VenaSeal™ closure system for varicose veins
Columbus, Ohio, US
Abstract
Endothermal treatments are now considered the new gold-standard treatment for eliminating venous reflux in patients with chronic venous insufficiency. In a quest to minimize the invasiveness, nonthermal techniques that do not require tumescent anesthesia have been developed in the last decade. These new nonthermal, tumescent-less techniques are well tolerated and result in equivalent outcomes compared with endothermal ablations. VenaSealTM, one such technique, utilizes a proprietary cyanoacrylate glue to occlude the saphenous vein. Studies using VenaSealTM have demonstrated high anatomic success rates with closure rates >90% reported at 3 years. Sustained improvements in patient-reported clinical outcomes have been reported up to 36 months. No major adverse events or thrombotic complications have been reported with this procedure. Phlebitis and skin reactions are the most common minor adverse events. Adoption of a particular nonthermal procedure depends on several factors, such as the learning curve, initial set-up costs, overall cost-effectiveness, and reimbursement. VenaSealTM does not have any other initial set-up costs, and the procedure is simple, consistent, and easy to learn. To date, no data regarding cost-effectiveness are available. The purpose of this document is to summarize the VenaSealTM data and discuss the procedural steps.
Introduction
Chronic venous disease is a fairly common condition, with the prevalence estimated at 175 million in the US.1 However, the prevalence of advanced, symptomatic venous disease (chronic venous insufficiency) is relatively lower; it is estimated to be 5% of the population.2 Advanced stages of chronic venous insufficiency can result in significant disability and affect quality of life.3 The last 20 years have seen a major transformation in the management of chronic venous insufficiency, with guidelines recommending endovenous therapies as the preferred methods of treatment over surgical vein stripping.4,5
The newer nonthermal nontumescent techniques do not require the use of tumescent anesthesia and include cyanoacrylate glue, VenaSealTM, mechanochemical ablation, Clarivein, and the proprietary endovenous microfoam, Varithena. All of these techniques are approved for use in saphenous veins. The advantagesof nonthermal nontumescent techniques, apart from fewer needle sticks and the discomfort associated with tumescent anesthesia, include the lack of heat-induced thrombosis and skin injuries. Treatment from the saphenofemoral junction to the most distal refluxing portion of the saphenous veins, without concern for nerve injury is also an advantage of the nonthermal nontumescent technologies.
Cyanoacrylate glue has long been used in the management of intracranial arteriovenous malformations, pelvic variceal, and gastric variceal treatments.6 VenaSealTM, a proprietary cyanoacrylate glue, is an n-butyl cyanoacrylate with unique properties, including quick polymerization upon contact with blood and high viscosity. These properties help prevent embolization. VenaSealTM cyanoacrylate glue is also designed to be pliable and to allow flexion and torsion once solidified.
A preclinical swine model demonstrated that induction of an inflammatory reaction in the vein wall led to fibrotic occlusion of the vein over the implant, which is a distinctly different finding compared with the endothelial injury caused by thermal ablation or sclerotherapy.7 VenaSealTM received the CE mark in 2011 and it was approved by the US Food and Drug Administration (FDA) in February 2015. Table I shows the current worldwide availability of VenaSealTM.
Clinical Studies
In an initial preclinical feasibility study, Almeida et al studied the VenaSealTM procedure in 38 patients.8 No postprocedural compression therapy was used and no adjunctive therapies were allowed for 6 months after the incident procedure. The maximum diameter of the saphenofemoral junction in this study was 8±2.2 cm. At 3 years, a 94.7% occlusion rate was noted. The mean Venous Clinical Severity Score (VCSS) improved from baseline (6.1±2.7) to 3 years (2.2±0.4) (P<0.0001). While no major complications were noted, mild and self-limited phlebitis was reported in 15.8% of patients, which was responsive to nonsteroidal anti-inflammatory treatment. Postprocedural duplex ultrasounds also demonstrated thread-like thrombus and glue extensions into the common femoral vein in 8 patients (21.1%), which resolved after a few weeks of anticoagulation therapy. In this study, the catheter tip was positioned 1.5 to 2 cm away from the saphenofemoral junction and the first two aliquots of cyanoacrylate glue injected simultaneously. The technique was modified for all subsequent trials by positioning the catheter tip 5 cm away from the saphenofemoral junction and by injecting the first two aliquots of the cyanoacrylate glue 1 cm apart, rather than simultaneously.9 This modification was made in the VenaSeal™ instructions-for-use document as well.10
The next VenaSealTM study was eSCOPE, a European prospective multicenter registry involving 70 subjects. VenaSeal™ treatment resulted in a 92.9% closure rate at the 12-month end point.11 The study design was similar to the feasibility study; no postprocedure compression socks were used for this study. The average volume of glue used in this study was 1.58 mL. Adverse events included a mild, self-limited phlebitis in 11.4% of the patients. No thrombotic events were reported.
