Where do lymph and tissue fluid flow during intermittent pneumatic massage of lower limbs with obstructive lymphedema?
Waldemar L. OLSZEWSKI1,4, Jaroslaw B. CWIKLA2, Marzanna ZALESKA1, Anna DOMASZEWSKA-SZOSTEK1, Tomasz GRADALSKI3, Sylwia SZOPINSKA1
SUMMARY
In obstructive lymphedema of the lower limbs, lymphatic collectors are obliterated and lymph and tissue fluid accumulate in the spontaneously formed tissue spaces. Thus, the transport of stagnant fluid should be supported by external mechanical forces. Sequential intermittent pneumatic massage serves this purpose. The effectiveness of pneumatic massage is assessed by change in limb volume. The open questions remain where does the fluid flow to, where does it accumulate, and can it pass to the nonswollen tissues of hypogastrium and gluteal regions?
We investigated during pneumatic massage of the limb the pathways of lymph and mobile tissue fluid flow: a) from the calf and thigh across the inguinal and gluteal regions to the healthy non-swollen tissues of hypogastrium, and b) in the hypogastrium to the lateral and upper abdominal quadrants, with use of lymphoscintigraphy. To prove that there was effective fluid flow during pneumatic massage, plethysmographic flow measurements were carried out. We showed that: (i) pneumatic compression propelled isotope in lymph in the remaining patent lymphatics and tissue fluid in the interstitial space toward the inguinal region and femoral channel, (ii) no isotope crossing the inguinal crease or running to the gluteal area could be detected, (iii) no isotope flow to the hypogastrium was observed in the lymphatics or in tissue fluid, and (iv) isotope injected intradermally in the hypogastrium did not spread during massage to the upper and contralateral abdominal quadrants.
In conclusion, intermittent pneumatic compression is effective in propelling mobile tissue fluid and translocating large fluid volumes toward the groin. However, there remains the question of how to facilitate further flow toward the non-swollen tissues and local absorption of fluid.
INTRODUCTION
In obstructive lymphedema of the lower limbs, most or all lymphatic collectors leading to the superficial and deep inguinal lymph nodes are obstructed following infections, trauma, surgery, or irradiation.1 The limited hydraulic function of the damaged collecting lymphatics results in accumulation in the interstitial space of capillary filtrate containing plasma humoral and cellular components, as well as parenchymal cell products. Impairment of flow away of plasma-filtered macromolecules and protein-bound ions generates high osmotic pressure. This attracts water from the vascular compartment and further increases the stagnant tissue fluid volume. Furthermore, the accumulating fluid deforms the soft tissue structure and spontaneously creates fluid conducting channels in the subcutaneous tissue.2
This natural hydraulic process still remains insufficient in transporting fluid away from swollen regions and should be supported by external mechanical forces. Such forces can be applied to the swollen limb by massage to squeeze the mobile edema fluid toward the root of the extremity.
In the calf and thigh the spontaneous fluid channels form along large blood vessels, as the saphenous, popliteal, and femoral veins, and also around small unnamed vessels, leading to the groin region. There they end up at the inguinal crease where skin is connected with the inguinal ligament and external oblique muscle by natural elastic fibers.3
The question arises whether the accumulated tissue fluid can form spontaneous subcutaneous channels across the inguinal crease to the hypogastrium. This would facilitate absorption of fluid in normal hypogastrium tissues, presumably forming connections with normal lymphatics. Such newly formed flow pathways would justify the common practice of treating the core (truncal) lymphatics as a major therapy component before limb massage.4,5 The essence of this concept is that treatment must first be directed at lymphatic territories, such as the hypogastrium and trunk, so that they are adequately prepared to receive lymph (tissue fluid) from swollen regions.
In this study we investigated with use of lymphoscintigraphy the pathways of lymph and mobiletissue fluid flow: a) across the inguinal and gluteal regions to the healthy non-swollen tissues of hypogastrium, and b) in the hypogastrium to the lateral and upper abdominal quadrants, during pneumatic massage of the limb. To prove that there was effective fluid flow during pneumatic massage, plethysmographic flow measurements were carried out.
