Venous thromboembolic disease and pregnancy: prevention and treatment
Laboratoire d’Hématologie
Montpellier, France
ABSTRACT
The management of venous thromboembolism (VTE) during pregnancy is challenging for several reasons. In this article, we address the following questions: in pregnant women, how do we (i) treat VTE once a diagnosis is confirmed? (ii) assess the risk of VTE, and (iii) manage women with a high risk of VTE? When anticoagulants are required in pregnancy and the puerperium, low-molecular-weight heparin (LMWH) is now the preferred drug, but optimal dosage and monitoring remain unresolved issues. In addition, there is a paucity of reliable information about the risk of VTE in women with thrombophilia (asymptomatic or with a previous deep venous thrombosis or pulmonary embolism). Recommendations and limitations of the literature are highlighted.
INTRODUCTION
Pulmonary embolism (PE) is the leading cause of maternal mortality in Western countries.1-4 The incidence of pregnancy-related venous thromboembolism (VTE) is not known precisely, and depending on the study varies from 0.13 to 2.3 episodes per 1000 deliveries.5-7 Although these absolute rates are low, the risk of VTE is threefold to tenfold higher than in nonpregnant woman of similar age.6 A meta-analysis showed that two-thirds of cases of deep vein thrombosis (DVT) occur antepartum, distributed equally throughout all three trimesters.8 In contrast, 43% to 60% of pregnancyrelated PEs occur 4 to 6 weeks postpartum.9
I – WHICH ANTICOAGULANT CAN BE USED DURING
PREGNANCY?
Is there any place for vitamin K antagonists?
Warfarin readily crosses the placenta and has been associated with congenital malformation (exposure from 6 to 12 weeks) and fetal and neonatal bleeding.10 It should therefore be avoided in the management of VTE during pregnancy, preference being given to unfractionated heparin (UFH) and lowmolecular- weight heparin (LMWH), which do not cross the placenta and have no teratogenic effects. Warfarin can, however, be considered during pregnancy in women with high-risk valves.
Management of women receiving long-term vitamin K antagonists
In such women who want to become pregnant, repeat pregnancy tests should be proposed and warfarin should be replaced by full-dose LMWH when pregnancy is confirmed.11
Low-molecular-weight heparin or unfractionated heparin?
LMWH is now the most commonly used anticoagulant for prophylaxis and treatment of VTE in pregnancy and the puerperium.12 LMWH is preferred to UFH for several reasons. At least outside pregnancy, LMWHs are as effective as UFH for prevention or treatment of DVT and PE.13-15 It has a better safety profile both for the fetus and the mother16 and there is no evidence of teratogenicity or risk of fetal bleeding or that LMWH crosses the placenta.17 One of the advantages of LMWH is the potentially reduced risk of bleeding. This is of particular relevance in obstetric practice where postpartum bleeding remains the most frequent cause of severe obstetric morbidity. LMWHs are not associated with an increased risk of severe peripartum bleeding. In one systematic review, the frequencies of antenatal bleeding, postnatal bleeding, and wound hematoma were 0.43%, 0.94%, and 0.61%, respectively leading to an overall frequency of 1.98% (95% confidence interval [CI], 1.5- 2.57).18 The observed rate of major bleeding compares favorably with the rate of massive bleeding (0.7%) from one prospective study without the use of LMWH.19 In their review of 277 pregnancies in which LMWH was used, Greer and Nelson-Piercy noted no case of heparininduced thrombocytopenia.18 The reliable pharma – cokinetics of LMWHs and their long half-life, which means injections can be less frequent, make them attractive for practical use during several months of pregnancy. Significantly lower bone density in patients receiving UFH than in those receiving LMWHs, and no statistically significant difference between patients receiving LMWHs and untreated patients, suggest that bone loss associated with LMWHs is not different from physiologic bone loss during pregnancy.17,18
Is it possible to use danaparoid in pregnant women?
