Venous edema of the lower limbs
St Joseph Hospital Foundation
Paris, France
Chronic venous disorders of the lower limbs are manifest by many noticeable, but few specific, clinical signs. In France, some 18 million persons suffer from pain in the legs and 12 million may have varicose veins. Over 200 000 hospital admissions per year may involve venous disease, two-thirds of which require surgical management. Slightly more than 5% of cases of sick leave from work may be related to venous disease and the functional signs that accompany it, amounting to millions of lost workdays. Thus, without a doubt, venous disease is a problem of public health.
VENOUS EDEMA OF THE LOWER LIMBS – WHICH MECHANISM IS INVOLVED?
Edema is an accumulation of fluid in the extracellular compartment, which results in an increase in the volume of interstitial fluid. Physiologically, a balance exists between intravascular hydrostatic pressure and oncotic pressure on the one hand, and interstitial pressure on the other. Transcapillary hydrostatic pressure tends to drain fluid from blood vessels, whereas oncotic pressure, which is associated with protein concentrations, tends to produce fluid retention in blood vessels. A decrease in oncotic pressure (hypoalbuminemia) and/or an increase in hydrostatic pressure (heart failure) lead to an increase in salt and water in the interstitial compartment. Proteins pass through the wall of the blood vessel when venous and lymphatic microcirculation is impaired.1
CAUSES OF LOWER-LIMB EDEMA
Generally, the causes of lower-limb edema are suggested by the clinical examination of the patient. The clinical interview should aim to identify a previous history of the condition, evaluate the acute or chronic feature of edema, and look for possible precipitating factors. Clinically, the examination should identify whether the edema is isolated or diffuse, painful or not painful, its consistency, the existence of urticaria-like lesions suggesting angioedema, the existence of a serous effusion (peritoneum, pleura), signs of thromboembolic disease, and clinical evidence of internal organ disease (heart, kidney, liver).1,2
Diffuse edema
In heart failure, increased ventricular filling pressure exists as well as increased pressure in the entire upstream plasma compartment. Furthermore, decreased cardiac output produces renal hypoperfusion, which worsens salt and water retention. Cardiac edema is related to right ventricu- lar failure. The latter can complicate left ventricular failure, pulmonary disease, or pericardial disease. The first signs of salt and water retention are weight gain and oliguria. Edema subsequently occurs and, as a result of orthostatic posture, most often remains confined to the lower limbs. Edema may also be localized to the patient’s back in the case of bed rest. Other signs of right ventricular failure are painful hepatomegalia with hepatojugular reflux and turgescent jugular veins.
In hepatic cirrhosis, the mechanism responsible for edema is multifactorial. The decreased oncotic pressure associated with hypoalbuminemia resulting from a lack of protein synthesis due to hepatocellular failure is the initial cause of salt and water retention. Its association with ascites related to portal hypertension amplifies the process by creating an obstacle to venous return. The existence of kidney disease also contributes to formation of edema. Clinically, this type of edema has the same features as cardiac edema and is associated with signs of hepatocellular failure and portal hypertension.
The primary causes of lower-limb edema related to kidney disease are nephritic syndrome, nephritis, and chronic renal failure.
• Nephritic syndrome is defined as proteinuria greater than 3 g/24 hr, hypoalbuminemia less than 30 g/L, and hypoproteinemia less than 60 g/L. Loss of albumin via the kidney is responsible for the decrease in oncotic pressure with salt and water retention. This form of edema involves the lower limbs, but also the upper limbs and face.
• Nephritis is characterized by a primary abnormality in the nephron, which causes fluid retention, and is mainly related to kidney disease. Increased intravascular hydrostatic pressure produces passage of fluid into the interstitial compartment. Generally, proteinuria remains moderate, and hematuria, hypertension, and organic renal failure may occur. This edema has the same features as nephritic syndrome–related edema.
• In the event of chronic renal failure, the existence of edema reflects a salt and water intake exceeding losses. It develops at a late stage in the course of renal failure.
