What is the best method of imaging in iliofemoral venous obstruction?
MB, BCh, BAO, FRCR
Ireland
Abstract
Iliofemoral venous obstruction is increasingly recognized as a major cause of post-thrombotic syndrome. Patients can be left with significant symptoms after just one episode of iliofemoral deep-vein thrombosis; ranging from milder problems, such as varicose veins, to itching, leg swelling, and even venous ulceration. With the advent of endovascular techniques to reconstruct the iliofemoral segment has come an understanding that accurate recognition and diagnosis form a central part of the puzzle. Clinical evaluation is limited, and imaging has assumed a central role. This article looks at the optimal method for imaging the iliofemoral venous segment.
Introduction
Iliofemoral venous obstruction (IF-VO) has emerged as one of the principal causes of lower extremity post-thrombotic syndrome (PTS), following iliofemoral deep-vein thrombosis (IF-DVT).1
A landmark study by Tom O’Donnell and Norman Browse in 1977 detailed the socioeconomic effects after IF-DVT; it demonstrated that 50% of men were unable to hold down a job just 5 years after IF-DVT, and after 10 years the majority had venous ulcers.2 Adherence to anticoagulation treatment, the clinical efficacy of the newer anticoagulants, and the use of compression stockings (the latter have come under scrutiny after the large SOX trial [Compression Stockings to Prevent Post-Thrombotic Syndrome]3) have all somewhat improved this dismal outcome; nonetheless, the morbidity from this condition is severe. The majority of post IFDVT patients who go on to develop venous ulceration typically pass through the following stages (although the order may vary): venous claudication, weight gain, development of varicose veins, skin changes, and eventually venous ulceration. The first two problems are not captured at all by current clinical methods for evaluation of venous problems.4,5 It is fairly obvious that one of the reasons patients continue to do so poorly clinically is that we are identifying patients much too late in their clinical trajectory–when they already have established PTS as opposed to earlier in its course. There is a mindset among physicians that all DVTs result in similar outcomes for patients, and the ATTRACT trial (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis) did not help to alter this perception.6,7
Over the last quarter of a century, the advent of endovascular stenting has meant that there are now treatment options that are less invasive than an open surgical procedure to bypass the affected area.8-11 Therefore, there is increasing interest in identifying the best imaging options to identify this critical condition at an earlier stage in their disease trajectory.12-16 Table I shows advantages and disadvantages of various imaging modalities.
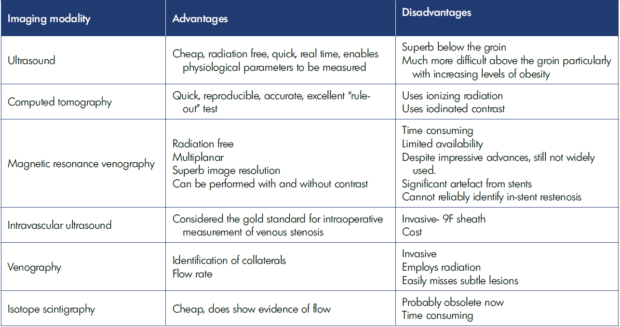
Table I. Advantages and disadvantages of imaging modalities for evaluating venous segments in post-thrombotic syndrome.
Ultrasound
Color Doppler ultrasound (CDUS) by skilled operators is an excellent method (Figure 1). Although identification of occluded iliofemoral venous segments on its own may be difficult, CDUS is certainly excellent in identifying physiological flow issues below it, in terms of lack of respiratory variability and lack of response to augmentation.17-19
With IF-VO, the external iliac or common femoral vein Doppler waveform is typically flat and shows minimal to no change during respiration, deep Valsalva or a Müller maneuver (Figure 2).20
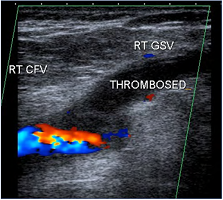
Figure 1. Longitudinal ultrasound image demonstrating color flow in the main section of the right common femoral vein (RT CFV) and thrombosis extending from the right greater saphenous vein (RT GSV) just into the CFV.