A review of 51 pregnancies in 49 danaparoid-treated patients between 1981 and 2004,20 showed that all patients developed heparin intolerance (32 due to heparin-induced thrombocytopenia,19 mainly due to heparin-induced rash) and had current or past VTE complications or both. The median duration of danaparoid use was 10 weeks. Danaparoid was used until delivery of a healthy infant in 37 pregnancies. In the remaining 14 pregnancies it was stopped earlier (anticoagulant treatment no longer required n=3; adverse event leading to treatment discontinuation n=11). Four maternal bleeding events were recorded during pregnancy, delivery or postpartum, two of which were fatal due to placental problems. Three fetal deaths associated with maternal complications antedating danaparoid use were recorded. Anti-Xa activity transfer was not observed in any of five fetal cord blood and three maternal breast milk samples. The authors concluded that danaparoid can be used as an alternative antithrombotic agent in pregnant women with high thrombotic risk and intolerance to heparins.
Is it possible to use pentasaccharide in pregnant women?
Although there have been some reports of the successful use of pentasaccharide in pregnant women, the quality of available evidence is very low. Therefore, the American College of Chest Physicians states that clinicians should avoid the use of fondaparinux and should only discuss its use for those with heparininduced thrombocytopenia or a history of heparininduced thrombocytopenia who cannot receive danaparoid.11
New anticoagulants
There are insufficient data to evaluate the safety of direct thrombins or anti-Xa inhibitors in pregnant women.
Which anticoagulant can be used in nursing women?
For most anticoagulants, data are limited. There were two early convincing reports about the absence of detection in breast milk and anticoagulant effect of warfarin in breastfed infants.21,22 Because of its high molecular weight and strong negative charge, UFH does not pass into breast milk. In a study of 15 patients, small amounts of LMWH were found in breast milk.23 However, due to the low bioavailability of orally ingested LMWH, a clinically relevant effect on the nursing infant is unlikely.11
II – HOW WE CAN TREAT VTE DURING
PREGNANCY?
The absence of randomized trials in pregnancy complicates the VTE treatment recommendations in pregnancy. It is therefore important to emphasize the need for coordination of physicians, including the hematologist, to establish clear local guidelines for VTE treatment during pregnancy (Table I).
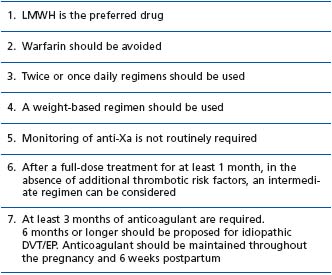
Table I : Treatment of acute VTE (DVT and/or PE) during pregnancy
Initial anticoagulant treatment
According to the last American College of Chest Physicians (ACCP) recommendations, LMWH is the preferred drug for the treatment of VTE during pregnancy (grade 1A), with a weight-adjusted dosing regimen (as per the manufacturer’s recommendations) (Table II).

Table II : LMWH full-dosing regimens
Once or twice daily dosing regimen?
During pregnancy, physiologic changes affect the pharmacokinetics of LMWH:24 60% expansion of intravascular plasma volume, 50% increase in glomerular filtration rate. Are these changes significant enough to modify the dosing regimen during pregnancy? A 2004 study in a relatively small number of women has suggested that once daily administration of tinzaparin may be appropriate in the treatment of VTE in pregnancy, despite some criticisms concerning the anti-Xa level.25 However, the United Kingdom Royal College of Obstetricians and Gynaecologists (RCOG) in 2001 and 2007 and the ACCP in 2004 have suggested a twice daily regimen. In 2008 the ACCP stated that a once daily regimen is acceptable for the treatment of VTE,11 on the basis of data published by Voke et al26 who surveyed antenatal VTE practice in the UK and Ireland, and Knight et al,27 who reported a population-based national case-control study evaluating the incidence and management of obstetric PE in the UK.
Is anti-Xa monitoring necessary during treatment of VTE in pregnancy?
