What a phlebologist should know about the anterior accessory saphenous vein?
Erasmus University Rotterdam, Rotterdam,
The Netherlands
Abstract
The anterior accessory saphenous vein (AASV) is not only a tributary of the saphenofemoral junction, but it is one of the saphenous trunks, situated in its own saphenous compartment in the thigh, lateral to the great saphenous vein (GSV). Incompetence of the AASV, often without GSV incompetence, is found in about 10% of limbs with varicose veins. As to the clinical appearance of isolated AASV incompetence, this typically presents as varicose veins, coursing from the anterior thigh to the lateral knee and calf. The vast majority of these limbs can be classified as C2 according to the clinical, etiological, anatomical, and pathophysiological (CEAP) classification. Duplex ultrasound of patients with varicose veins should always include investigation of the AASV, before planning any treatment. Treatment of AASV incompetence used to be a classic high ligation procedure with ligation of the GSV and stripping of the AASV, in combination with phlebectomies. Nowadays, this has been replaced by endovenous thermal ablation with subsequent ultrasound-guided foam sclerotherapy (UGFS) or in combination with phlebectomies. A useful alternative is to perform UGFS of the AASV and its varicose tributaries. Both strategies received a recommendation grade I C in recent guidelines for AASV treatment. Another possibility is to perform only phlebectomies of the visible varicose veins. In patients with recurrent varicose veins both after surgery and endovenous ablation of the GSV, the AASV is often involved. The optimal strategy for prevention of such AASV recurrence is still a matter of debate.
Anatomy and duplex anatomy of the AASV
While the anatomy of the saphenofemoral junction (SFJ) and the great saphenous vein (GSV) in the thigh has been extensively described based on anatomical dissections, surgical findings, and duplex ultrasound (DUS) findings, and hence is obvious to all practitioners, there is a lot of persisting confusion about the anterior accessory saphenous vein (AASV). The reason for this confusion is mainly because Caggiati et al1, in a consensus document, used cadaver dissection–based anatomical definitions to define accessory veins as “venous segments that ascend parallel to the saphenous veins, either anterior, posterior, or more superficial to the main trunk.” According to this rather vague definition, it was not clear whether accessory saphenous veins were running inside or outside of the saphenous compartment. Subsequently, along with increasing understanding of the so-called “duplex anatomy,” a refinement of the anatomical nomenclature was developed and published again in the Journal of Vascular Surgery in 2005.2 It stated that the AASV “at the upper thigh courses deeply (superficial to the muscular fascia, like the GSV) to a hyperechoic fascia that resembles the GSV covering. However the AASV can be easily identified, because it courses more anteriorly with respect to the GSV, with a path corresponding to that of the underlying femoral artery and veins.”2 This means that, on DUS, the AASV can easily be recognized in its own saphenous compartment or “saphenous eye,” which can be clearly distinguished from the saphenous compartment of the GSV.3 In the upper third of the thigh, two saphenous eyes can often be distinguished, one of the GSV and the other one of the AASV, the latter being recognizable by the alignment sign (Figure 1).3 Duplex ultrasound investigation made it clear that the AASV is a real truncal saphenous vein, different from the GSV. Therefore, the often erroneously used name “anterior accessory great saphenous vein” and its abbreviation (“AAGSV”) should be completely abandoned. Nowadays, investigation of the AASV has become an intrinsic part of the routine DUS of patients with chronic venous disease before treatment.4
In very exceptional cases (less than 1% of GSVs), there is a duplication of the GSV itself, with two parallel veins runningin a unique saphenous compartment. In such rare cases, the more anteriorly situated vein of the two is not called the AASV, but is one of the two GSVs of a duplicated GSV.