Edema occurring in the context of gastrointestinal disease is associated with hypoalbuminemia, which can be related to either inadequate protein intake as a result of malnutrition (Kwashiorkor, marasmus) or to excessive losses due to malabsorption (inflammatory bowel disease, surgical resection, exocrine pancreatic failure), or due to increased venous-lymphatic pressure in the setting of exudative enteropathy. In the case of malabsorption, hypoalbuminemia is associated with iron deficiency, hypocalcemia, and vitamin B, D, and K deficiencies. Useful laboratory tests include the measurement of steatorrhea, the d-xylose test, and the Schilling test. Increased clearance of _1-antitrypsin establishes the diagnosis of exudative enteropathy.
Idiopathic cyclic edema, an entity that affects young women of childbearing potential, produces edema in the face and upper extremities in the morning, and subsequently affects the lower limbs later in the day. The pathophysiology of this disorder has not been elucidated, but capillary hyperpermeability exists.
Hereditary angioneurotic edema, associated with C1-esterase inhibitor, manifests itself as more or less acute episodes of diffuse edema. Decreased titers of the C4 fraction of complement at the time of an acute episode should lead to a measurement of C1-esterase inhibitor.
Drugs can cause edema that is often moderate and remains limited to the lowermost, dependent anatomical areas. Certain drugs alter capillary permeability (calcium channel blockers, nitrates). Others produce salt and water retention of renal origin: nonsteroidal antiinflammatory drugs (NSAIDs), steroids, and estrogenprogestin combinations. Finally, some drugs directly provide a sodium quantity (intravenous penicillin, oral alkalinizing agents, gastric mucosa protective agents, lithium).
Hypo- and hyperthyroidism, and increased plasma estrogen concentrations during the premenstrual syndrome (PMS) can also be accompanied by edema. Longterm intake of diuretics may affect the renin-angiotensin regulation and lead to resistant edema.
Edema limited to the lower limbs
An acute, swollen, red leg with fever, generally above 38.5°C (101.3°F), and laboratory data evidencing infection (leukocytes, inflammatory syndrome) suggest the diagnosis of erysipelas. The causative organisms most commonly responsible are Streptococcus and Staphylococcus aureus. Necrotizing fasciitis is a very serious complication with signs of sepsis and local progression marked by the occurrence of blisters and skin necrosis.
Edema can be of venous origin as a result of primary venous disease, venous thrombosis, an extrinsic obstacle to venous return, or a syndrome with malformations such as Klippel-Trenaunay syndrome. The clinical examination supplemented by duplex ultrasonography confirms the suggested diagnosis. Measurement of ddimers is useful when a venous thrombosis is suspected.
Lymphedema of the lower limbs, whether congenital, primary, or secondary, is a cause of increased limb size.3 Congenital lymphedema may be hereditary (Millroy’s syndrome) and starts at birth. Primary lymphedema develops in childhood or adolescence (Lymphedema praecox), or after the age of 35 years (Lymphedema tardum). Hereditary lymphedema starting in puberty has been called Meige’s syndrome. In some forms of lymphedema, genetic defects could be detected (eg, Turner’s syndrome, yellow nails syndrome). Secondary lymphedema occurs following surgery (lymph node dissection, bypass grafting, venous surgery), radiotherapy, or due to tumor extension. It can also be related to compression of a structure or occur in the context of venous disease, whether advanced primary venous insufficiency or related to postthrombotic syndrome. Lastly, a specific context can guide the physician to the possible diagnosis of filariasis.
Other causes
In other cases, the recent history will guide the clinician, eg, a notion of pain and edema that occurred suddenly following exertion suggests a muscle strain, or tendon rupture, or a muscle compartment syndrome if an interval exists between the exertion and pain, and edema. In the absence of prior exertion, the possibility of a popliteal cyst rupture, a popliteal aneurysm rupture with signs of ischemia, or revascularization-related edema following late-stage elimination of ischemia should be considered.
Algoneurodystrophy, Lyme disease, periarteritis nodosa, bone tumors, muscle sarcomas, arthritis, and retroperitoneal fibrosis are other causes of lower-limb edema. Charcot’s blue edema associated with self-inflicted injury by placement of a tourniquet is a differential diagnosis.