Often, the most useful aspect of ultrasound is the ability to compare one side with the other. In a patient with an occluded left iliofemoral venous segment, the right side will typically have grown to accommodate the extra flow, and then by comparison, the left will clearly be abnormally small. Obviously, this becomes more important in subtle lesions, where the discrepancy from side to side may well be less obvious. In slim patients and in skilled hands, it is the method of choice and should be used in every patient as an initial screening tool. If it is clearly normal, then the patient may not require any further investigation. If abnormal, the patient may require further investigation depending on symptoms, etc. Ultrasound is operator dependent, and if not personally performed by the endovascular specialist, the sonographer needs a special understanding of precisely what the specialist requires (inflow, outflow, potential access sites, etc). This information may not typically be obtained during a standard lower limb venous ultrasound, which for obvious reasons, tends to concentrate on reflux more than obstruction. Comparison between sides (right groin vs left groin) and identification of the profunda femoris vein (Figure 3) are both often neglected unless specifically requested.
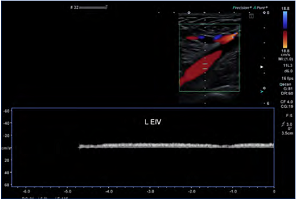
Figure 2. Although there is normal color flow in the left external iliac vein (L EIV), the Doppler signal suggests otherwise–note the flat tracing with minimal to no respiratory variability. There is a more central inferior vena cava (IVC) and iliac venous obstruction. Ultrasound demonstrates physiological changes as well as simple anatomical abnormalities.
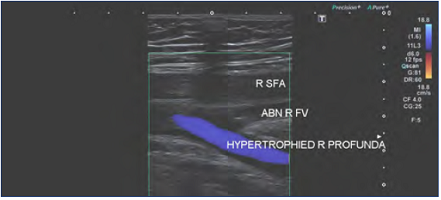
Figure 3. Previous right iliofemoral deep-vein thrombosis with extension into the right femoral vein. Note the compensatory hypertrophy of the profunda femoris vein. Abbreviations: ABN R FV, abnormal right femoral vein; R SFA, right superficial femoral artery; hypertrophied R profunda, hypertrophied (enlarged) right profunda femoris vein.
Ultrasound is the imaging modality of choice in follow-up of previously stented areas because the stent segment can usually be identified with certainty (unlike in unstented iliac veins) and, obviously, it is radiation free. The evaluation of instent restenosis is also possible (Figure 4) with this method, and it is the most practical for performing serial follow up on patients.21 Computed tomographic (CT) venography (discussed below), which provides excellent data, might be considered–but not repeatedly–because of its high radiation dose. Magnetic resonance (MR) venography (discussed below) achieves variable results depending on the composition of the stented segment; ie, those stents with less stainless steel give rise to less artefact, and thus MR venography may be somewhat useful, whereas with those stents containing more stainless steel, the signal drop out means that establishing stent patency is not possible. In-stent restenosis cannot currently be quantified on MR venography, whereas it is possible on CT venography and CDUS.
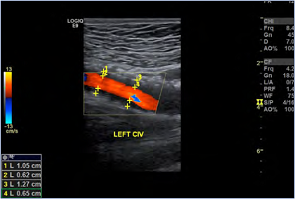
Figure 4. The degree of in-stent restenosis after iliac venous stenting can be quantified: (1.05-0.62)/1.05=40.9% stenosis. This patient was symptomatic with recurrent venous claudication at this time. Abbreviations: CIV, common iliac vein.
CT venography
CT venography is probably the most widely used imaging modality for investigation of iliofemoral and inferior vena cava pathology worldwide. It is quick, simple, reproducible, and most radiologists are comfortable interpreting it. CT venography may be divided into “indirect CT venography” and “direct CT venography.”22-25
Indirect CT venography is the more commonly used modality; the aim is to achieve systemic levels of opacification. This is performed by injection through a peripherally sited IV cannula (arm, usually) at a rate of 3 cc to 5 cc/sec, typically with 100 to 150 cc of iodinated contrast at a delay of 60 to 120 seconds depending on cardiac output and the rate of injection.