MATERIAL AND METHODS
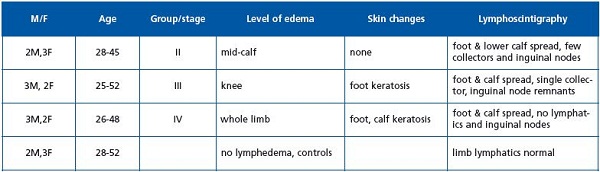
The study was carried out in 15 patients, aged 28-56 years, mean weight 65 kg (58-72), mean height 168 cm (161-178), with a diagnosis of lymphedema of one lower limb, stage II to IV, duration of 2 to 15 years (Table 1). Eleven patients reported small foot skin abrasions or light trauma of foot in the past followed by development of foot and calf transient edema. More severe edema developed months to years later and in 50% of cases was complicated by 1 to 3 attacks of dermatolymphangio – adenitis. In 4 patients edema developed for no detectable reason. Patients with acute inflammation, chronic venous insufficiency, and systemic etiology of edema were excluded from the study. Five patients without lymphedema with suspected enlarged abdominal lymph nodes served as controls. Lymphoscintigraphy and tissue fluid flow measurements are, in our hospital, the mandatory diagnostic procedures in all cases of lymphedema.
The consent of patients was obtained and the study was approved by the Warsaw Medical University Ethics Committee.
Staging was based on clinical evaluation: level of edema embracing limb from foot to groin and degree of skin keratosis and fibrosis. Briefly, in stage II pitting edema affected the foot and the lower half of the calf, in stage III the foot and calf were involved, with hard foot and ankle area skin, in stage IV the whole limb was edematous with foot and calf skin hyperkeratosis and papillomatosis of the toes.1
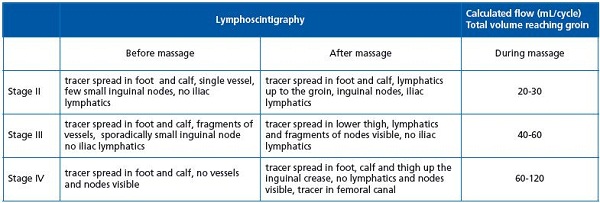
Lymphatic pathways were evaluated on lymphoscintigraphic images (Table 1), which revealed in stage II spread of tracer in the foot and lower calf, and an interrupted outline of a single lymphatic and a few smallinguinal nodes with irregular outline. In stage III no draining lymphatics were seen, with some inguinal nodes of irregular outline appearing 2 hours after isotope injection. Stage IV was characterized by spread of tracer in the foot and the entire calf without visualization of collecting lymphatics and nodes.
Lower limb lymphoscintigraphy was carried out in each patient in two sessions, the first without pneumatic massage and the second days later following a 45-minute limb pneumatic massage. An intradermal injection of 99mTc-Nanocoll (3 mCi) (Amersham, Switzerland) was given between the first, second, and third toes (to visualize the superficial lymphatic system) and in the subcutis of the mid-portion of the sole (to visualize the deep system). Imaging was performed using a gamma camera (Orbiter ZLC 750, Siemens, Germany) immediately after isotope injection and after 45 minutes of pneumatic massage. The images were classified according to the stage of lymphedema. In 5 of these patients (3 stage II, and 2 stage IV), lymphoscintigraphy of the skin and subcutis of hypogastrium was additionally performed by intradermal injection of 1/10th of the Nanocoll dose used for limb scintigraphy. The spread of isotope in the limb and its movement toward the groin were observed simultaneously with spread of isotope injected into the hypogastrium.
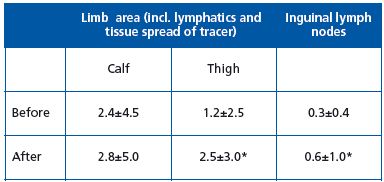
For semiquantitative evaluation of scintigrams, the images of the lower leg and thigh lymphatics and lymph nodes were evaluated quantitatively. Lymphoscintigrams were scanned and analyzed using specialized PC software (Olympus Micro ImageTM ver. 3.0.0., Olympus Optical Co., Hamburg, Germany). The surface area of the lymphatics (Lv) and inguinal lymph nodes (LN) of both extremities was evaluated in the inguinal area, thigh, and calf. Data were expressed as indices obtained from the equations ILv or LN=SL LV or LN/SCLv or LN, where SLv or LN was the surface area of lymph vessels or lymph nodes measured for the lymphedema (L) and contralateral normal (C) extremity.
