Venous malformations of the hand: surgical treatment
Piero Di GIUSEPPE, MD2;
1Director, Specialist in Vascular Surgery;
2Hand surgeon, Specialist in Plastic
Surgery; Center for Vascular
Malformations “Stefan Belov”, Humanitas
“Mater Domini” Hospital, Castellanza
(Varese), Italy
Abstract
Congenital vascular malformations (CVM) of the hand are extremely variable and difficult to treat. The correct approach first requires a complete, stepwise diagnostic procedure from clinical examination to echo Doppler (ECD) examination followed by magnetic resonance (MR) imaging, which can be performed without contrast if ECD demonstrated a slow-flow malformation. Treatment can be performed via sclerosis or surgery. Sclerosis has the advantage of being less invasive but the disadvantage of having a significant incidence of recurrence and the risk of nerve damage. Here, we report the results from a group of 115 patients with venous malformation (VM), all treated by surgery. The results were good for limited forms, with a high incidence of complete defect removal and healing (75% healed, 14% improved), and good improvement was observed for infiltrating forms (15% healed and 74% improved). Recurrence was 11% for limited forms and 10% for extended forms. Complications were few: 3 with temporary paresthesia and 2 with postoperative pain. Surgery is a good option in VM of the hand. However, experience with the surgical approach for the area and for VM is required. Teamwork between hand and vascular surgeons is a good option.
Introduction
Congenital vascular malformations (CVM) are inborn errors in the process of angiogenesis and vasculogenesis during fetal life. The result can be a defect in main vessels, such as the absence of (aplasia) vessels, reduction in lumen diameter (stenosis), dilatation (aneurysm), and remnants of embryonal, immature vascular masses in tissues.1 The last type is by far the most common.
According to modern classification by the International Society for the Study of Vascular Anomalies (ISSVA), CVM can be of different types depending on the vessels involved. There are venous malformations (VMs), arteriovenous malformations (AVMs), lymphatic malformations (LMs), and a combination of these or a combination with other defects, which are called “syndromes.”2
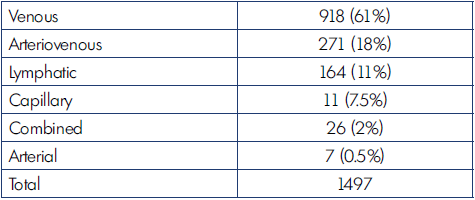
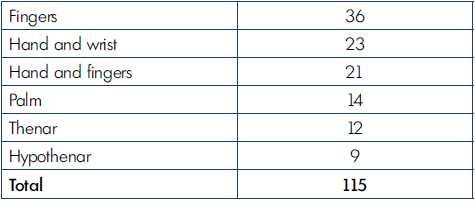
The most common type of CVM is the venous form, as shown in Table I.
VM can be located in almost any part of the body and can differ in extension, infiltration of tissues, and involvement of other structures, making it an extremely variable disease to the point that it may be difficult to find 2 similar cases. That variability makes it difficult to have a clear understanding of any particular case and to choose the strategy for approach.
