from a variety of practice types.
The results of this observational research capture important large-scale data on the management of patients with CVD in routine Moroccan clinical practice, a country for which there is a sparsity of data on the management of CVD. VEIN STEP is an international program, and the results for Morocco will soon be complemented by those from other countries to provide an up-to-date overview of treatment practices and their efficacy across the globe. It is hoped that enhanced awareness among physicians will result in more patients receiving effective treatment earlier in the course of CVD to alleviate symptoms and delay or prevent disease progression and the development of severe complications.
Conclusions
VEIN STEP provides large-scale data from a real-life setting on the current management of CVD with conservative treatments. Results from Morocco reinforce that treatment with VADs and in particular MPFF, is associated with improvements in symptom intensity and QOL at all stages of the disease.

REFERENCES
1. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F; VCP Coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31(2):105-115.
2. Vuylsteke ME, Colman R, Thomis S, Guillaume G, Van Quickenborne D, Staelens I. An epidemiological survey of venous disease among general practitioner attendees in different geographical regions on the globe: the final results of the Vein Consult Program. Angiology. 2018;69(9):779-785.
3. Carradice D, Mazari FA, Samuel N, Allgar V, Hatfield J, Chetter IC. Modelling the effect of venous disease on quality of life. Br J Surg. 2011;98(8):1089-1098.
4. Labropoulos, N. How does chronic venous disease progress from the first symptoms to the advanced stages? A review. Adv Ther. 2019;36:13-19.
5. Nicolaides AN, Labropoulos N. Burden and suffering in chronic venous disease. Adv Ther. 2019;36:1-4.
6. Benigni JP, Bihari I, Rabe E, et al; UIP – Union Internationale de Phlébologie. Venous symptoms in C0 and C1 patients: UIP consensus document. Int Angiol. 2013;32(3):261-265.
7. Bogachev V, Arribas JMJ, Baila S, Ulloa Dominguez J, Walter J. Management and evaluation of treatment adherence and effectiveness in chronic venous disorders: results of the international study VEIN Act Program. Drugs Ther Perspect. 2019;35:396-404.
8. Tsoukanov YT, Tsoukanov AY, Nikoiaychuk A. Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment. Phlebolymphology. 2017;22(1):3-11.
9. Labropoulos N, Leon L, Kwon S, et al. Study of the venous reflux progression. J Vasc Surg. 2005;41(2):291-295.
10. Mansilha A. Early stages of chronic venous disease: medical treatment alone or in addition to endovenous treatments. Adv Ther. 2020;37:13-18.
11. Kuet ML, Lane TR, Anwar MA, Davies AH. Comparison of disease-specific quality of life tools in patients with chronic venous disease. Phlebology. 2014;29(10):648- 653.
12. Launois R. Health-related quality-of-life scales specific for chronic venous disorders of the lower limbs. J Vasc Surg Venous Lymphat Disord. 2015;3(2):219-227.
13. Rabe E, Pannier F. Societal costs of chronic venous disease in CEAP C4, C5, C6 disease. Phlebology. 2010;25(suppl 1):64- 67.
14. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence part I. Int Angiol. 2018;37(3):181-254.
15. Takase S, Pascarella L, Bergan JJ, Schmid- Schonbein GW. Hypertension-induced venous valve remodeling. J Vasc Surg. 2004;39:1329-1334.
16. Pascarella L, Schmid-Schonbein GW, Bergan J. An animal model of venous hypertension: the role of inflammation in venous valve failure. J Vasc Surg. 2005;41:303-311.
17. Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1- 59.
18. Kearon C, Kahn SR, Agnelli G, et al; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):454S- 545S.
19. Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19(6):1669.
20. Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (MPFF) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebocontrolled trials. Int Angiol. 2018;37(2):143- 154.
21. Eklöf B, Rutherford R, Bergan J, Carpentier P, Gloviczki P. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248-1252.
22. Launois R, Le Moine JG, Lozano FS, Mansilha A. Construction and international validation of CIVIQ-14 (a short form of CIVIQ-20), a new questionnaire with a stable factorial structure. Qual Life Res. 2012;21(6):1051-1058.
23. Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52(5):1387-1396.
24. Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158(5):448-456.
25. Raju S, Hollis K, Neglen P. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Ann Vasc Surg. 2007;21(6):790-795.
26. Moffatt C, Kommala D, Dourdin N, et al. Venous leg ulcers: patient concordance with compression therapy and its impact on healing and prevention of recurrence. Int Wound J. 2009;6(5):386-393.
27. Heinen MM, van der Vleuten C, de Rios MJM, Uden CJT, Evers AWM, van Achtenberg T. Physical activity and adherence to compression therapy in patients with venous leg ulcers. Arch Dermatol. 2007;143(10):1283-1288.
28. Branisteanu DE, Feodor T, Baila S, Mitea IA, Vittos O. Impact of chronic venous disease on quality of life: results of vein alarm study. Exp Ther Med. 2019;17(2):1091-1096.
29. Lugli M, Maleti O, Iabichella ML, Perrin M. Investigation of non-saphenous veins in C0S patients. Int Angiol. 2018;37(2):169- 175.
30. Vital A, Carles D, Serise JM, Boisseau MR. Evidence for unmyelinated C fibres and inflammatory cells in human varicose saphenous vein. Int J Angiol. 2010;19:e73- 77.
31. Davies AH. The seriousness of chronic venous disease: a review of real-world evidence. Adv Ther. 2019;36(suppl 1):5-12.