One of the typical characteristics of the AASV is that it has a relatively short course (5 to 20 cm from the SFJ) and it never reaches below the knee. If the AASV is incompetent, it typically results in visible varicose veins that are often coursing obliquely on the anterior side of the thigh to the lateral side of the knee and lower leg (Figure 2). These varicosities should not be called extrafascial AASV, but rather anterolateral tributaries of the AASV. Previously, these tortuous tributaries of the AASV were also described as the “vena circumflexa femoris anterior” or “varix semicircularis anterior,” and French phlebologists used to call it “la cravate antérieure,” all outdated terminology from the era before DUS investigation was introduced.
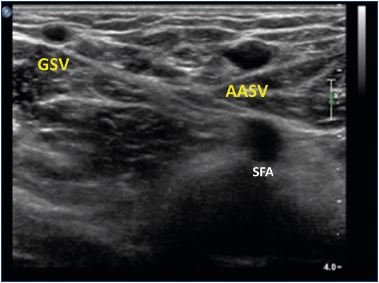
Figure 1. Duplex ultrasound of the left thigh (transverse view),
about 5 cm under the groin.
The GSV has a smaller caliber than the AASV. The AASV is
situated in front of the SFA and femoral vein (“alignment sign”).
Abbreviations: AASV, anterior accessory saphenous vein; GSV,
great saphenous vein; SFA, superficial femoral artery.
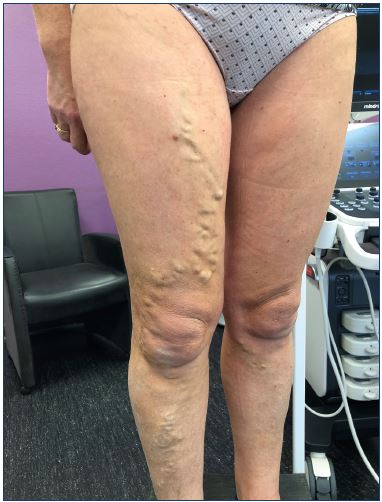
Figure 2. Typical clinical appearance of isolated incompetence
of the right anterior accessory saphenous vein.
Image courtesy of Dr. Claudine Hamel-Desnos
The AASV usually joins the GSV between 0.5 and 2 cm from the SFJ. On DUS, the “Mickey Mouse” image may be seen with a “double ear” at one side if the junction between the AASV and the GSV is very close to the SFJ (Figure 3). In some rare cases, the AASV even has a separate junction with the common femoral vein, which results in a “double” SFJ. It is important to mention that veins from the lymph nodes in the groin are not only draining into the GSV, but also into the AASV.5 Near the SFJ, lymph nodes can be typically seen on ultrasound, surrounding the AASV.
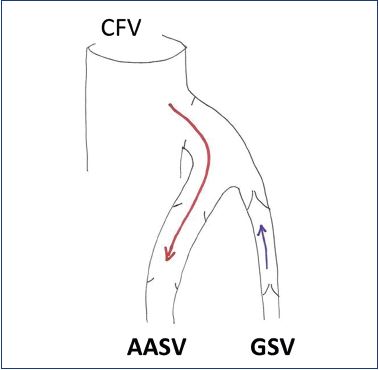
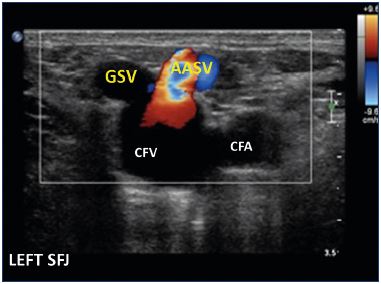
Figure 3. Duplex ultrasound of the left saphenofemoral junction
(transverse view) illustrating the junction of the GSV and the
AASV, the latter showing reflux at Valsalva maneuver.
Abbreviations: AASV, anterior accessory saphenous vein; CFA,
common femoral artery; CFV, common femoral vein; GSV, great
saphenous vein; SFJ, saphenofemoral junction.

Figure 4. Diagram of the right incompetent saphenofemoral
junction with isolated incompetence of the AASV. The red arrow
indicates the course of reflux.
Abbreviations: AASV, anterior accessory saphenous vein; CFV,
common femoral vein; GSV, great saphenous vein.