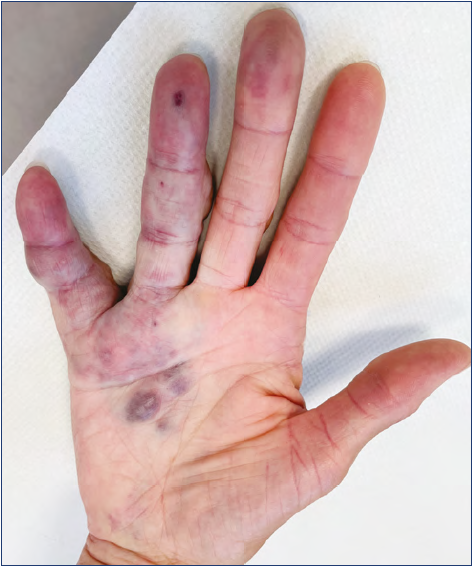
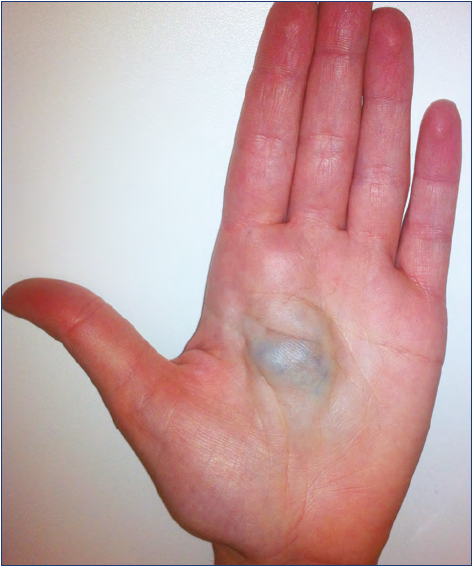
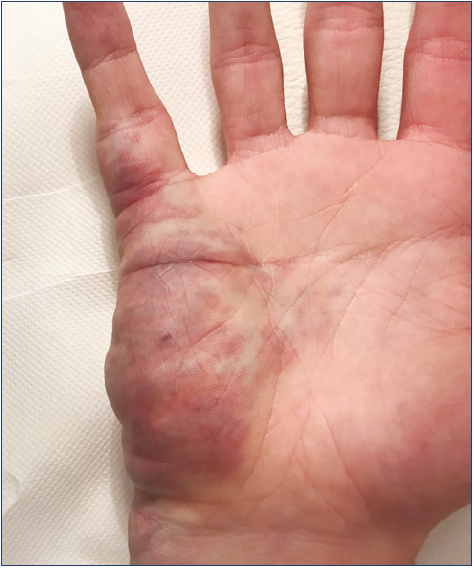
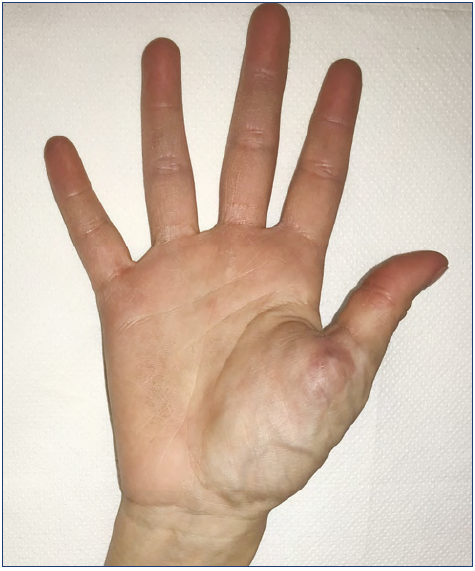
The hand is a very particular structure with complex anatomy in which many different tissues of high functional value (fine nerves, vessels, small muscles, tendons) are located within a small space. The hand is not rarely affected by VM, which may involve limited areas or infiltrate the entire hand even with extension to the wrist and forearm (Figures 1-5).
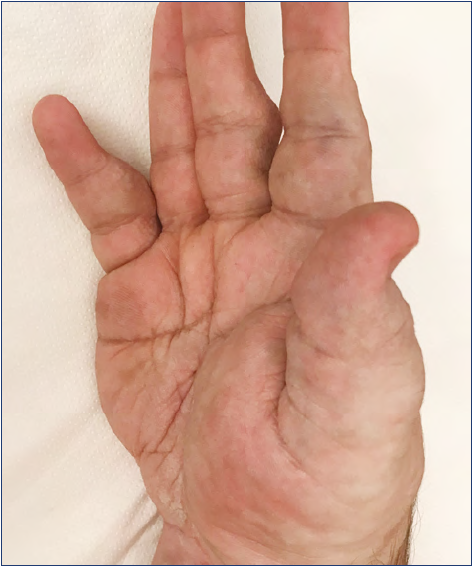
Figure 5. Extended venous malformations of the hand: the malformation extended to the wrist and forearm.
Great variability in VM makes it difficult to approach those cases, as the malformation may involve fine structures that should be spared during treatment. Moreover, as these diseases are rare and as few centers are specialized in the approach for treatment of VM, few papers, mainly with limited numbers of cases, have been published.