Lipedema denotes a symmetrically increased lowerlimb size associated with excessive adipose tissue extending from the hips to the ankles, but sparing the dorsal aspect of the foot. This condition is observed mostly in obese women, starting at puberty. It is a clinical entity different from lymphedema, but which can be confused with it. The dorsal aspect of the foot is not affected, at least in the early stage of development. The infiltrated area shows soft, nonpitting edema that is painful to the touch, which differentiates lipedema from lymphedema.4
Venous edema
Venous dysfunction is related to a primary defect in the venous valves of the superficial or deep veins, postthrombotic deep vein valvular incompetence, incompetent distal perforator veins, deep vein valvular dysgenesis, dysfunctioning of the muscle pump, or compression that impedes venous return. Venous insufficiency generates venous stasis and increases ambulatory distal pressure, which is the cause of the vicious circle of events. Induced venous dilatation results in a defect in valvular coaptation. Increased venous pressure is the cause of microangiopathy, which is the source of skin trophic changes.5,6
The patient with venous disease is evaluated by means of an interview, to look for factors precipitating the condition, and a clinical examination, with the patient erect, preferably on a platform, and alternating his or her weight on one foot, to examine the entire limb, the suprapubic area, and abdomen.
Edema of chronic venous disease, in particular, is sporadic, unilateral or bilateral, has no component of inflammation, is limited to the legs, but may also involve proximal parts of the lower extremity, is enhanced by prolonged orthostatic posture, and is improved by raising the legs.
Venous edema manifests itself as a “large cold leg” whose etiological diagnosis, guided by the clinical context in which the edema is progressing, can be extensively investigated with duplex ultrasonography. On examination, venous edema is variable in intensity, tending to be soft. Increased warmth of the skin often exists, as well as a cyanotic presentation when the patient is in an orthostatic position. When edema becomes chronic, thickening of the skin and lymphedema may develop. The combination of edema and varicose veins, ankle telangiectasia, hyperpigmented dermatitis, white atrophy, cutaneous sclerosis, and eczema or venous ulcers on the lower leg, is a good indication of the venous origin of the edema.
Sequelae of deep vein thrombosis with destruction or alteration of valvular functioning—an obstacle to venous return due to occlusion, or more rarely, compression— generate venous-related edema. In postthrombotic disease, edema is dependent on the degree of venous recanalization, the extent of valvular incompetence, and the development of a collateral circulation. The diagnosis is established relatively easily when the patient is known to have a documented past history of venous thrombosis. When thrombosis has gone unnoticed clinically or an episode of edema has not led to laboratory investigations, in some cases, duplex ultrasonography can identify sequelae of thrombosis and guide the diagnosis. Valvular hypogenesis or agenesis is a rare cause of edema. Postthrombotic disease is not the only possible cause of a “large cold leg” of venous origin. A compressive cause should also be sought, in particular, compression from a tumor, benign or malignant, located in the pelvis.
May-Thurner syndrome (venous spur, iliac compression syndrome), corresponds to compression of the left common iliac vein by the right common iliac artery. This anatomical abnormality is asymptomatic in most cases, or can manifest itself as unilateral edema of the left lower limb and can be enhanced by exertion. It is also responsible for the predominant occurrence of iliofemoral deep vein thrombosis on the left side.
Deep vein thrombosis is often responsible for unilateral edema of recent and rapid onset. It can be accompanied by a slight fever. Clinical signs, while nonspecific and not always present, can guide the clinician and include pain, collateral venous circulation, increased local warmth, loss of mobility, and cyanosis. Use of a clinical score allows the clinician to evaluate the probability of the diagnosis, but it is essential to confirm it with duplex ultrasonography within the first 36 hours, after initiating emergency anticoagulant therapy. A low clinical probability in combination with negative d-dimers, determined by the enzyme-linked immunosorbent assay (ELISA) method, rules out the diagnosis of venous thrombosis as a result of the excellent negative predictive value of d-dimer.
The heavy legs syndrome
Heavy legs syndrome is one of the most common manifestations of chronic venous disease; it is a sensitive, but nonspecific symptom (Table I). It is described more often by women than men with a prevalence of 30% to 50%.7,8 It is not always associated with the existence of varicose veins, but sometimes with superficial venous reflux.9 This subjective impairment tends to affect young women. It can be constant, occur solely on exertion, or in orthostatic posture, particularly at the end of the day, and can be isolated or accompanied by edema and other signs of venous insufficiency.