While in the vast majority of cases the AASV diameter is smaller than the GSV diameter, it may be the other way round, mainly when the AASV is incompetent (Figure 1). According to thorough DUS studies in more than 2000 limbs of patients with varicose veins, in about 10% of cases, there was incompetence of an accessory saphenous vein, mostly the AASV, without involvement of the GSV.6 In most such cases, the SFJ is incompetent and reflux is seen from the SFJ into the AASV during a Valsalva maneuver, while the preterminal valve of the GSV is competent and hence there is no reflux in the GSV (Figure 4).
Symptoms and clinical presentation of AASV incompetence
Although the varices may be quite prominent, not all patients with AASV incompetence present with typical venous symptoms like heaviness, pain, nocturnal cramps, itching, and paresthesia. Many patients are mainly worried about the cosmetic aspect, complaining about their “ugly legs.”
By far, the most typical presentation of AASV incompetence is varicose veins (C2, according to the clinical, etiological, anatomical, and pathophysiological [CEAP] classification7) that are visible on the anterior thigh, typically running obliquely to the lateral side of the knee and further down to the lateral calf (Figure 2), but other varices may also be seen (Figure 5, right leg). The most cranial varix indicates the connection with the AASV, which may be situated somewhere between the SFJ (or close to it) and the midthigh, as described above. In some cases, AASV tributaries connect with either the GSV (Figure 5, left leg) or the small saphenous vein (SSV), which may explain the presence of varicosities in the GSV or SSV territory as well. Development of edema (C3) or more advanced stages of chronic venous disease (C4-C6) are not very common in limbs with AASV incompetence. Unfortunately, detailed data, mentioning the CEAP classification in AASV incompetence in particular, have been rarely reported in the literature. In a study on 63 consecutive limbs in 62 patients (58 female, 5 male) with isolated AASV incompetence without GSV involvement, 57 limbs were classified as C2 (90%), 2 were classified as C3 (3%), 3 limbs as C4 (5%), and 1 with an ulcer (C6) in a morbidly obese patient (own series, unpublished data). In the majority of these cases, the venous clinical severity score ranged between 1 and 4. Theivacumar et al8 published a small series of 33 patients with primary or recurrent varicose veins due to isolated AASV incompetence and they classified 28 patients as C2 (85%), 4 as C3 (12%), and 1 as C4 (3%). The Aberdeen Varicose Veins Symptoms Severity Score in this group was similar to an age- and sex-matched control group with GSV incompetence. Equally, a recent study did not find any difference in clinical severity between limbs with refluxing SFJ and AASV (n=23) vs limbs with refluxing SFJ and GSV (n=145); they found a higher percentage of C4-C6 (22%) limbs in the AASV group than in the two studies mentioned above.9 In this study, the AASV group comprised significantly more females and the BMI was higher than in the GSV group.9 The latter may also have influenced disease severity in the AASV group.
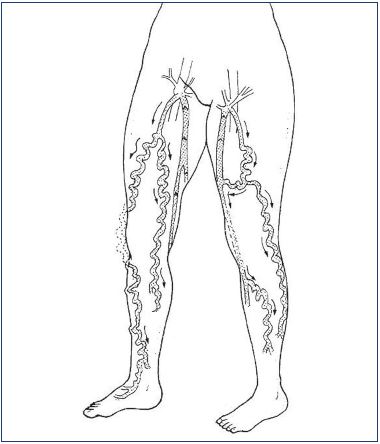
Figure 5. Diagram of a patient with bilateral incompetence of
the AASV with different patterns right/left of refluxing tributaries
and course of reflux.
In the right leg, there are varicose veins both on the lateral and
anterior side of the knee. In the left leg, there are varicose veins
on the lateral side of the leg, but there is also a connection with
the great saphenous vein, which becomes incompetent and
gives rise to anterior varicose veins of the lower leg as well.
Abbreviations: AASV, anterior accessory saphenous vein.