A consensus and general guidelines about treatment of VM have been published.3,4 However, due to the anatomical peculiarities of the hand, general principles need to be adapted for that specific location. Unfortunately, there is a lack of practical information in the literature about that topic. With that in mind, here we discuss the diagnostic process and treatment of VM in the hand based on our experience from our VM-dedicated center. We also provide a retrospective analysis of the cases treated and the results of our approach.
Diagnosis
A complete diagnosis of VM in the hand is mandatory because of the variability of the disease and because precise data about VM location and extension are important in choosing treatment strategy.
The diagnostic process for this disease should progress in a stepwise manner, from the simplest test to the most invasive.5 As a first step, clinical examination should determine the location of the disease and make the differential diagnosis with AVM and LM and the effects of the defect on hand function. Typically, VMs are masses that are not pulsating and that are compressible with slow refilling. By lifting the limb, an emptying effect is sometimes recognizable. Pressing the mass may be painful. Sometimes, solid masses may be perceptible in the context of the malformation: these are due to phleboliths, calcified areas, which are typical of VM.
The second step of the diagnostic process is an echo Doppler (ECD) examination, which can provide several main data and should not be ignored. Firstly, ECD shows the type of flow in the mass, allowing differential diagnosis with AVM (high flow) and LM (cysts with no flow); VM has a typical slow flow. Secondly, ECD provides information about the location, whether deep or superficial, and the involvement of tissues. By differentiating among an intramuscular, a superficial, and an interstitial form, ECD is useful for treatment planning (Figure 6). Phleboliths may be visible inside the malformation. Moreover, ECD may show whether the malformation is formed with venous lakes or with a more compact mass with small vessels: this difference is useful for deciding whether to try sclerosis or not as in the second type (which is not uncommon) sclerosis is less effective.
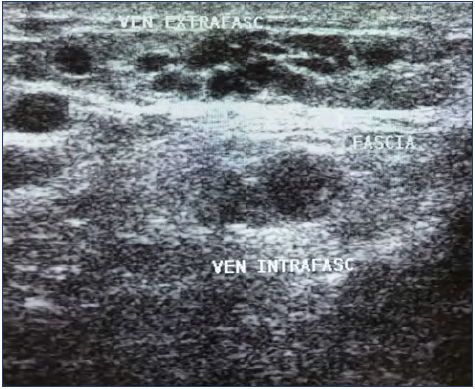
Figure 6. Echo Doppler of the palm of the hand in a case of venous malformation. Subcutaneous malformations are visible together with subfascial (intramuscular) forms.
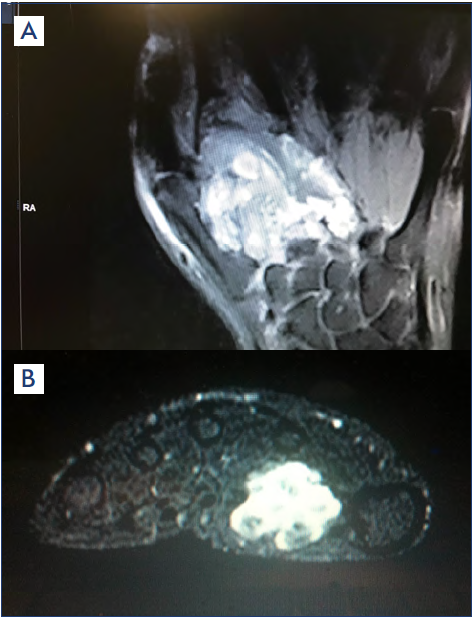
Figure 7. Magnetic resonance imaging in (A) frontal and (B) transversal views showing the location of venous malformations (bright areas).
The third step in the diagnostic process uses imaging procedures. Magnetic resonance (MR) imaging will clearly show the site and extension of the malformation. As VM has slow flow, recognized by the ECD performed before, contrast injection would not be necessary (Figure 7). Computed tomography (CT) with contrast may be useful; however, CT images are not as clear as those obtained by MR. Angio CT should be avoided because this test mainly shows the arterial tree, which is not pathologic or may show slight arteriovenous (AV) fistulas, whereas the VM is not as well shown as with MR.