The dose adjustments over the course of pregnancy remain controversial: some authors suggest that dose should be increased in proportion to change in weight; others suggest adjustment using the assay of anti-Xa levels 4 to 6 after the injection (0.5 to 1.2 anti-Xa/ml for a twice daily regimen or 1 to 2 anti- Xa/ml for a once daily regimen).28 The ACCP considers that definitive advice cannot be provided.11 The experience of the RCOG indicates that using a weight-based regimen is satisfactory and that anti-Xa monitoring is not routinely required in women with therapeutic doses of LMWH, particularly as there are concerns over the accuracy of anti-Xa monitoring.29 A study from the UK National External Quality Assessment Scheme (NEQAS) has demonstrated extremely wide coefficients of variation.30
In France, routine platelet count monitoring (every 2-3 days up to day 21 and then every 2 weeks) is required in all patients receiving UFH or LMWH, including pregnant women. In the UK, RCOG guidelines advise against routine platelet count monitoring in pregnant women who have received only LMWH as there have been no cases of heparininduced thrombocytopenia in pregnancies managed with LMWH.
Massive life-threatening VTE
Intravenous UFH is the preferred treatment in massive VTE with cardiovascular compromise.29 There is also a case for considering thrombolytic therapy, as anticoagulant treatment will not reduce obstruction of the pulmonary circulation. Data on thrombolytic therapy in pregnancy are limited, with concerns about maternal bleeding and adverse fetal effects.
Vena cava filter
Removable vena cava filters are a reasonable approach to women who have a transient contraindication to anticoagulants, such as the development of a VTE near the time (within 1 to 2 weeks) of delivery.31
After the initial period, is it possible to reduce the dose?
There is no clear consensus. Many experts continue with the full treatment dose while others switch to an intermediate regimen. The rationale of the former option is based on the safety of LMWHs during pregnancy and the continuing risk of VTE during pregnancy.5,16 In contrast, the other option is based on successful intermediate regimens used in patients with contraindications to warfarin or with underlying malignancy.32,33 In these two studies, patients received dalteparin once daily, which corresponds to 50% (10 000 U/24 h) of full-dose treatment in the first study and 75% (150 U/kg/24 h) in the second. Rodger et al (Canada) treat acute VTE in pregnancy with full-dose LMWH for 3 weeks and then halve the dose throughout the rest of the pregnancy and at least throughout the post-partum period.34 They argue that the efficacy and safety of the prophylactic dose of LMWH (which is not exactly half the full dose) are comparable to those of warfarin (INR 2-3) for acute DVT.35 Greer et al (UK) suggest a full dose for a minimum of one month before reducing to an intermediate dose of LMWH in the absence of additional risk factors such as underlying thrombophilia, immobility, and obesity.12 The ACCP recommends intermediate-dose LMWH: dalteparin 5000 U/12 h or enoxaparin 1 mg/kg/24 h. Intermediate regimens therefore range from 50% to 75% of the full treatment dose.
Finally, these modified regimens could be of interest in women at increased risk of bleeding and perhaps of osteoporosis.
What is the maintenance treatment of VTE in pregnancy?
It is currently admitted that treatment should be employed during the remainder of the pregnancy and for at least 6 weeks postnatally. The rationale for this position is based on the continuing risk of recurrent VTE during pregnancy and the postpartum period since pregnancy is itself a risk factor for VTE. Published recommendations usually advise at least 3 months; a minimum of 3 months of anticoagulation can be proposed for secondary VTE and longer anticoagulation, 6 months, should be considered for idiopathic VTE. The last ACCP guidelines, published in 2008, emphasize that there are no appropriately designed trials to define the duration of anticoagulation for women with VTE during pregnancy and suggest that at least 6 months is a “reasonable duration”.11
Additional therapy
To our knowledge, there is no study in pregnant women, but in a randomized, controlled trial in nonpregnant patients the incidence of postthrombotic syndrome after a first proximal DVT was reduced from 23% to 11%.36 Therefore, mobilization with graduated elastic stockings (at least class II) should be encouraged to reduce pain and swelling and also to reduce the risk of postthrombotic syndrome for 2 years after the occurrence of VTE.
Management of anticoagulant therapy at the time of delivery
Women requiring therapeutic doses of LMWH should be counseled before delivery, which should be planned with a team of specialists (obstetrician, hematologist, anesthesiologist, cardiologist).
Spontaneous or planned delivery? Cesarean section?