The VeClose study, a pivotal trial in the US, is a prospective, multicenter, randomized (1:1) clinical trial comparing VenaSealTM cyanoacrylate glue (n=108) with radiofrequency ablation (n=112). This study was designed to demonstrate statistical noninferiority of cyanoacrylate glue to radiofrequency ablation. Both arms received compression therapy to avoid any confounding factors. No adjunctive therapies were allowed up to 3 months after the incident procedure. Data collection included closure rates, patient-reported quality-of-life scores, including the clinical, etiological, anatomical, and pathophysiological (CEAP) classification, VCSS, EuroQual-5D (EQ-5D), and Aberdeen Varicose Vein Questionnaire (AVVQ). The primary end point of the study, complete closure of the great saphenous vein at the end of 3 months, was achieved in 99% of patients in the VenaSealTM group and 96% in the radiofrequency ablation group (adjudicated by Core lab). At 36 months, these closure rates were 94.4% and 91.9%, respectively.12
Surprisingly, the intraoperative pain scores were not statistically different between the two groups, despite the use of tumescent anesthesia in the radiofrequency ablation group. Ecchymosis rates in the treated segment were significantly lower in the cyanoacrylate glue group compared with the radiofrequency ablation group. The VCSS and AVVQ scores improved significantly at 3 months and were sustained over the study time (up to 36 months) in both groups without any significant between group differences. Multiple imputation models (optimistic and pessimistic models) showed that cyanoacrylate glue was noninferior to radiofrequency ablation. No deep vein thrombosis (DVT) or pulmonary embolus (PE) occurred. Phlebitis occurred in 20% of the patients in the VenaSealTM group and 14% of the patients in the radiofrequency ablation group (P=0.36). In both groups, most of these episodes were mild, transient, and treated successfully with anti-inflammatory therapy. At 36 months, late-onset phlebitis was reported in one patient and an access site scar was reported in the VenaSealTM group, while no adverse events were reported in the radiofrequency ablation group.
The VeClose group also published results from the 20-patient “roll-in” cohort. These patients were enrolled in the roll-in phase of the trial. In order to train the investigators in the procedural details, the study mandated VenaSealTM treatment for two patients at each of the 10 participating sites, prior to randomization. Occlusion rates in this cohort were 100% at the 12-month follow-up and the clinical results (VCSS, AVVQ, and EQ-5D) were similar to the randomized clinical trial cohorts.13
All of the previously discussed studies were industry initiated and sponsored. The WAVES study (Lake Washington Vascular VenaSeal Post-Market Evaluation) is an investigator initiated, single-center study that assessed the use of VenaSealTM in great saphenous veins (n=48), small saphenous veins (n=8), and accessory saphenous veins (n=14).14 The study also specifically included larger saphenous veins with diameters up to 20 mm. No compression therapy was used postprocedure. The primary end point was closure of the saphenous vein at 3 months. Intraoperative pain scores, and the VCSS, AVVQ, and EQ-5D scores were similar to the VeClose study. The overall vein closure rate was 99% at 3 months, and the VCSS, AVVQ, and EQ-5D scores all improved at 3 months compared with baseline. Mild phlebitis, which was either self-limiting or resolved with anti-inflammatory treatment, occurred in 10 patients (7%). Allergic reactions were reported in one patient requiring antihistamine and oral corticosteroid use. No deep vein thrombosis or pulmonary embolism occurred.
Another study from Hong Kong reported a lower anatomic success of 78.5% at the 12-month follow-up.15 However, all patients showed improvements in the VCSS and AVVQ scores. However, 60% of the enrolled patients were lost to follow-up at 12 months, which is one of the criticisms of this study. No major adverse events were reported.