Indirect CT venography is excellent for evaluation of acute deep-vein thrombosis to “rule- out” swollen legs (if normal, the deep venous system is unlikely to be at fault; this is especially so in bilateral lower-extremity swelling), in malignancy,26 and during stent follow-up in certain patients in whom CDUS is difficult (scarring/obesity, etc). However, if patients’ symptoms are unremitting or otherwise inexplicable with a “normal” indirect CT venography, we have learned to favor either direct CT venography or intravascular ultrasound (IVUS) (Figures 5-7).
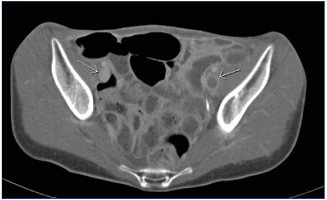
Figure 5. Indirect computed tomographic (CT) venography. Acute left iliofemoral deep-vein thrombosis; note the difference in attenuation between the two sets of external iliac veins.
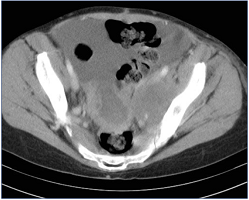
Figure 6. Indirect computed tomographic (CT) venography. Large centrally necrotic midline pelvic mass with nearly confluent left external iliac vein lymph node mass: compare external iliac veins—right normal; left stretched over lymph node.
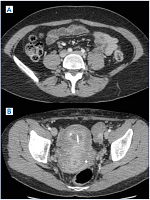
Figure 7. A) Indirect computed tomographic (CT) venography. Left iliac system appears nearly the same as the right. B) Unremitting symptoms prompted us to perform a direct CT venography.
Direct CT venography is performed by injection of contrast into the affected limb, typically into the foot (Figures 8-9). The purpose of direct CT venography is to achieve a high level of contrast concentration in the venous system of the affected extremity; essentially, a “venous angiogram” can be obtained and therefore multiplanar reformats (MPRs) and maximum intensity projection (MIPs) images can be performed on the data set required.
Direct CT venography was first shown to us by the experts from Grenoble, France; it was a “Road to Damascus” moment; we immediately realized this would be an extremely useful technique in the investigation of patients with post-thrombotic venous obstruction prior to venous reconstruction.27 In fact, it has proven to be exactly this; namely, it identifies which of the veins leading into the common femoral venous “sump” carries the most blood, what we call the “dominant inflow”; we believe this is critical for planning ahead of endovascular reconstruction, in that subsection of post IF-DVT patients whose scarring extends down to the common femoral vein or below.28
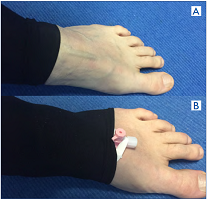
Figure 8. How to perform a direct computed tomographic (CT) venography. A) Roll up the Class 2 compression stocking to expose a vein. B) Place 20G IV cannula with injection port into vein; tape it down; roll stocking back over the IV cannula. It is now ready for injection.
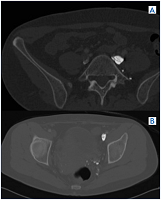
Figure 9. A and B) Note synechiae/scars inside the vein lumen in same patient from Figures 7 A and B, which was read as “normal.”
We reserve direct CT venography for those patients whom we consider potential candidates for endovascular reconstruction, ie, their symptoms need to merit it (significant venous claudication, severe weight gain related to same, venous ulceration).
Magnetic resonance venography
MR venography is now finally achieving the prominence it deserves in investigation of the iliofemoral venous segments.16,29-32 The techniques have improved considerably in the last decade and it has gone from being a cumbersome, slow, poorly performed, and difficult-to-reproduce technique to one that is now achieving mainstream acceptance and applicability. It offers huge advantages in terms of lack of radiation and lack of iodinated contrast. With increasing knowledge, it will, in my opinion, become the go-to method for investigation of IF-VO. In certain centers, it has already achieved that distinction, and the images acquired are excellent in terms of spatial resolution, identification of scarring, wall thickness, intraluminal synechiae, and so on.