Delivery by cesarean section should only be decided on the basis of obstetric indications. It should be emphasized that induction of labor in a patient with an unfavorable cervix may increase the risk of cesarean delivery, which should be avoided because of the risk of VTE.37 Therefore, spontaneous vaginal delivery is preferable.
Time off anticoagulation
To avoid unwanted anticoagulant effects during delivery in women receiving therapeutic doses of LMWH, LMWH should be discontinued before elective induction of labor or cesarean section. A woman taking LMWH should be advised that once she thinks that she is in labor, she should not inject any further LMWH. According to the recommendations of the various societies, it is recommended to stop for 24 hours. The latest ACCP recommendations advise stopping 24 to 36 hours before elective induction of labor or cesarean section.11 The approach taken if spontaneous labor occurs in women receiving therapeutic doses of LMWH depends on the proximity of the last dose to the expected time of delivery and, if available, the anti-Xa level.38
Is it possible to stop anticoagulation for 24 hours in all women? This depends on the characteristics of the VTE. If the patient is considered to be at high risk (ie, VTE within 4 weeks), it is important to minimize the time off anticoagulation. Several approaches can be discussed. It has been proposed to replace LMWH by intravenous UFH, due to a shorter half-life, and to discontinue treatment 4 to 6 hours prior to the expected time of delivery.39 If spontaneous labor occurs, careful monitoring with aPTT is required and protamine sulfate may be needed to reduce the risk of bleeding.40 Full anticoagulation with LMWH has been maintained during labor and delivery in women with recent (within 4 weeks) VTE.41 Dulitzki et al reported no increased risk of major bleeding during cesarean delivery in 41 patients treated with LMWH.42 Epidural analgesia should be avoided.
If the patient is not considered to be at high risk (VTE during the last 3 months and fully anticoagulated), some experts propose switching to prophylactic doses of LMWH at 36 weeks of gestation.43 In this case, it is usually recommended that LMWH should be stopped as soon as a woman is (or thinks she is) in labor. No increased bleeding is expected with this approach.39
Regional anesthesia
There are several recommendations that have been devised to help anesthesiologists reduce the risk of spinal hematoma.44 However, although the consensus statements are based on evaluation of the available information, data are scarce, especially considering the obstetric population.37 Two studies showed no complications when using the following recommendations.42,45 Generally the consensus statements suggest that epidural analgesia should be avoided for at least 24 hours after the last dose of therapeutic LMWH or UFH.37,46 According to the RCOG, LMWH should not be given for at least 4 hours after the epidural catheter has been removed, and the cannula should not be removed within 12 hours of the most recent injection.29 Epidural analgesia should be avoided for at least 12 hours after the last dose of prophylactic LMWH.37
Immediate postnatal anticoagulation
There is a paucity of data that can be used to guide postnatal anticoagulation. Bates and Ginsberg consider that LMWH should be restarted as soon as it is safe to do so, usually within 12 hours of delivery, and warfarin can be started at the same time.38 The ACCP does not address this issue.11 The RCOG considers that “if the woman chooses warfarin postpartum, this should be avoided until at least the third postnatal day”. A thromboprophylactic dose of LMWH should be given by 3 hours postoperatively (more than 4 hours after removal of the epidural catheter, if appropriate).29 The Obstetric Medicine Group of Australasia suggests that prophylactic doses can be recommenced within 2-6 hours of both vaginal and cesarean deliveries, and therapeutic doses at least 24 hours after surgical delivery.37
III – HOW WE CAN ASSESS THE RISK OF VTE
DURING PREGNANCY?
Despite decreased mortality over the last 70 years, PE continues to be one of the most common causes of maternal death in developing countries. The ageadjusted incidence of VTE ranges from 5 to 50 times higher in pregnant versus nonpregnant women. The clinician dealing with the risk of VTE and prophylaxis in pregnancy and postpartum faces several questions: Are women who are at greatest risk identifiable? Is pregnancy-related VTE preventable? When is the best time to start prophylaxis? Unfortunately, there has been no large clinical study of the benefit of thromboprophylaxis during pregnancy.47,48 However, in 2002, Rodger et al found that most Canadian clinicians favor intervening with thromboprophylaxis rather than observing without prophylaxis in pregnant women, asymptomatic or with previous VTE, with thrombophilia.49 Hence, in the absence of evidence, the default recommendation becomes intervention. But do all women need thromboprophylaxis?