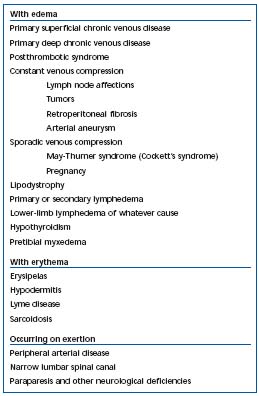
Table I. Primary causes of heaviness in the legs (based on ref. 11).
It involves a sensation of heaviness or tiredness and swelling of the legs that is exacerbated at the end of the day, after prolonged standing, and pacing back and forth. Heavy legs are frequent symptoms in patients with occupations requiring prolonged sitting, or those who experience weight gain, a sudden reduction in muscular activity, or exposure to heat. Severe premenstrual syndrome, pregnancy, and estrogen-progestin combinations (the “pill”) are factors that amplify such disorders.
The pathophysiology of heavy legs syndrome has not been elucidated with certainty. The circumstances of occurrence suggest a link with venous stasis, especially since symptoms are improved by a healthy lifestyle (not being overweight, regular physical activity, etc), methods of hydrotherapy, and venous tonic medicinal products. An epidemiological link exists with edema, independent of the severity of varicose veins and the existence of skin-trophic changes. Thus, in many cases, subclinical edema may exist, but also concomitant minimal functional lymphatic insufficiency, which can formally be demonstrated.10
All of the causes of venous edema mentioned in the above can be responsible for the sensation of heaviness in the legs: superficial or deep primary venous insufficiency, postthrombotic disease, and venous compression.11 Lymphedema can also generate lower-limb heaviness. The mechanisms involved in the symptoms of chronic venous disease are poorly understood. In this field, epidemiology may detect several risk factors. This approach must be further improved by the use of methods that investigate venous physiology and microcirculation. In addition to good clinical evaluation and the use of ultrasonographic methods, when jointly applicable to a decision regarding treatment, management of chronic venous disease should include knowledge of the patient’s socio-occupational context, as well as a documented approach to treatments, their usefulness, their mechanisms of action, long-term efficacy, and limits. Elastic compression therapy is useful whatever the clinical presentation of the disease and may by used in combination with measures to promote a healthy lifestyle and diet. Phlebotropic agents can be started as soon as symptoms occur.

REFERENCES
2. Blétry O, Vignes S, Priollet P. OEdèmes. In: Godeau P, Herson S, Piette J-C, eds. Traité de Médecine. Paris, France: Flammarion Médecine-Sciences; 2005:95-101.
3. Boursier V, Vignes S, Priollet P. Lymphoedèmes. In: Encyclopédie Médico- Chirurgicale. Traité de Médecine. Paris, France: AKOS; 2004:2-0535-2-0540.
4. Boursier V, Pecking A, Vignes S. Analyse comparative de la lymphoscintigraphie au cours de lipoedèmes et des lymphoedèmes primitifs des membres inférieurs. J Mal Vasc. 2004;29:257-261.
5. Priollet P. Insuffisance veineuse chronique: aspects cliniques. Presse Med. 1994;23:229- 235.
6. Collège des Enseignants de Médecine Vasculaire. Conduite à tenir devant une insuffisance veineuse chronique des membres inférieurs. In: Veines, Artères, Lymphatiques, Microcirculation (VALMI). Paris, France: 2M2 eds; 2004:99-102.
7. Bradbury A, Evans C, Allan P, et al. What are the symptoms of varicose veins? Edinburgh Vein Study cross sectional population survey. BMJ. 1999;318:353- 356.
8. Carpentier PH, Hildegard RM, Biro C, et al. Prevalence, risk factors and clinical patterns of chronic venous disease of the lower limbs: a population-based study in France. J Vasc Surg. 2004;40:650-659.
9. Bradbury A, Evans CJ, Allan P, et al. The relationship between lower limb symptoms and superficial and deep venous reflux on duplex ultrasonography: the Edinburgh Vein Study. J Vasc Surg. 2000;32:921-931.
10. Carpentier PH. Epidémiologie et physiopathologie des maladies veineuses chroniques des membres inférieurs. Rev Prat. 2000;50:1176-1181.
11. Lévesque H, Cailleux N. Jambes lourdes et grosses jambes. Rev Prat. 2000;50:1183- 1188.