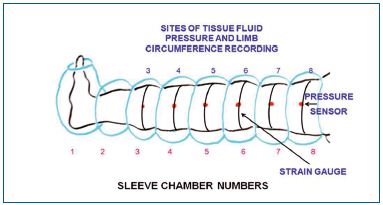
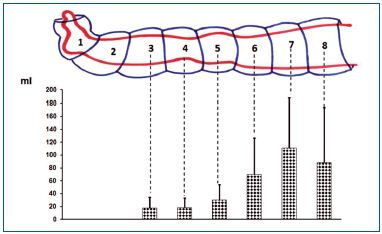
We used a device produced for us by Biocompression (Moonachie, NJ) (Figure 1). The sleeve was composed of 8 segments each 9 cm long and was sequentially inflated at inflation pressures from 50 to 125 mm Hg. Gradient pressures were decreased proximally by 20%, inflation time of each chamber was 50s, total inflation time equaled 400s, there was no deflation of distal chambers, deflation time of all chambers was 50s at the end of each cycle. The sleeve embraced the whole limb to the inguinal crease.
Manual massage for hypogastrium fluid clearance was done for 10 minutes at the site of isotope injection in the upper and lateral direction.
Strain gauge plethysmography was used to measure sequential changes of circumference in the calf and thigh during massage (Figure 1). The data obtained were used to calculate volume changes of the massaged limb segments. A plethysmograph (Hokanson, Bellevue, WA, type EC6) in a recording vein mode was applied. Six mercury strain gauges of a length of 22 cm to 53 cm were put around limb at chamber levels 3 to 8 (Figure 1). Increase in circumference caused elongation of the gauge which was read off on the recorder graph scale in mm. The numerical data obtained were used to calculate volume by multiplying the cross area of limb segments by 90 mm (length of the compressing chamber). Subtracting the volume before compression from that during compression provided data on the proximally transferred volume.
For comparison of numerical data on lymphoscintigram densitometry, Student’s t-test was applied with significance at P<0.05.
RESULTS
Lymphoscintigraphic evaluation of lymph and tissue fluid flow in the massaged limb
Lymphoscintigraphic and volumetric evaluation of lymph and tissue fluid flow is presented in Tables 2 and 3.
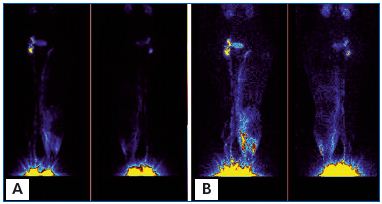
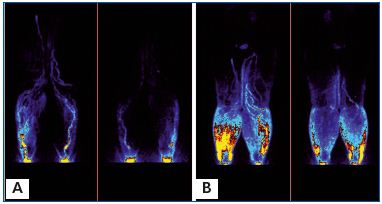
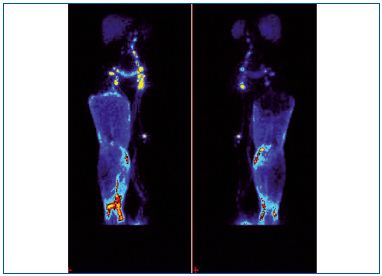
The pathways of lymph and tissue fluid flow during pneumatic massage are shown in Figures 2-7 and the evaluation is presented in Table 2. After massage in stage II (Figure 2) and some cases in stage III (Figures 3, 4), the tracer filled the upper parts of the thigh tissues. It flowed along the lymphatics to the femoral canal and retroperitoneal space. In stage IV, it reached the inguinal crease and accumulated in the upper thigh (Figures 5, 6, 7). No pictures of isotope flow from the thigh across the inguinal crease to the lower abdominal quadrant were observed. In normal limbs, isotope flowed along the superficial and deep lymphatic system to the inguinal nodes and through the femoral canal to iliac lymph nodes.
Semiquantitative evaluation of lymphoscintigrams before and after intermittent compression.
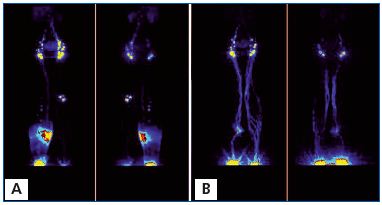
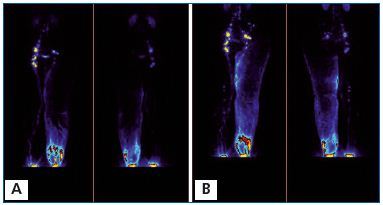
In the whole group of patients there was an increase in the spread of tracer to the thigh and inguinal region including the inguinal lymph nodes (Table 3). There were wide individual variations.