Plain rx of the hand is an additional exam that is useful for recognizing bone involvement and showing phleboliths (Figure 8).7
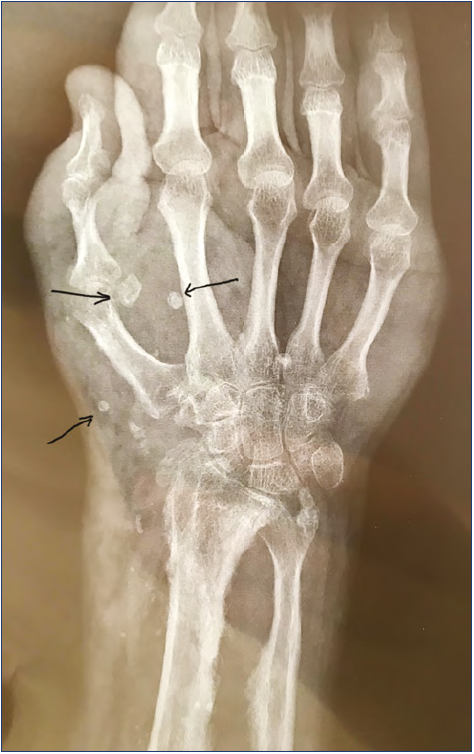
Figure 8. Plain rx of the hand in a case of extended vascular malformation: notice phleboliths (arrows).
Treatment
Treatment of VM is based on the following procedures: sclerosis, surgery, and laser.
Sclerosis is traditionally the first-line treatment for dilated veins, like in varicose veins. Several substances are available, such as polidocanol, sodium tetradecyl sulfate (TDSS) or ethanol, even in the form of ethanol gel.8
Sclerosis has been shown to be less effective for treatment of VM, with a high incidence of recurrence, except with the use of ethanol, pure or jellified.4 Risks of sclerosis are skin necrosis, nerve damage (sometimes permanent), and muscle contracture.9 An experimental study demonstrated that ethanol may exit the venous walls and cause damage in adjacent nerves, demonstrated by a reduction in nerve axons.10 In small anatomic compartments, like the hand, the risk of alcohol migration outside the wall producing nerve damage is theoretically increased. Ethanol gel may have a lower incidence of complications.
The topic of risk for nerve damage is highly relevant with regard to the hand because finger sensibility is crucial for hand function: a loss of sensibility in the fingertips of the thumb or index finger is a main disability (blind finger). Some small case series studies report good results for sclerosis in treatment of VM in the hand,11,12 but the topic of risk of complications remains open.
An alternative procedure is the surgical removal of the malformation. However, due to the peculiar anatomy of the hand, surgery requires specific experience in the approach for that area and in the management of its specific fine structures. Correct choice of skin incision, skin-sparing technique, tourniquet use, microscope use, and in how to handle the involved structures, such as bone, nerves, vessels, muscles, and tendons are all technical skills that may be crucial for a successful surgical treatment.13 Unfortunately, reports of technical data from surgical treatment of VM in the hand are lacking in the literature. In the case of extended, infiltrating forms, a step-by-step procedure is indicated, in order to avoid long-duration surgery, which may lead to blood loss and poorer results. An approach by a multidisciplinary team may offer the best results.
Catheter embolization is not indicated in VM (unlike AVM), as that method can only occlude afferent arteries and is ineffective in reducing or occluding the vascular venous mass.4 Unfortunately, even today, embolizations are sometimes performed in VM; the results are poor and may be dangerous if arteries of the fingers are occluded.
Laser treatment has a very limited application in this area because of the risk of structure damage.
Materials and methods
To obtain data about the characteristics of VM localized in the hand, the treatment performed, and the results, a group of patients treated in a single center were analyzed retrospectively.
In the period of 1986 to 2019, 115 patients with vascular malformation of the hand were treated. During the period of 1986 to 2010, they were treated in a vascular surgery division and from 2010 to 2019, in a center dedicated to vascular malformation.