Which prophylaxis for which patients?
It is essential when assessing thrombotic risk associated with pregnancy to take into account acquired factors, as well as genetic predisposition (Table III).
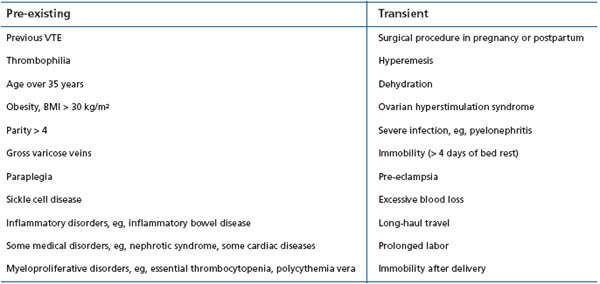
Table III : Risk factors for VTE in pregnancy and postpartum period
Pregnant women with previous VTE
The limitations of historical data hamper reliable estimation of the risk of recurrence during pregnancy and puerperium in women with previous VTE. In 2000, Brill-Edwards et al conducted a multicenter prospective study of 125 pregnant women with a previous single VTE.50 Women had antenatal prophylaxis withheld but were given prophylaxis in the postpartum period. Overall, 3 of the women with either abnormal thrombophilia screening or idiopathic previous VTE had an antepartum recurrence (5.9%; 95% CI, 1.2-16.2%). In contrast, there were no recurrences among the 44 women without thrombophilia or a previous VTE with a transient risk factor (relative risk 0, 95% CI, 0-8%). More recently, in 2007, a prospective observational study in the UK and a large Italian cohort study demonstrated a significantly increased risk of recurrence if the previous VTE was unprovoked, related to pregnancy or oral contraceptives, while thrombophilia screening was of limited benefit except in identifying antithrombin (AT) deficiency.26,51 It is clear that women with thrombophilia have an increased risk of VTE in pregnancy, but this risk varies depending upon the specific thrombophilia (Table IV). Current evidence and existing guidelines recommend that women with previous VTE and thrombophilia should receive antenatal thromboprophylaxis with LMWH continued for 6 weeks postpartum.
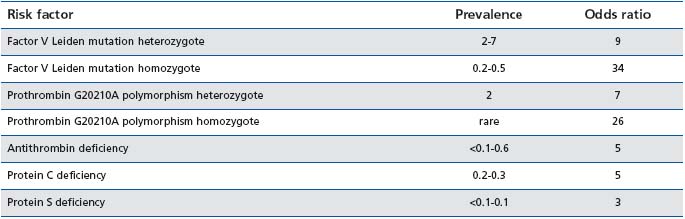
Table IV : Estimated prevalence of congenital thrombophilia and the associated risk of thromboembolism during pregnancy in a European population58
Whether thromboprophylaxis is warranted in these women identifiable as at high risk remains to be determined. Several nonrandomized studies have reported low VTE rates with the use of prophylactic doses.45 Only 2 randomized studies evaluating the efficacy and safety of prophylaxis, with major limitations, have been reported.52,53 Gates et al performed the only randomized, controlled trial comparing antenatal LMWH with placebo.52 Unfortunately, its sample size was too small to draw any definitive conclusion. Poor recruitment in this study indicates that large-scale trials using such a design would be difficult to run. In a prospective, nonrandomized study, Bauersachs et al recently showed that risk-stratified heparin prophylaxis is associated with a low incidence of VTE during pregnancy.54 An alternative way to assess the value of prophylaxis is to examine the balance of risks and benefits using a Markov model to compare prophylactic LMWH with expectant management.55 In this study, for high-risk women, antepartum prophylaxis is a cost-effective strategy, while for lowrisk women expectant management leads to better outcomes than use of LMWH. However, the definition of low- and high-risk women in this study is questionable.