The technique for performing MR venography is beyond the scope of this chapter. Interested readers are directed toward the references; it does require an interested and expert radiologist, as well as well-trained radiographic staff and top-of-the-line machines with the appropriate coils and software algorithms to produce diagnostic images. Many patients undergo MR imaging; as yet, unfortunately, relatively few patients have a good quality MR venogram (Figures 10, 11).
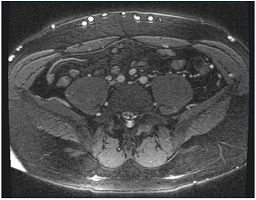
Figure 10. Magnetic resonance venography. Note multiple collaterals along the skin’s surface. The common iliac arteries are readily identified, but the veins are occluded and not seen.
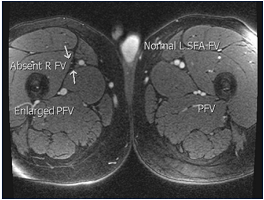
Figure 11. Magnetic resonance venography is the best noninvasive imaging modality; in many centers, it is already the dominant modality. It provides excellent anatomical detail and uses zero radiation. Sequences are becoming faster. Abbreviations: PFV, profunda femoris vein; R FV, right femoral vein; SFA FV, superficial femoral artery, femoral vein.
Intravascular ultrasound
IVUS is an invasive technique that provides superb imaging of the internal lumen and surrounds of the iliofemoral venous system. IVUS has been around for quite some time,33,34 and its use has been extensively documented in the coronary circulation and more recently in both the venous and arterial systems. In some ways it was a technique looking for a “home,” but it has most assuredly found that in the iliofemoral venous segment, as well as through much of the peripheral vasculature, particularly to guide therapy.35 It offers huge advantages in terms of identification of luminal problems that are not readily seen on venography, even multiplanar venography.36-38
The VIDIO trial (Venogram vs IVUS for Diagnosing Iliac vein Obstruction)38 has demonstrated quite eloquently that IVUS changes the decision algorithms in a significant percentage of patients, and it is far superior even to multiplanar venography in identifying subtle lesions. It is fair to say that in patients with marked obstruction–for instance, due to tumor compression–IVUS may not be required (Figure 12); nonetheless, it improves many aspects of endovascular reconstruction, including stent diameter, stent length, and accurate identification of landing zones; post treatment, it can confirm adequate wall apposition and expansion as well as measure the stent diameter and area (Figure 13). In the future, it will almost certainly provide physiological data in the same way that standard transabdominal ultrasound currently offers, demonstrating Doppler characteristics as well as color flow.
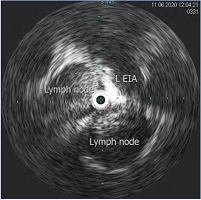
Figure 12. Intravascular ultrasound (IVUS) demonstrating compression of the left external iliac vein by surrounding lymph nodes. Abbreviation: L EIA, left external iliac artery.
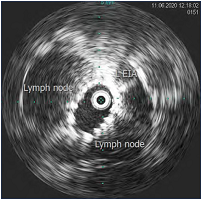
Figure 13. Same patient post stent placement; lymph nodes have been pushed aside and stent expansion is confirmed; area can be measured. Abbreviation: L EIA, left external iliac artery
Venography
Venography for many years was the gold standard in identification of iliofemoral venous pathology but has gradually been replaced by cross sectional methods and now by IVUS. It is still very useful for identifying flow rate during a procedure, but more skill is required in identification of more subtle characteristics, in particular, subtle compression of the vein in an anteroposterior fashion, which can easily be missed even by the experienced observer unless a lateral projection is equally obtained.