Lymphoscintigraphic evaluation of lymph flow from the hypogastrium during limb massage
There was minimal radial spread of the isotope from the site of injection with no signs of its movement toward the upper or contralateral quadrants (Figure 7). In two patients flow was directed toward the inguinal nodes.
Tissue fluid volume transfer during pneumatic massage
Continuous recording of circumference changes during sequential compression gave indirect insight into the volumes of fluid translocated from the compressed segments to the proximal ones. The increase in circumference at each level was recalculated as increase in volume. Summarized data of 15 patients are presented in Figure 8. The tissue fluid flow ranged from 20-30 mL/cycle in the calf to 60-105 mL/cycle in the thigh.
Relationship between the isotope spread and tissue fluid flow
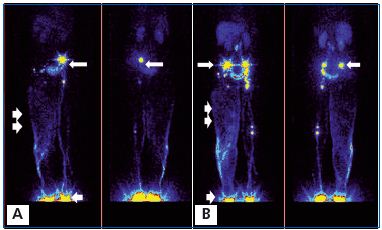
A. Before massage. Left normal side. 99Tc Nanocoll was injected into the toe web of the foot (arrow) and it visualized the superficial and deep lymphatics. To visualize lymph drainage from the hypogastrium, another dose of Nanocoll was injected intradermally in the left lower abdominal quadrant (arrow). Right lymphedema side. Nanocoll injected into right foot (with lymphedema) showed few calf lymphatics (two arrows).
B. After 1 hour of pneumatic sequential massage of the lymphedematous right limb, isotope spread in the subcutaneous tissue of the calf and part of the thigh, but did not cross the groin level despite high pressure sequential compression. Isotope did not move to the abdominal area. Moreover, isotope injected bilaterally into the hypogastrium spread slightly radially, but did not flow to the upper or lateral quadrant.
Pneumatic compression caused flow of tracer toward the groin in both the lymphatics and interstitial space (stages II and III). In stage IV, tracer spread in the interstitial space of the entire limb with a sharp border at the inguinal crease, not entering the femoral channel. The proximal flow of tracer was accompanied by tissue fluid flow (Table 2, Figure 8). Most tracer and massaged fluid accumulated in the thigh.
DISCUSSION
This study provided the following information: (i) pneumatic compression of the lower limb propelled isotope in lymph in the remnant lymphatics and tissue fluid in the interstitial space toward the inguinal region and femoral canal, (ii) no isotope filling fluid channels crossing the inguinal crease or running to the gluteal area was visualized, (iii) there was no isotope flow to the hypogastrium in either lymphatics or tissue fluid, and (iv) the isotope injected intradermally in the hypogastrium did not spread during massage to the upper and contralateral abdominal quadrants.
Our study showed that sequential pneumatic compression with high inflation pressure is very effective in propelling stagnant lymph and tissue fluid in cases of obstructive lymphedema. The lymphoscintigrams showed that tissue fluid finds its way toward the root of the extremity along the natural pathways with least hydraulic resistance. These are the perivascular spaces and are spontaneously formed by tissue deformation subcutaneous channels.2 Isotope accumulated along the great saphenous vein and internal aspect of the thigh. Even if there was some flow along femoral and iliac lymphatics, tissue fluid reaching the inguinal crease did not pass it. In advanced stage IV, tissue fluid moved to the knee or lower thigh level only.
During pneumatic compression the flow of tracer followed the tissue fluid flow. Large volumes of proximally moved fluid indicated that it was not only lymph in the subepidermal plexus and the remaining patent lymphatics, but also tissue fluid accumulating in the spontaneously formed tissue spaces, where the bulk of edema fluid is usually found.2 The qualitative comparison of lymphoscintigraphic pictures and flow data clearly showed that sequential compression propelled lymph and stagnant tissue fluid toward the groin. Most of the fluid accumulated in the groin, which was expected. However, in contrast to the general view, it did not move toward the hypogastrium, but rather to the femoral channel.