The 8th ACCP recommendations concerning the prevention of VTE in pregnant women with previous VTE are detailed in Table V. I feel they are of limited interest for the clinician since several approaches (from clinical surveillance to intermediate-dose) are proposed for each group of patients, as mentioned in the table.
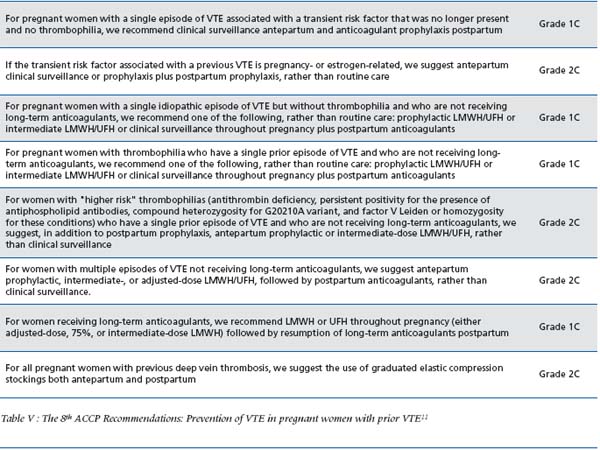
Pregnant women without previous VTE
It is increasingly common for pregnant women to present with known thrombophilia, usually detected because of screening following identification of inherited thrombophilia in a family member. As previously mentioned, the risk of VTE varies greatly depending upon the specific thrombophilia, but the absolute risk remains low. As an example, the results from cohorts, which are likely to be more reliable, show a pooled odds ratio of 4.46 (95% CI, 1.82- 10.94; 7879 pooled women), with no evidence of statistical heterogeneity (p = 0.36), for the risk of a first VTE during pregnancy or the postpartum period associated with the factor V Leiden heterozygous mutation. Case-control studies revealed a higher risk (odds ratio 8.6, 95% CI, 5.85-12.63; 1,433 [corrected] pooled women) with significant heterogeneity (P< 0.005). Since the risk of VTE is lower in women with no history of VTE, antenatal thrombo - prophylaxis does not always seem necessary, even if the women are receiving postpartum thrombo - prophylaxis for 4 to 6 weeks. In existing guidelines (ACCP, RCOG), women with AT deficiency, those with combined defects, and those homozygous for defects should receive antepartum and postpartum thromboprophylaxis. However, this approach needs further clinical investigation. As an example, in a cohort of 96 women homozygous for the factor V Leiden mutation, the risk of a first symptomatic pregnancy-related VTE was found to be 12.1% per pregnancy (95% CI: 6.3-22.1), 9.1% (95% CI: 4.2- 18.4) in the postpartum period and 3.0% (95% CI: 0.8-10.4) during pregnancy.56 Thrombosis occurred principally in the postnatal period, as already published, whether or not thrombophilia was present. This result reinforces the widely accepted fact that anticoagulants have to be given during the postpartum period for 4 to 6 weeks. On the other hand, there is room for debate regarding antepartum anticoagulant prophylaxis, even if the incidence of pregnancy-related VTE in factor V Leiden homozygotes seems higher than the best estimated incidence observed in an overall population of pregnant women (3% in the present study vs 0.06%; relative risk 10.7;95% CI 9.7-11.7).7 Studies measuring the effectiveness of prophylactic interventions are lacking.48 It remains to be established whether intervention with LMWH is of benefit in women at “high” risk.
The relatively equal distribution of VTE throughout all 3 trimesters suggests that when antepartum prophylaxis is used, it should be started early in the first trimester.8,57
CONCLUSION
The use of anticoagulant therapy during pregnancy is challenging. LMWHs are now the most commonly used anticoagulant for prophylaxis and treatment of VTE. However, the optimal strategy remains unclear due to the limitations of the available data.

REFERENCES
1. Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance—United States, 1991- 1999. MMWR Surveill Summ. 2003;52:1-8.
2. Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet. 1999;353:1258-1265.
3. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066-1074.
4. Ragusa A, Pisoni M, Wetzl R, Maccario L. Maternal mortality and thromboembolic risk in pregnancy. Haematologica. 2005;1:22-29.