The first Korean report on the use of VenaSealTM in great and small saphenous veins was recently published. Cyanoacrylate glue was used to treat 47 great and 16 small saphenous veins. At 3 months, a closure rate of 100% was reported. The VCSS score improved during the followup period. Adverse events included phlebitis-like “abnormal skin reactions” in 8 patients (23.5%) with a full recovery at 2 weeks.16
A Canadian study compared outcomes of VenaSealTM (n=148) with radiofrequency ablation (n=328) in a single-center, nonrandomized setting. The VenaSealTM group included great saphenous veins (n=112), small saphenous veins (n=24), and accessory saphenous veins (n=2). “Treatment success” (not defined in the publication) was 100% in the VenaSealTM group and 99% in the radiofrequency ablation group. Superficial phlebitis was noted in 5% of the patients in the VenaSealTM group and 16% of the patients in the radiofrequency ablation group.17
Off-label use of VenaSealTM in incompetent perforator veins was studied in a small feasibility study in The Netherlands. A total of 33 perforator veins from 27 limbs in 23 patients were treated with a modified off-label technique, with a 76% (25/33) occlusion rate at the 3-month follow-up. No major complications occurred. A larger study is needed for further assessment.18
Indications and contraindications
Indications for using cyanoacrylate glue treatment are no different from the indications for other ablative therapies. However, it is important to discuss procedural outcomes and set appropriate expectations with the patient. In asymptomatic patients with documented reflux, the goal is to improve cosmesis. In symptomatic patients, the goal is to improve symptoms, speed up ulcer healing, and reduce recurrence rates. As per the FDA approved instructions for use document,10 absolute contraindications include previous hypersensitivity reactions to cyanoacrylate glue or cyanoacrylates, acute superficial thrombophlebitis, thrombophlebitis migrans, and the presence of acute sepsis.
Procedure
The VenaSeal closure system (Figure 1) procedure pack is a self-contained sterile, single-patient kit comprised of the cyanoacrylate glue and the cyanoacrylate glue delivery system components, including a glue disperser gun, 5 mL of the cyanoacrylate glue in a small bottle, 5-F delivery catheter, 7-F introducer/dilator, 2 dispenser tips (blunt tip needles), two 3-mL syringes, and a 0.035” J-wire guidewire. Other required tools that are not included in the procedure pack include a micropuncture set for Seldinger access, sterile ultrasound gel packs, ultrasound probe covers, and 10 mL syringes for flushing.
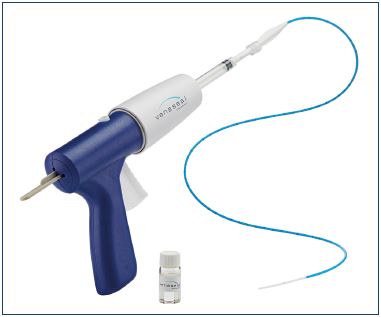
Figure 1. The Venaseal glue dispenser with the cyanoacrylate
glue primed catheter, housed in a 7-F dilator. Courtesy of
Medtronic 2018.
Standard patient-procedure preparation for endovascular procedures is followed for VenaSeal™ procedure. The patient is placed in a prone or supine position for small saphenous vein or great saphenous vein treatment, respectively. The area to be treated is disinfected and a sterile drape is applied. At the author’s institution, the setup uses two tables–“dry” and “wet.” The cyanoacrylate glue container, dispenser gun, dispenser tips, and the 5-F delivery sheath are placed on the “dry” table (Figure 2). Careful precautions are taken to avoid any contact with saline or blood to prevent polymerization of the cyanoacrylate glue.
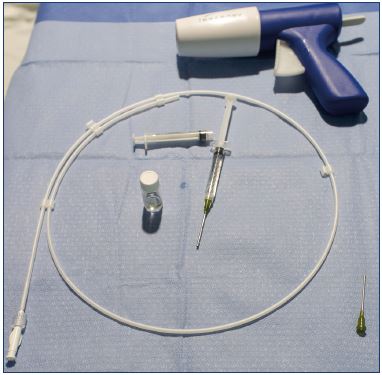
Figure 2. The dry” table with the cyanoacrylate glue, dispenser,
3-mL syringes, and the 5-F catheter.