For this reason, it is mainly reserved simply for identification of the correct path to follow during iliofemoral venous reconstruction, (Figure 14) and obliques are often essential for this. After stent placement, demonstration of rapid inline flow with abolition of collaterals is an excellent marker of success (Figure 15). Most experienced workers in this field do not perform catheter venography as a preoperative imaging investigation.
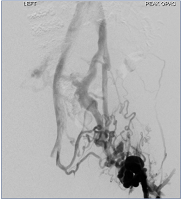
Figure 14. Initial venogram in left groin showing large collaterals and no in-line flow from left common femoral vein to inferior vena cava.
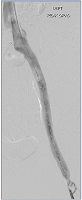
Figure 15. Completion image demonstrating perfect in-line flow from left common femoral vein (LCFV) to inferior vena cava (IVC); abolition of collaterals, rapid passage of contrast.
Conclusion
We are fortunate to have so many excellent methods of imaging of the iliofemoral venous segment. Currently, CT venography is probably the work horse, but in experienced centers, MR venography is already, rightfully, taking over this role owing to its many advantages.
Intraoperatively, a combination of IVUS and venography is the current ideal combination, whereas post operatively, follow-up of the patient with ultrasound appears the most prudent and sensible option.
REFERENCES
1. Comerota AJ, Gravett MH. Iliofemoral venous thrombosis. J Vasc Surgery. 2007;46(5):1065-1076.
2. O’Donnell TF Jr, Browse NL, Burnand KG, Thomas ML. The socioeconomic effects of an iliofemoral venous thrombosis. J Surg Res. 1977;22(5):483-488. doi:10.1016/0022-4804(77)90030-0.
3. Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent postthrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383(9920):880-888.
4. Prandoni P, Villata S, Rossi L, et al. The clinical course of deep-vein thrombosis. Prospective long term follow up of 528 symptomatic patients. Haematologica. 1997;82:423-428.
5. Soosainathan A, Moore HM, Gohel MS, Davies AH, et al. Scoring systems for the post-thrombotic syndrome. J Vasc Surg. 2013;57:254-261.
6. Stone J, Hangge P, Albadawi H, et al Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284. doi:10.21037/ cdt.2017.09.01.
7. O’Sullivan GJ, de Graaf R, Black SA. Just how attractive is the ATTRACT trial? Cardiovasc Intervent Radiol. 2018;41(9):1313-1317. doi:10.1007/ s00270-018-2016.
8. O’Sullivan GJ. Endovascular management of chronic iliac vein occlusion. Tech Vasc Interv Radiol. 2000;3:45-53.
9. Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57:1163-1169.
10. Lou WS, Gu JP, He X, et al. Endovascular treatment for iliac vein compression syndrome: a comparison between the presence and absence of secondary thrombosis. Korean J Radiol. 2009;10(2):135-143. doi:10.3348/ kjr.2009.10.2.135.
11. O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11(7):823-836. doi:10.1016/ s1051-0443(07)61796-5.
12. Brinegar KN, Sheth RA, Khademhosseini A, Bautista J, Oklu R. Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J Radiol. 2015;7(11):375-381.
13. Butros SR, Liu R, Oliveira GR, Ganguli S, Kalva S. Venous compression syndromes: clinical features, imaging findings and management. Br J Radiol. 2013;86(1030):20130284. doi:10.1259/ bjr.20130284.
14. Zucker EJ, Ganguli S, Ghoshhajra BB, Gupta R, Prabhakar AM. Imaging of venous compression syndromes. Cardiovasc Diagn Ther. 2016;6(6):519- 532. doi:10.21037/cdt.2016.11.19.
15. Gozzo C, Giambelluca D, Cannella R, et al. CT imaging findings of abdominopelvic vascular compression syndromes: what the radiologist needs to know. Insights Imaging. 2020;11(1):48. doi:10.1186/s13244-020-00852-z.
16. Baekgaard N, Fanelli F, O’Sullivan GJ, et al. New Horizons in Deep Venous Disease Management. Torino, Italy: Edizioni Minerva Medica; 2017.