Finally, in some exceptional cases, patients with AASV incompetence may present with a bulging mass in the groin due to an aneurysm of the proximal AASV, which may even mimic an inguinal hernia and may be complicated by thrombosis of the aneurysm.10
Diagnosis of AASV incompetence by duplex ultrasound
As for the diagnosis of all other patients with chronic venous disease (C2-C6), duplex ultrasound is mandatory, both for documenting the course of reflux and for planning an adequate treatment strategy. Duplex ultrasound investigation is performed with the patient standing. The AASV is identified as per the above-mentioned description. The diameter of the AASV can usually not be measured at mid-thigh, as is done for the GSV, but rather more cranially, preferably at about 5 cm from the SFJ and in a straight part of the vein (away from any focal dilatation or aneurysm). When there is a focal dilatation, exceeding 20 mm, this is defined as an AASV aneurysm, most frequently situated close to the SFJ.
At the time of publication of the revision of the CEAP classification,7 the AASV was not really recognized yet as a separate saphenous trunk (see above). This causes a problem when trying to describe chronic venous disease properly in a limb presenting with symptomatic varicose veins due to isolated AASV incompetence, in particular to locate the reflux in the advanced CEAP classification. Some colleagues claimed C2S As Ep Pr5 was the correct way to describe this, whereas others were more in favor of mentioning it as C2S As Ep Pr2. The latter seemed more logical, as the AASV is a saphenous trunk above the knee, so the CEAP classification would be similar to the classification used for the GSV above the knee. The ongoing new revision of the CEAP classification will undoubtedly propose a solution to this problem.
Treatment options for incompetence of the AASV and tributaries
Whereas the literature on the treatment of the GSV is quite extensive, not many studies have focused on the AASV; in addition, AASVs have probably been included as GSVs in clinical trials without distinction. On the other hand, in several recent randomized clinical trials, limbs with preoperative AASV incompetence have been excluded in order to study a homogeneous group of limbs with GSV incompetence and evaluate the postoperative fate of the AASV, in particular after thermal ablation of the GSV. In general, whenever AASV treatment is separately included in a series of GSV/AASV treatments, limbs with isolated AASV incompetence represent about 5% to 7% of all included limbs.11,12
Recently, the American College of Phlebology (present name: American Vein and Lymphatic Society [AVLS]) Guidelines Committee developed the guidelines for the “treatment of refluxing accessory veins,” under the lead of Kathleen Gibson.13 Recommendations were based on both a literature review and expert opinions to help physicians make evidence-based decisions (as much as possible) for the benefit of patients with chronic venous disease related to AASV incompetence (treatment of the posterior accessory saphenous vein was also included).
In practice, several treatment options are available and the choice of treatment in an individual patient will mainly depend on the patient’s expectations, symptoms, clinical observations, and DUS findings.
Before the introduction of endovenous ablation techniques, the treatment of choice for isolated AASV incompetence was high ligation at the SFJ, division and ligation of the GSV, and then stripping of the AASV (or just excision if it was very short, eg, only 5 cm). This treatment was combined with extensive phlebectomies of all tributaries; the intervention was usually performed under general anesthesia. Prinz et al14 published a small retrospective study on a series of 30 patients treated with this technique. In two cases, the GSV thrombosed postoperatively and therefore it was decided to remove it. After a 3-year follow-up, the authors found reflux in the above-knee GSV at DUS in one-third of the limbs; however, the clinical results were not mentioned. In my own unpublished series mentioned above (62 patients, 63 limbs), the clinical results were excellent after a 1-year follow-up in all, but 1, patient. One patient developed neovascularization at the SFJ and symptomatic reflux in the GSV; she underwent additional stripping of the GSV. Duplex ultrasound after 1 year showed grade 2 neovascularization (more than 4 mm in diameter, with reflux) at the SFJ in 3 cases (5%), occlusion or absence of the proximal half of the GSV in 4 cases (6%), and asymptomatic reflux in the GSV from mid-thigh due to incompetence of a femoral vein perforator in another 4 cases (6%). Patient satisfaction was very high in this selected group of patients.