Of the 115 patients, 74 (64%) were female and 41 (36%) were male. Mean age was 29.6 years and ages ranged from 5 to 70.
Extension of the lesions were recorded, dividing the cases into 2 groups: limited and extended forms. The extended forms included multiple-sited lesions and infiltrating forms of different areas of the hand. It was difficult to decide if limited lesions localized to 2 different areas, should be classified as “limited” or “extended.” The decision was made to include these with multiple sites within the “extended” forms group because of the necessity to perform more operations.
All patients of this group were treated by surgery alone in a single operation or by 2 or more steps, according to the extension and to the symptoms. Nonsymptomatic areas were for the most part not treated. All operations were done in teamwork by the same vascular and hand surgeons.
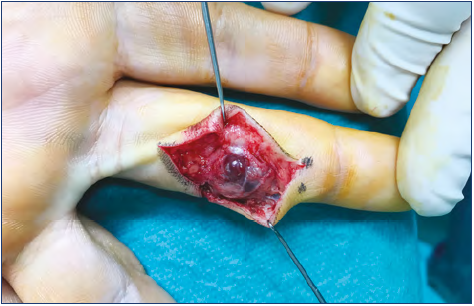
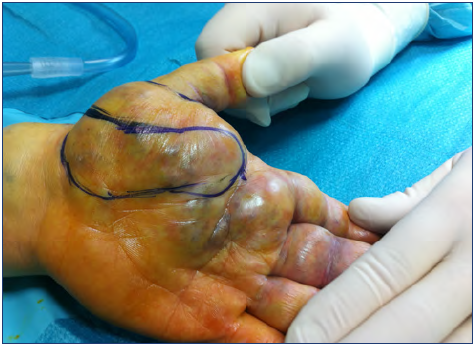
Technically, the resection was done with the aim of avoiding damage to vessels, nerves, and tendons. For this reason, in case of VM surrounding these structures, the surgeries were planned in detail and included use of magnifying glasses or a microscope to aid in separating the malformed tissue from the fine structures (Figure 9).13
All patients were controlled after treatment by clinical examination and by ECD performed by the same investigator.
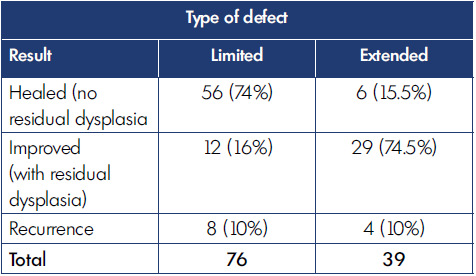
Results were divided thusly: healed (no residual VM), improved (absence or significant reduction in symptoms, but with residual VM), and recurrence (new growth of malformation in the operated area), with distinction between limited and extended forms.
The label of “healed” in the extended group was given only in relation to the operated area and did not consider other involved areas that were not treated.
Results
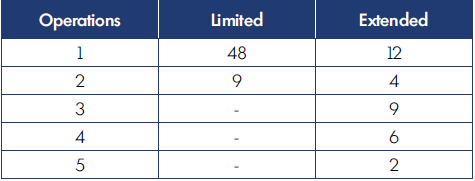
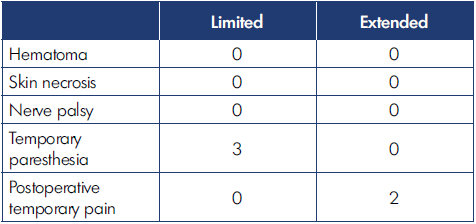
Location of the malformations in the hand are shown in Table II; notice that the fingers were the most common site of VM. According to the extension of the defect, we registered 76 limited and 39 infiltrating cases. The number of operations for limited and extended forms are reported in Table III. Complications of surgery are shown in Table IV. The results of surgical treatment, divided by limited and extended forms, is shown in Table V.
Discussion
Analysis of these series demonstrated that VM may be located in various sites in the hand. The most common site is the fingers, which is also a location where surgery may be possible, whereas sclerosis has a higher probability of producing nerve damage (Figure 10). The distinction between limited and infiltrating forms is crucial, as the approach, the number of operations involved, and the results differ, as seen in Tables III-V.