5. Andersen BS, Steffensen FH, Sorensen HT, Nielsen GL, Olsen J. The cumulative incidence of venous thromboembolism during pregnancy and puerperium—an 11 year Danish population-based study of 63,300 pregnancies. Acta Obstet Gynecol Scand. 1998;77: 170-173.
6. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ, 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year populationbased study. Ann Intern Med. 2005;143:697-706.
7. Lindqvist P, Dahlback B, Marsal K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999;94:595-599.
8. Ray JG, Chan WS, Chan WS, Ray JG. Deep vein thrombosis during pregnancy and the puerperium: a meta-analysis of the period of risk and the leg of presentation low molecular weight heparin use during pregnancy: issues of safety and practicality. Obstet Gynecol Surv. 1999;54:265-271.
9. Gherman RB, Goodwin TM, Leung B, Byrne JD, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Prim Care Update Ob Gyns. 1998;5:155- 156.
10. Ginsberg JS, Hirsh J, Turner DC, Levine MN, Burrows R. Risks to the fetus of anticoagulant therapy during pregnancy. Thromb Haemost. 1989;61:197-203.
11. Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:844S-886S.
12. Greer I, Hunt BJ. Low molecular weight heparin in pregnancy: current issues. Br J Haematol. 2005;128:593-601.
13. Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecularweight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A metaanalysis of randomized, controlled trials. Ann Intern Med. 1999;130:800- 809.
14. Leizorovicz A, Simonneau G, Decousus H, Boissel JP. Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: a meta-analysis. BMJ. 1994;309:299-304.
15. Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140:175-183.
16. Sanson BJ, Lensing AW, Prins MH, et al. Safety of low-molecular-weight heparin in pregnancy: a systematic review. Thromb Haemost. 1999;81:668- 672.
17. Lepercq J, Conard J, Borel-Derlon A, et al. Venous thromboembolism during pregnancy: a retrospective study of enoxaparin safety in 624 pregnancies. BJOG. 2001;108:1134-1140.
18. Greer IA, Nelson-Piercy C. Lowmolecular- weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106:401-407.
19. Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. BMJ. 2001;322:1089-1093; discussion 1093-1094.
20. Lindhoff-Last E, Kreutzenbeck HJ, Magnani HN. Treatment of 51 pregnancies with danaparoid because of heparin intolerance. Thromb Haemost. 2005;93:63-69.
21. McKenna R, Cole ER, Vasan U. Is warfarin sodium contraindicated in the lactating mother? J Pediatr. 1983;103:325-327.
22. Orme ML, Lewis PJ, de Swiet M, et al. May mothers given warfarin breastfeed their infants? Br Med J. 1977;1:1564-1565.
23. Richter C, Sitzmann J, Lang P, Weitzel H, Huch A, Huch, R. Excretion of low molecular weight heparin in human milk. Br J Clin Pharmacol. 2001;52:708- 710.
24. Patel JP, Hunt BJ. Where do we go now with low molecular weight heparin use in obstetric care? J Thromb Haemost. 2008;6:1461-1467.
25. Smith MP, Norris LA, Steer PJ, Savidge GF, Bonnar J. Tinzaparin sodium for thrombosis treatment and prevention during pregnancy. Am J Obstet Gynecol. 2004;190:495-501.
26. Voke J, Keidan J, Pavord S, Spencer NH, Hunt BJ. The management of antenatal venous thromboembolism in the UK and Ireland: a prospective multicentre observational survey. Br J Haematol. 2007;139:545-558.
27. Knight M. Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG. 2008;115:453- 461.
28. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecularweight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S-94S.
29. Royal College of Obstetricians and Gynaecologists (RCOG). Thromboembolic disease in pregnancy and the puerperium: acute management. London (UK): Royal College of Obstetricians and Gynaecologists (RCOG);2007 Feb. 17 p. (Green-top guideline; no. 28). [99 references].
30. Kitchen S, Iampietro R, Woolley AM, Preston FE. Anti Xa monitoring during treatment with low molecular weight heparin or danaparoid: inter-assay variability. Thromb Haemost. 1999;82:1289-1293.