The rest of the components, including the micropuncture set, saline container, lidocaine container, gauze pads, towel, gel packs, saline syringes, 7-F introducer sheath, and a 0.035” J-wire guidewire, are placed on the “wet” table (Figure 3). Once the set-up is complete, the 3-mL syringe is filled with cyanoacrylate glue and attached to the disperser gun. The 5-F delivery catheter is then connected to the syringe and is primed with cyanoacrylate glue (by squeezing the dispenser gun plunger) up to a mark that is 3 cm from the tip of the catheter.
1. Identify the most caudad point of reflux in the target vein with ultrasound and administer topical anesthetic.
2. Access the vein using an ultrasound-guided Seldinger technique, and a micropuncture needle with a 0.018’’ wire.
3. Place a 7-F introducer sheath over the 0.018’’ wire and pass a dilator into the introducer sheath.
4. Exchange the 0.018’’wire for a 0.035” J-wire guidewire (Figure 4), pass a 7-F dilator over the guidewire (Figure 5), and use a saline-filled syringe to flush the dilator to prevent backwash of any blood into the dilator (Figure 6).
5. Prime a 5-F introducer catheter with cyanoacrylate glue (described above) and advance the catheter to the saphenofemoral junction (Figure 7). Under ultrasound guidance, position the catheter tip 5.0 cm caudal to the saphenofemoral junction.
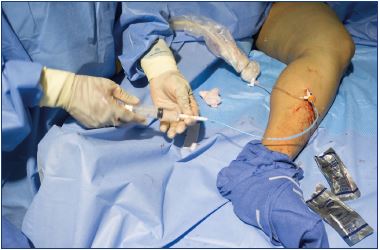
Figure 6. Dilator is flushed with saline and the syringe is locked
to the dilator until the 5-F catheter is ready to be inserted.
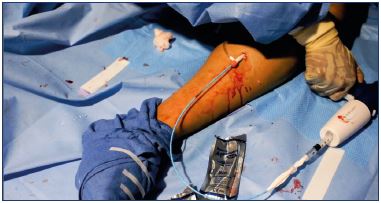
Figure 8. A cyanoacrylate glue aliquot is delivered by squeezing
the handle of the dispenser gun for 3 seconds.
6. Use an ultrasound probe to apply pressure 2 to 3 cm cephalad to the tip of the catheter.
7. Make two injections with approximately 0.10 mL cyanoacrylate glue (achieved by squeezing the dispenser gun handle for 3 seconds) (Figure 8); these injections should be given 1 cm apart at this location.
8. Maintain pressure with the ultrasound probe for 3 minutes.
9. Pull the catheter back 3 cm and inject another 0.10 mL of cyanoacrylate glue.
10. Maintain manual compression 30 seconds.
11. Continue the procedure every 3 cm with cyanoacrylate glue injection and the 30-second ultrasound probe / manual compression sequences until the entire length of the target vein segment is treated.
12. Remove the sheath and catheter and apply compression at the access site until hemostasis is achieved.
13. Apply an adhesive bandage at the access site.
14. Confirm venous occlusion using duplex ultrasound.
Adjunctive procedures for tributary vein treatments are either performed in the same setting or staged based on several clinical factors. Compression therapy is not needed after the procedure unless concomitant phlebectomy or sclerotherapy are performed. Follow-up requirements for the clinical exam and venous duplex vary based on the provider’s personal preference, patient complaints, and patient risk factors for venous thrombosis. As noted in the animal histologic exams, follow-up ultrasound exams demonstrate no evidence of thrombotic occlusion of the veins. There seems to be a collapse of the vein over the VenaSealTM implant. Ultrasound images of the treated vein demonstrate a hyperechoic vein with a nonsignificant reduction in diameter, even at the 1-year follow-up (Figure 9), unlike the veins treated with thermal ablations or sclerotherapy.
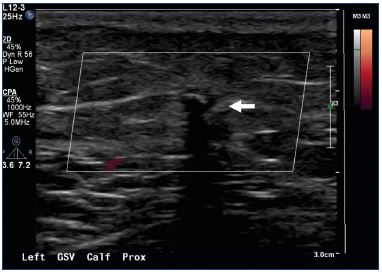
Figure 9. A duplex image acquired 6 months after the
VenaSeal™ procedure.