According to the American College of Phlebology guidelines, endovenous laser ablation (EVLA) or radiofrequency ablation (RFA) of the refluxing saphenous trunk is recommended for the treatment of symptomatic isolated AASV incompetence (recommendation grade I C).13 This intervention is performed under local tumescent anesthesia and can be combined with either miniphlebectomies or ultrasound-guided foam sclerotherapy (UGFS) to treat the usually extensive varicose tributaries. In the previously mentioned prospective study by Theivacumar et al,8 two similar groups of patients, having either GSV or AASV incompetence, were treated with EVLA of the refluxing saphenous trunk. Six weeks after EVLA of the AASV and GSV, additional UGFS was required in 61% and 42% of patients, respectively (nonsignificant difference). The same group also reported good results of EVLA and UGFS in recurrent varicose veins related to AASV incompetence after previous stripping of the GSV.15 Other investigators reported excellent 1-year results of EVLA with concomitant UGFS in case of AASV incompetence (56 limbs).16 The intervention was considered safe and the incidence of endovenous heat-induced thrombosis (EHIT) was not greater after EVLA or RFA of the AASV than of the GSV.17,18
In many patients with isolated AASV incompetence, UGFS may offer a simple and suitable solution, which can be performed in one (Figure 6) or more sessions and can easily be repeated. In the American College of Phlebology guidelines, it received a recommendation of grade I C, the same as endovenous thermal ablation.13
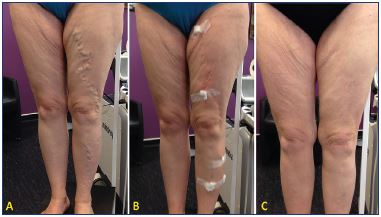
Figure 6. Treatment of a refluxing anterior accessory saphenous
vein and its tributaries by means of ultrasound-guided foam
sclerotherapy in one session.
Panel A. Before treatment; Panel B. Immediately after foam
injections. Panel C. Clinical appearance after 6 months.
Image courtesy of Dr. Claudine Hamel-Desnos.
In a large retrospective study by Bradbury et al19 using foam sclerotherapy in more than 1200 limbs with varicose veins, 139 limbs were treated for AASV incompetence (93 primary, 46 recurrent varicose veins). After a mean follow-up time of more than 2 years, recurrent reflux of the AASV was present in only 3.6% of cases.
An alternative strategy for patients with symptomatic AASV incompetence consists of performing single ambulatory phlebectomies under local tumescent anesthesia, without high ligation (contrary to the case with classic surgery – see above). This technique is known by the acronym ASVAL (Ambulatory Selective Varicose vein Ablation under Local anesthesia) and it has mainly been studied in the GSV, although the findings can easily be applied to the AASV.20,21 Recently, a Spanish group reported results of what they described as a new strategy (modified CHIVA) for the refluxing AASV, consisting of single phlebectomies without high ligation, in the same way as the ASVAL technique prescribes.22 They included 65 patients in a prospective study with a 1-year follow-up to analyze efficacy and safety. After 1 year, varicose veins recurred in only 8% of the patients. The mean diameter of the AASV was significantly reduced (from 6.4 to 3.4 mm) and the reflux was abolished in 82% of treated limbs.
In another small retrospective study, an interesting combination of techniques was reported. In 28 patients (29 legs) with isolated AASV incompetence and varicose tributaries, phlebectomies of all varicosities were performed under local tumescent anesthesia, which was followed by foam sclerotherapy of the refluxing AASV using polidocanol foam (1%, 2%, or 3%) that was injected 5 to 8 cm from the SFJ.23 The authors mainly focused on the immediate follow-up, in view of potential thrombotic complications. At 1 week, DUS showed occlusion of the AASV and no deep vein thrombosis. In one case, there was a mild inflammatory reaction and, in another case, there was a more pronounced inflammatory reaction at the site of AASV treatment. Unfortunately, further follow-up was not well documented.