31. Kawamata K, Chiba Y, Tanaka R, Higashi M, Nishigami K. Experience of temporary inferior vena cava filters inserted in the perinatal period to prevent pulmonary embolism in pregnant women with deep vein thrombosis. J Vasc Surg. 2005;41:652- 656.
32. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.
33. Monreal M, Roncales FJ, Ruiz J, et al. Secondary prevention of venous thromboembolism: A role for lowmolecular- weight heparin. Haemostasis. 1998;28:236-243.
34. Rodger MA, Walker M, Wells PS. Diagnosis and treatment of venous thromboembolism in pregnancy. Best Pract Res Clin Haematol. 2003;16:279- 296.
35. Marchetti M, Pistorio A, Barone M, Serafini S, Barosi G. Low-molecularweight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis. Am J Med. 2001;111:130-139.
36. Brandjes DP, Buller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759-762.
37. Kopp SL, Horlocker TT. Anticoagulation in pregnancy and neuraxial blocks. Anesthesiol Clin. 2008;26:1-22.
38. Bates SM, Ginsberg JS. How we manage venous thromboembolism during pregnancy. Blood. 2002;100:3470-3478.
39. Demers C, Ginsberg, JS. Deep venous thrombosis and pulmonary embolism in pregnancy. Clin Chest Med. 1992;13:645-656.
40. Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996;93:2212-2245.
41. Hague WM, North RA, Gallus AS, et al. Anticoagulation in pregnancy and the puerperium. Med J Aust. 2001;175:258-263.
42. Dulitzki M, Pauzner R, Langevitz P, Pras M, Many A, Schiff E. Lowmolecular- weight heparin during pregnancy and delivery: preliminary experience with 41 pregnancies. Obstet Gynecol. 2002;87:380-383.
43. Bates SM. Treatment and prophylaxis of venous thromboembolism during pregnancy. Thromb Res. 2002;108:97- 106.
44. Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28:172-197.
45. Nelson-Piercy C, Letsky EA, de Swiet M. Low-molecular-weight heparin for obstetric thromboprophylaxis: experience of sixty-nine pregnancies in sixty-one women at high risk. Am J Obstet Gynecol. 1997;176:1062-1068.
46. Checketts MR, Wildsmith JA. Central nerve block and thromboprophylaxis—is there a problem? Br J Anaesth. 1999;82:164- 167.
47. Gates S, Brocklehurst P, Davis L. Prophylaxis for venous thromboembolic disease in pegnancy and the early postnatal period (Cochrane review). 2003.
48. Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132:171-196.
49. Rodger MA, Carrier M, Keely E, Karovitch A, Nimrod C, Walker M, Wells PS. The management of thrombophilia during pregnancy: a Canadian survey. J Obstet Gynaecol Can. 2002;24:946-952.
50. Brill-Edwards P, Ginsberg JS, Gent M et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343:1439-1444.
51. De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol. 2006;135:386-391.
52. Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low-molecularweight heparin. Am J Obstet Gynecol. 2004;191:1296-1303.
53. Howell R, Fidler J, Letsky E, de Swiet M. The risks of antenatal subcutaneous heparin prophylaxis: a controlled trial. Br J Obstet Gynaecol. 1983;90:1124- 1128.
54. Bauersachs RM, Dudenhausen J, Faridi A, et al. Risk stratification and heparin prophylaxis to prevent venous thromboembolism in pregnant women. Thromb Haemost. 2007;98: 1237-1245.
55. Johnston JA, Brill-Edwards P, Ginsberg JS, Pauker SG, Eckman MH. Costeffectiveness of prophylactic low molecular weight heparin in pregnant women with a prior history of venous thromboembolism. Am J Med. 2005;118:503-514.
56. Procare. Risk of venous thromboembolism during pregnancy in homozygous carriers of the factor V Leiden mutation: are there any predictive factors? J Thromb Haemost. 2004;2:359-360.
57. Blanco-Molina A, Trujillo-Santos J, Tirado R, et al. Venous thromboembolism in women using hormonal contraceptives. Findings from the RIETE Registry. Thromb Haemost. 2009; 101:478-482.
58. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359:2025-2033.