The decision to operate in all cases here instead of choosing sclerosis was based on our former experience in which we noticed a significant recurrence or even no effect after sclerosis with classical substances, even using the foam technique. Over the years, we’ve had several other cases treated elsewhere via sclerosis, mainly with polidocanol or TDSS with very poor results, such as no effect or early recurrence. We have also tried ethanol sclerosis, which is known to be much more effective. However, we noticed some cases of nerve damage, even permanent damage, which discouraged us from continuing with this treatment.
For these reasons, we have chosen to approach VM of the hand mainly via surgery in teamwork.
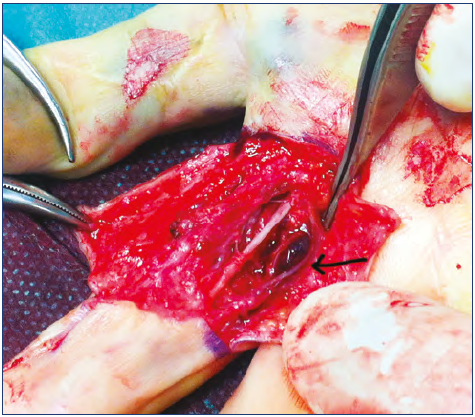
Figure 10. Surgical approach to a limited venous malformation of a finger: notice that nerve (front) and vessels (arrow) have been isolated before removing the malformation (dark area).
We are aware that some other groups have a preference for sclerosis. However, the lack of data about surgery and the good results we observed in our early experience encouraged us to continue with the surgical strategy.
During surgery, we have noticed that VMs often are not typical vessels but are blackberry-like, crumbly tissue, sometimes containing phleboliths (Figures 11, 12, 13). Moreover, these malformations may be irregular and infiltrate deeply into muscles or surrounding nerves, tendons, and vessels. These characteristics made us question whether such tissue could be successfully sclerosed. Regardless, we found that removal of limited malformations by surgery were mainly successful.
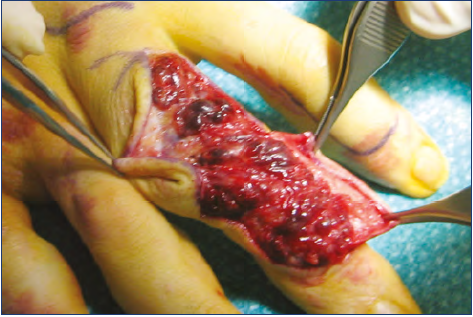
Figure 12. Resection of limited venous malformations of the
dorsum of the finger: notice the aspect of several small, limited masses; sclerosis would probably be ineffective in this case.
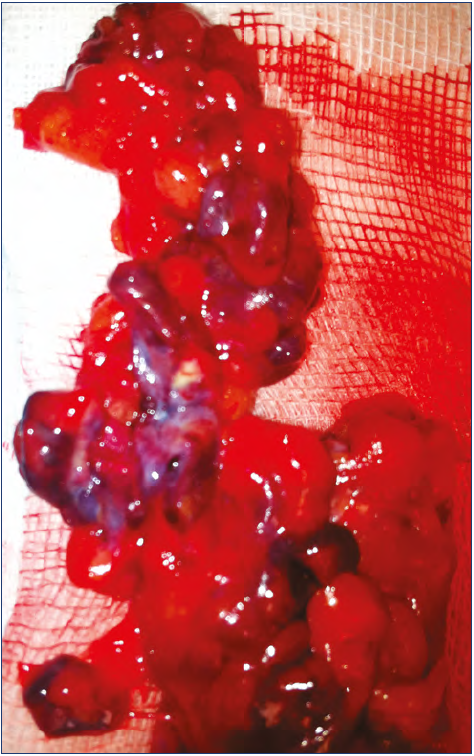
Figure 13. Tissue specimen of removed venous malformation: notice several blue masses mixed with normal fat. This patient is the same as for Figure 12. This is not the aspect in all cases: other (more common) forms arise mainly by a vascular mass without fat.