Finally, the nonthermal, nontumescent techniques (mechanochemical ablation, cyanoacrylate glue) have not yet been studied in isolated AASV incompetence, but the promising results of these techniques in GSV and SSV incompetence could probably also be achieved in isolated AASV incompetence.
Role of the AASV in recurrent varicose veins after surgery and endovenous ablation: the eternal culprit?
After previous surgical or endovenous treatment of the GSV, recurrent varicose veins often originate from the groin, with recurrent or persisting reflux of the SFJ, new refluxing tributaries, and/or neovascularization.24 One of the most “popular” pathways of recurrence, causing varicose veins at the thigh level, is an incompetence of the SFJ with reflux of the AASV.
Garner et al25 evaluated a series of patients with recurrent varicose veins by means of DUS and found 141 groin recurrences, where a refluxing AASV was involved in 61 (43%) of these recurrences. They concluded that the AASV should be routinely looked at during preoperative DUS scanning and they advocated more thorough surgery at the SFJ, including dissecting/dividing the AASV beyond the side branches or stripping of the AASV, if identified during operation.
Nowadays, the situation is not that different after EVTA of the GSV. Several studies have reported new incompetence of the AASV to be responsible for recurrence of varicose veins in 8% to 35% of cases.26-28 This is probably related to persisting incompetence of the SFJ after EVTA in certain cases, although the pathophysiology is not completely clear. Future studies will be needed, including prospective DUS follow-up, to unravel this issue further. Whether EVTA at the very SFJ (sometimes called laser or RFA crossectomy) or preventive ablation of a nonrefluxing AASV would reduce the number of recurrences from the groin and in particular involvement of the AASV in such recurrences remains unclear. Nevertheless, it can be concluded that the AASV remains an important culprit, so, nihil novi sub sole – nothing new under the sun.

REFERENCES
1. Caggiati A, Bergan JJ, Gloviczki P, Jantet G, Wendell-Smith CP, Partsch H. International Interdisciplinary Consensus Committee on Venous Anatomical Terminology. J Vasc Surg. 2002;36(2):416-422.
2. Caggiati A, Bergan JJ, Gloviczki P, Eklof B, Allegra C, Partsch H. International Interdisciplinary Consensus Committee on VENOUS ANATOMICAL TERminology. J Vasc Surg. 2005;41(4):719-724.
3. Cavezzi A, Labropoulos N, Partsch H, et al. A duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs – UIP consensus document. Part II. Anatomy. Eur J Vasc Endovasc Surg. 2006;31:288-299.
4. De Maeseneer M, Pichot O, Cavezzi A, et al. Duplex ultrasound investigation of the veins of the lower limbs after treatment for varicose veins – UIP consensus document. Eur J Vasc Endovasc Surg. 2011;42(1):89-102.
5. Uhl JF, Lo Vuolo M, Labropoulos N. Anatomy of the lymph node venous networks of the groin and their investigation by ultrasonography. Phlebology. 2016;31:334-343.
6. García-Gimeno M, Rodríguez-Camarero S, Tagarro-Villalba S, et al. Duplex mapping of 2036 primary varicose veins. J Vasc Surg. 2009;49(3):681-689.
7. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248- 1252.
8. Theivacumar NS, Darwood RJ, Gough MJ. Endovenous laser ablation (EVLA) of the anterior accessory great saphenous vein (AAGSV): abolition of saphenofemoral reflux with preservation of the great saphenous vein. Eur J Vasc Endovasc Surg. 2009;37:477-481.
9. Schul MW, Schloerke B, Gomes GM. The refluxing anterior accessory saphenous vein demonstrates similar clinical severity when compared to the refluxing great saphenous vein. Phlebology. 2016;31:654-659.
10. Bush RG, Bush P. Aneurysms of the superficial venous system: classification and treatment. Veins Lymphatics. 2014;3(5403):61-63.
11. De Maeseneer MG, Philipsen TE, Vandenbroeck CP, et al. Closure of the cribriform fascia: an efficient anatomical barrier against postoperative neovascularisation at the saphenofemoral junction? A prospective study. Eur J Vasc Endovasc Surg. 2007;34:361-366.