Our results again raise the question: What is the fate of fluid accumulating in the groin? Does it find its way to the pelvis through the femoral and obturator channels? How large a portion of fluid is absorbed in the upper thigh and genitals? It cannot be water only as tissue fluid protein concentration remains at a low level and at the same concentration as in the calf fluid (personal observations)
There are few publications on lymph and tissue fluid flow during pneumatic compression of swollen limbs.6,7 Based on the results of lymphatic vascular factorial analysis, a beneficial effect of intermittent pneumatic compression was detected in 18 of 22 limbs examined. It facilitated radiocolloid transport in the proximal portion of the limb and also propelled tracer from the injection site toward the lymphatics. The effect was evident as soon as external compression therapy began.6 In another study, pneumatic compression brought about decrease in the volume of the massaged limb, although no flow of tracer toward the groin was observed.7 The authors concluded that water was absorbed but that fluid proteins remained in the massaged regions. No data on applied pressure and inflation timing were presented that would allow analysis of tissue fluid flow. None of these papers addressed the problem of massaged fluid flow through the groin to the hypogastrium or pelvis through the femoral channel.
There is a widely accepted notion that emptying of the hypogastrium by manual or pneumatic massage prior to limb massage creates space for lymph and tissue fluid from the massaged tissues. The essence of this concept is that treatment must first be directed at lymphatic territories, such as the trunk, so that they are adequately prepared to receive lymph from subsequently treated lymphedematous regions such as the arm or leg. This truncal clearance or decongestion approach makes intuitive sense to most intensively trained practitioners.
According to Földi,4 lymphatic tributary regions or territories are separated by lymphatic watersheds. The term watershed is borrowed from hydrology, where it can be thought of as a drainage basin usually bounded by ridges of higher ground. Although lymphatic watersheds are not true anatomical structures, their dividing lines delineate the direction of lymph flow.5 Although treatment of the trunk has long been a standard manual lymph drainage process, there have been no anatomical studies confirming the presence of watersheds or randomized clinical trials comparing manual lymphatic drainage with and without truncal decongestion.
In our study we were not able to confirm lymph and tissue fluid flow to the hypogastrium and from there to the neighboring quadrants. The isotope-containing lymph and tissue fluid were stopped at the inguinal crease and flow was directed toward the femoral channel. Also isotope flow away from the hypogastric subcutaneous tissue was not observed. Manual massage of this region revealed radial spread of isotope, but not flow to the upper or lateral quadrant. We think the concept of hypogastrium clearance should be reevaluated based on objective physiological studies of tissue fluid hydraulics.
Taken together, sequential intermittent compression of lymphedematous lower limbs is effective in propelling lymph and tissue fluid toward the groin. It is directed toward the femoral canal, but not to the hypogastrium. These findings point to the need to apply high compression pressures at the groin region and also to search for pharmacological and surgical methods to facilitate fluid flow across the inguinal crease.

REFERENCES
1. Olszewski WL. Clinical picture of lymphedema. In: Olszewski WL, ed. Lymph Stasis: Pathophysiology, Diagnosis and Treatment. Boca Raton, Fla: CRC Press; 1991:347- 378.
2. Olszewski WL, Jain P., Ambujam G, Zaleska M, Cakala M. Where do lymph and tissue fluid accumulate in lymphedema of lower limbs caused by obliteration of lymphatic collectors. Lymphology. 2009;42:105-111.
3. Lechner G, Jantsch H, Waneck R, Kretschmer G. The relationship between the common femoral artery, the inguinal crease, and the inguinal ligament: a guide to accurate angiographic puncture. Cardiovasc Intervent Radiol. 1988;11:165-169.
4. Földi E, Földi M, Clodius L. The lymphedema chaos: a lancet. Ann Plast Surg. 1989;22:505-515.
5. Földi E, Földi M. Conservative treatment of lymphedema. In: Olszewski WL, ed. Lymph Stasis: Pathophysiology, Diagnosis and Treatment. Boca Raton, FL: CRC Press; 1991:469- 473.
6. Baulieu F, Baulieu JL, Vaillant L, Secchi V, Barsotti J.Factorial analysis in radionuclide lymphography: assessment of the effects of sequential pneumatic compression. Lymphology. 1989;22:178- 185.
7. Miranda F Jr, Perez MC, Castiglioni ML, et al. Effect of sequential intermittent pneumatic compression on both leg lymphedema volume and on lymph transport as semi-quantitatively evaluated by lymphoscintigraphy. Lymphology. 2001;34:135-141.