Note the hyperechoic great saphenous vein (white arrow).
Discussion
VenaSealTM, a cyanoacrylate glue treatment of incompetent truncal veins, has been demonstrated to be a safe and effective treatment. Other than mild phlebitis episodes and rare reports of allergy that are self-limiting. No serious complications, particularly related to venous thrombosis are reported with this technique, making it an attractive option in patients with other comorbidities. Since there is no dosage limit for the cyanoacrylate glue, unlike other nonthermal nontumescent treatments, such as the sclerotherapy, multiple veins can be treated in the same setting. Except for the randomized control trial,19 all other trials required no postprocedural compression therapy. VenaSealTM is also an attractive option in patients with a disproportionately large thigh circumference (compared with the calf), which results in sliding of the postprocedure compression garments that are required for other nonthermal nontumescent treatments, such as foam sclerotherapy, mechanochemical ablation, and proprietary endovenous microfoam treatment. Young and active patients, who do not wish to wear postprocedural compression garments, prefer VenaSealTM treatment of multiple veins in a single session. Similarly, patients who fear needle sticks also prefer this treatment.
While saphenous occlusion rates are high in all except one study,16 long-term data in larger cohorts is lacking at this time. The improvement in patient-reported outcomes and quality measures, such as VCSS, AVVQ, and EuroQual scores is encouraging. Advancing the stiff VenaSealTM catheter is challenging in chronic postthrombotic veins, tortuous tributaries, and neovascularized veins, similar to thermal techniques. Foam sclerotherapy and phlebectomy remain the preferred treatments in this setting. Subdermal, superficial saphenous veins are also not ideal for VenaSealTM treatment due to the fibrotic changes in the treated veins, which is again similar to thermal ablation.
Venous ulcers are the most common leg ulcers,20 as 278 000 venous leg ulcers are reportedly managed by the UK National Health Services each year, at an annual cost of €1024 million.21 The landmark EVRA trial (Early Venous Reflux Ablation) demonstrated faster healing of venous leg ulcers and more ulcer-free time, with early endovenous ablation of superficial venous reflux rather than deferring these treatments.22 Epstein et al have also demonstrated that venous interventions are more effective and less expensive in the long run compared with compression therapy alone.21 There are no VenaSealTM outcome data in advanced venous disease and venous ulcerations at this time and further studies are needed in this population.
Finally, another n-butyl-cyanoacrylate based polymer with limited modifications, Biolas VariClose®, received the CE mark in 2013 and several studies have been reported from Turkey. Due to the limited modification, the glue is less viscous and polymerizes much quicker than VenaSealTM, which has the potential disadvantage of distal embolization and adhesion of the catheter tip to the vein wall during the procedure. While VenaSealTM is a segmental procedure with aliquots delivered every few centimeters, VariClose® requires continuous delivery of the low viscous cyanoacrylate glue. The VariClose® studies have also reported a high degree of anatomic success (>95%) at 12 months. The reported phlebitis rates are lower compared with VenaSeal.23,24 However, the definition of phlebitis and the rigor for monitoring these adverse effects vary significantly between the clinical studies reported on these 2 products. VariClose® is not available in the USA and there have been no head-to-head comparison studies of these products thus far. Conclusions
In summary, VenaSealTM is a simple procedure, with consistent procedural steps. No major adverse events have been noted. Minor complications include phlebitis episodes and rare reports of allergies to the cyanoacrylate glue. In the hands of experienced endovenous physicians without prior VenaSealTM experience, the procedure resulted in good anatomic and clinical success rates, along with a relatively short learning curve.13 Future VenaSealTM research should focus on treatment outcomes in late stage venous disease, venous ulcer healing and cost effectiveness.

REFERENCES
1. Business Wire. THE SAGE GROUP releases new estimates for the United States prevalence and incidence and costs of chronic venous disease (CVD), varicose veins and venous ulcers. https://www.businesswire.com/news/ home/20161220005781/en/SAGEGROUP- Releases-New-Estimates-United- States. Published December 20, 2016. Accessed June 7, 2018.
2. Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lowerlimb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care. 2003;16(6):305-316.