12. Chaar CI, Hirsch SA, Cwenar MT, Rhee RY, Chaer RA, Abu Hamad. Expanding the role of endovenous laser therapy: results in large diameter saphenous, small saphenous, and anterior accessory veins. Ann Vasc Surg. 2011;25:656-661.
13. Gibson K, Khilnani N, Schul M, Meissner M. American College of Phlebology guidelines – treatment of refluxing accessory saphenous veins. Phlebology. 2017;32:448-452.
14. Prinz N, Selzle K, Kamionek I, et al. Surgery of the lateral accessory saphenous vein [article in German]. Zentralbl Chir. 2001;126:526-527.
15. Theivacumar N, Gough M. Endovenous laser ablation (EVLA) to treat recurrent varicose veins. Eur J Vasc Endovasc Surg. 2011;41:691-696.
16. King T, Coulomb G, Goldman A, Sheen V, McWilliams S, Guptan RC. Experience with concomitant ultrasound-guided foam sclerotherapy and endovenous laser treatment in chronic venous disorder and its influence on Health Related Quality of Life: interim analysis of more than 1000 consecutive procedures. Int Angiol 2009;28:289-297.
17. Sufian S, Arnez A, Labropoulos N, Lakhanpal S. Endovenous heat-induced thrombosis after ablation with 1470 nm laser: incidence, progression, and risk factors. Phlebology. 2015;30:325-330.
18. Sufian S, Arnez A, Labropoulos N, Lakhanpal S. Incidence, progression and risk factors of endovenous heat-induced thrombosis after radiofrequency ablation. J Vasc Surg Venous Lymphat Disord. 2013;1:159-164.
19. Bradbury A, Bate G, Pang K, Darval KA, Adam DJ. Ultrasound guided foam sclerotherapy is a safe and clinically effective treatment for superficial venous reflux. J Vasc Surg. 2010;52:939-945.
20. Pittaluga P, Chastanet S, Locret T, Barbe R. The effect of isolated phlebectomy on reflux and diameter of the great saphenous vein: a prospective study. Eur J Vasc Endovasc Surg. 2010;40:122-128.
21. Biemans AA, van den Bos RR, Hollestein LM, et al. The effect of single phlebectomies of a large varicose tributary on great saphenous vein reflux. J Vasc Surg Venous Lymphat Disord. 2014;2:179-187.
22. Maldonado Fernández N, Linares- Palomino JP, López-Espada C, Martinez- Gámez FJ, Ros-Díe E. Clinical results of a new strategy (modified CHIVA) for surgical treatment of anterior accessory great saphenous varicose veins. Cir Esp. 2016;94:144-150.
23. Recke AL, Frendel A, Kahle B. Operative and postoperative risks of combined interventions with percutaneous phlebectomie and foam sclerotherapy. A retrospective analysis using the example of isolated anterior accessory vein insufficiency [article in German]. Phlebologie. 2017;46:75-80.
24. Fischer R, Chandler JG, De Maeseneer MG, et al. The unresolved problem of recurrent saphenofemoral reflux. J Am Coll Surg. 2002;195:80-94.
25. Garner J, Heppell P, Leopold P. The lateral accessory saphenous vein – a common cause of recurrent varicose veins. Ann R Coll Surg Engl. 2003;85:389-392.
26. Proebstle TM, Möhler T. A longitudinal single-center cohort study on the prevalence and risk of accessory saphenous vein reflux after radiofrequency segmental thermal ablation of great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015;3:265-269.
27. Winokur RS, Khilnani NM, Min RJ. Recurrence patterns after endovenous laser treatment of saphenous vein reflux. Phlebology. 2016;31:496-500.
28. Anwar MA, Idrees M, Aswini M, Theivacumar NS. Fate of the tributaries of saphenofemoral junction following endovenous thermal ablation of incompetent axial vein – a review article. Phlebology. 2019;34:151-155.