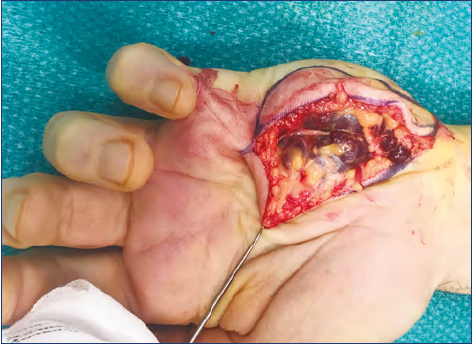
More difficult is the approach for infiltrating/extended cases. The aim for these was to reduce or eliminate symptoms, such as recurrent or continuous pain. The strategy in these cases was the tailored removal of the most symptomatic area of dysplastic vessels (Figure 14). In deep infiltrating forms, complete removal of the malformations located within the deep layers was not always possible. In many cases, several operations were required (Table III).
One main point is that VMs are often not only formed by vascular tissue: in many cases, fat hypertrophy was noticed, which is part of the mass effect and should be removed together with the malformation (Figure 15).
Complications were very few, as Table IV shows, even in the infiltrating group, and were even less than we expected. A main point here is that a stepwise strategy, splitting treatment into 2 or more surgical sessions rather than a single main session avoided significant blood loss, skin necrosis (except very limited necrosis of 1 edge of the wound, which healed spontaneously), complications of prolonged ischemia by tourniquet, and postoperative hematoma (we had not a single hematoma that required evacuation).
We saw good results for limited malformations, with a high incidence of healing without recurrence (Figure 16). The 75% of healed cases (no residual malformation) in the limited group indicates that surgery is likely to achieve a positive result in these cases. In our opinion, this is a better result than one would see with sclerosis, in which even occluded vessels may recur.
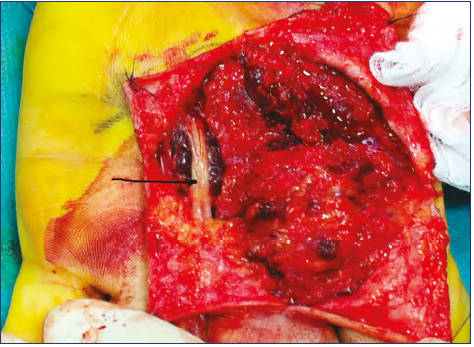
Figure 15. Surgery in a diffuse venous malformation of the
hand. Fat overgrowth is combined with dysplastic venous tissue. Notice a nerve (arrow) isolated over a mass of dysplasias.
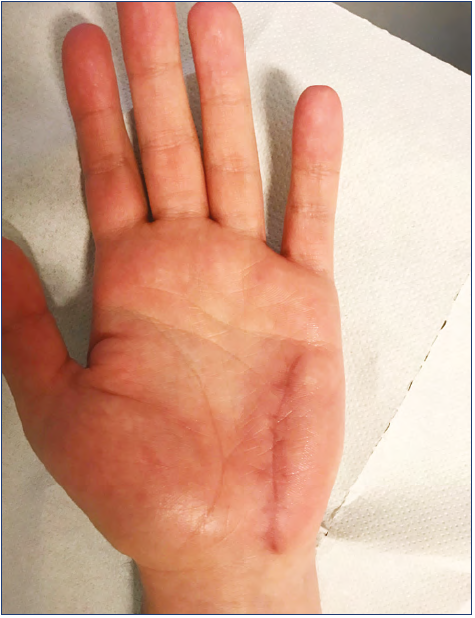
Figure 16. Postoperative control after removal of a venous malformation at hypothenar area: no residual malformation, confirmed by ultrasound exam. Same patient as for Figure 10.