17. Needleman L, Cronan JJ, Lilly MP, et al. Ultrasound for lower extremity deep venous thrombosis. Multidisciplinary recommendations from the Society of Radiologists in Ultrasound Consensus Conference. Circulation. 2018;137:1505- 1515.
18. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations. Radiology. 1996;199:45- 47.
19. Zitek T, Baydoun J, Yepez S, Forred W, Slattery DE. Mistakes and pitfalls associated with two-point compression ultrasound for deep vein thrombosis. West J Emerg Med. 2016;17:201-208.
20. Lin EP, Bhatt S, Rubens D, Dogra VS. The importance of monophasic Doppler waveforms in the common femoral vein: a retrospective study. J Ultrasound Med. 2007;26:885-891.
21. Raju S, Tackett P Jr, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg. 2009;49(2):511-518.
22. Wan-Yin S, Li-Wei W, Shao-Juan W, Xin-Dao Y, Jian-Ping G. Combined direct and indirect CT venography (combined CTV) in detecting lower extremity deep vein thrombosis. Medicine. 2016;95(11):e3010. doi:10.1097/ MD.0000000000003010.
23. Kulkarni NM, Sahani DV, Desai GS, et al. Indirect computed tomography venography of the lower extremities using single-source dual-energy computed tomography: advantage of low-kiloelectron volt monochromatic images. J Vasc Interv Radiol. 2012;23:879-886.
24. Ghaye B, Szapiro D, Willems V, et al. Pitfalls in CT venography of lower limbs and abdominal veins. Am J Roentgenol. 2002;178:1465-147.
25. Fujikawa A, Matsuoka S, Kuramochi K, et al. Vascular enhancement and image quality of CT venography: comparison of standard and low kilovoltage settings. AJR Am J Roentgenol. 2011;197(4):838- 843.
26. O’Sullivan GJ, Waldron D, Mannion E, Keane M, Donnellan PP. Thrombolysis and iliofemoral vein stent placement in cancer patients with lower extremity swelling attributed to lymphedema. J Vasc Interv Radiol. 2015;26(1):39-45. doi:10.1016/j.jvir.2014.10.010.
27. Menez C, Rodiere M, Ghelfi J, et al. Endovascular treatment of postthrombotic venous ilio-femoral occlusions: prognostic value of venous lesions caudal to the common femoral vein. Cardiovasc Intervent Radiol. 2019;42:1117-1127.
28. Coelho A, O’Sullivan G. Usefulness of direct computed tomography venography in predicting inflow for venous reconstruction in chronic postthrombotic syndrome. Cardiovasc Intervent Radiol. 2019;42(5):677-684.
29. van Langevelde K, Tan M, Srámek A, et al. Magnetic resonance imaging and computed tomography developments in imaging of venous thromboembolism. J Magn Reson Imaging. 2010;32:1302- 1312.
30. Tamura K, Nakahara H. MR Venography for the assessment of deep vein thrombosis in lower extremities with varicose veins. Ann Vasc Dis. 2014;7(4):399-403. doi:10.3400/avd. oa.14-00068.
31. Spritzer CE. Progress in MR imaging of the venous system. Perspect Vasc Surg Endovasc Ther. 2009;21:105-116.
32. Kearon C, Julian JA, Newman TE, et al. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med. 1998;128:663-677.
33. Neglen P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694-700.
34. Forauer AR, Joseph J. Gemmete JJ, Dasika NL, Cho KJ, Williams DM. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13(5):523-527.
35. Makris GC, Chrysafi P, Little M, et al. The role of intravascular ultrasound in lower limb revascularization in patients with peripheral arterial disease. Int Angiol. 2017;36(6):505-516. doi:10.23736/ S0392-9590.17.03866-4.
36. Montminy ML, Thomasson JD, Tanaka GJ, Lamanilao LM, Crim W, Raju S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord. 2019;7:801-807.
37. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6:48- 56.e1.
38. Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular Ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord. 2017;5:678-687