3. Vuylsteke ME, Thomis S, Guillaume G, Modliszewski ML, Weides N, Staelens I. Epidemiological study on chronic venous disease in Belgium and Luxembourg: prevalence, risk factors, and symptomatology. Eur J Vasc Endovasc Surg. 2015;49(4):432-439.
4. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(suppl 5):2S-48S.
5. National Institute for Health and Clinical Excellence. Varicose veins: diagnosis and management. Clinical guideline [CG168]. https://www.nice.org.uk/ guidance/cg168. Published July 2013. Accessed Jun 7, 2018.
6. Pollak JS, White RI Jr. The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001;12(8):907-913.
7. Almeida JI, Min RJ, Raabe R, McLean D, Madsen M. Cyanoacrylate adhesive for the closure of truncal veins: 60-day swine model results. Vasc Endovascular Surg. 2011;45(7):631-635.
8. Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg: Venous Lymphat Disord. 2013;1(2):174-180.
9. Almeida JI, Javier JJ, Mackay EG, Bautista C, Cher DJ, Proebstle TM. Thirty-sixth-month follow-up of first-inhuman use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg: Venous Lymphat Disord. 2017;5(5):658-666.
10. VenaSeal™ closure system product code: VS-402 https://www.accessdata.fda. gov/cdrh_docs/pdf14/P140018c.pdf. Accessed June 7, 2018.
11. Proebstle T, Alm J, Dimitri S, et al. Twelve-month follow-up of the European multicenter study on cyanoacrylate embolization of incompetent great saphenous veins. J Vasc Surg: Venous Lymphat Disord. 2014;2(1):105-106.
12. Kolluri R. Cyanoacrylate embolic adhesive vs. RFA: three-year follow-up pivotal trial. Paper presented at: the 2017 Veith Conference; November 16, 2017; New York, NY, US.
13. Kolluri R, Gibson K, Cher D, Madsen M, Weiss R, Morrison N. Roll-in phase analysis of clinical study of cyanoacrylate closure for incompetent great saphenous veins. J Vasc Surg: Venous Lymphat Disord. 2016;4(4):407-415.
14. Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular. 2017;25(2):149-156.
15. Chan YC, Law Y, Cheung GC, Ting AC, Cheng SW. Cyanoacrylate glue used to treat great saphenous reflux: measures of outcome. Phlebology. 2017;32(2):99- 106.
16. Park I. Initial outcomes of cyanoacrylate closure, VenaSeal system, for the treatment of the incompetent great and small saphenous veins. Vasc Endovascular Surg. 2017;51(8):545-549.
17. Yang GK, Parapini M, Gagnon J, Chen J. Comparison of cyanoacrylate (venaseal) and radiofrequency ablation for treatment of varicose veins in a Canadian population. J Vasc Surg. 2017;66(3):e65.
18. Boersma D, van Eekeren RR, Werson DA, van der Waal RI, Reijnen MM, de Vries JP. Mechanochemical endovenous ablation of small saphenous vein insufficiency using the ClariVein(®) device: one-year results of a prospective series. Eur J Vasc Endovasc Surg. 2013;45(3):299-303.
19. Morrison N, Gibson K, McEnroe S, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. 2015;61(4):985-994.
20. Margolisa DJ, Bilkerb W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46(3):381- 386.
21. Epstein D, Gohel M, Heatley F, Davies A. Cost-effectiveness of treatments for superficial venous reflux in patients with chronic venous ulceration. BJS Open. 2018 May 10. Epub ahead of print.
22. Gohel MS, Heatley F, Liu X, et al; EVRA Trial Investigators. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med. 2018;378(22):2105-2114.
23. Tok M, Tüydeş O, Yüksel A, Şenol S, Akarsu S. Early-term outcomes for treatment of saphenous vein insufficiency with N-butyl cyanoacrylate: a novel, non-thermal, and non-tumescent percutaneous embolization technique. Heart Surg Forum. 2016;19(3):E118-E122.
24. Yasim A, Eroglu E, Bozoglan O, Mese B, Acipayam M, Kara H. A new nontumescent endovenous ablation method for varicose vein treatment: early results of N-butyl cyanoacrylate (VariClose®). Phlebology. 2017;32(3):194-199.