In extended forms, we noticed that recurrence in the operated area was uncommon. We should point out that when speaking of “healing” and “recurrence” here, these refer to the operated area only. Even in these difficult cases, tailored surgery centered on the symptomatic points could improve patient condition (
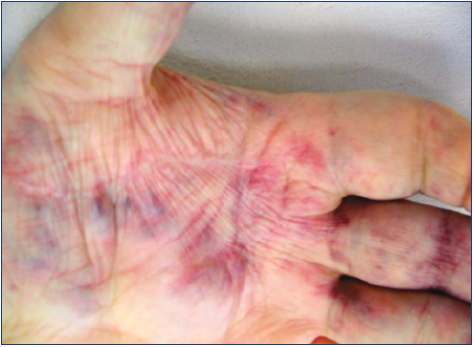
Figure 17. Postoperative result after successful tailored surgery
in an extended venous malformation case. There is no mass
recognizable in the operated area. Same patient as for Figure
14.
From our experience, we have learned several lessons. Firstly, VM can be removed successfully in many cases. Secondly, treatment should be chosen only after a complete diagnosis; for this, ECD was always very important for us. Thirdly, a team approach was crucial for us: the hand surgeon’s skill in anatomical knowledge coupled with the vascular surgeon’s skill in vessel management allowed us to successfully remove VM formerly considered inoperable.
Conclusion
Hand VM can be managed successfully by surgery if a careful and complete evaluation of the case is performed. Surgical treatment for hand VM is an effective and safe approach that reduces the incidence of recurrence. Complications are few if certain principles and techniques are followed.

REFERENCES
1. Belov S. Anatomopathological classification of congenital vascular defects. Semin Vasc Surg. 1993;6(4):219- 224.
2. Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(1):e203-e214. doi:10.1542/ peds.2014-3673.
3. Stillo F, Mattassi R, Diociaiuti A, et al: Guidelines for vascular anomalies by the Italian Society for the Study of Vascular Anomalus (SISAV). Int Angiol. 2022;41(2 suppl 1):1-130. doi:10.23736/S0392- 9590.22.04902-1.
4. Lee BB, Baumgartner I, Berlien P, et al. Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUO): updated 2013. Int Angiol. 2015;34(2):97- 149.
5. Stillo, F, Amato, B, Mattassi R, et al. Guidelines for vascular anomalies: venous malformations. Int Angiol. 2015;34 (suppl 1-2):18-23.
6. Vaghi M. Ultrasound diagnostic. In: Mattassi, R, Loose DA, Vaghi M. Hemangiomas and Vascular Malformations. An Atlas of Diagnosis and Treatment. Springer; 2015:207-211.
7. Marzella L, Atzei A, Cannavò F, et al. Upper limb vascular malformations guidelines: consensus conference in an expert panel. Chirurgia Della Mano. 2016;53(3):77-87.
8. Gorman J, Steven J, Zbarsky J, et al. Image guided sclerotherapy for the treatment of venous malformations. CVIR Endovascular. 2018;1:article 2. https://doi.org/10.1186/ s42155-018-0009-1
9. Odeyine SO, Kangesu L, Badran M. Sclerotherapy for vascular malformations: complications and review of techniques to avoid them. J Plast Reconstr Aesthet Surg. 2013;66(2):215-223. doi:10.1016/j. bjps.2012.09.002.
10. Fujiki M, Kurita M, Ozaki M, et al. Detrimental influences of intraluminallyadministered sclerotic agents on surrounding tissues and peripheral nerves: an experimental study. J Plast Surg Hand Surg. 2012;46(3-4):145-151. doi:10.3109/ 2000656X.2012.675881.
11. Guevara CJ, Gonzalez-Araiza G, Kim SK, et al. Sclerotherapy of diffuse and infiltrative venous malformations of the hand and distal forearm. Cardiovasc Intervent Radiol. 2016;39:705-710.
12. Schmidt VF, Masthoff M, Goldann C, et al. Percutaneous sclerotherapy of venous malformations of the hand: a multicenter study. Cardiovasc Intervent Radiol. 2021;44(10):1543-1550.
13. Di Giuseppe P. Treatment of vascular malformations in the hand. In: Mattassi, R, Loose DA, Vaghi M, eds. Hemangiomas and Vascular Malformations. Springer; 2015. https://doi.org/10.1007/978-88- 470-5673-2_44