Which venous patients need to be investigated with air plethysmography and why?
Erika MENDOZA4,5
Hospital, Middlesex, UK
2Department of Surgery and Cancer,
Imperial College, London, UK
3West London Vascular and Interventional
Centre, Northwick Park Hospital, Harrow,
UK
4Venenpraxis – Wunstorf, Speckenstrasse
10, 31515 Wunstorf, Germany
5General Secretary of the German Society
of Phlebology
Abstract
Air plethysmography (APG) is a small, lightweight device that measures change in calf volume in response to various maneuvers. Of these, elevation and dependency are the most important because they are gravitational maneuvers and, without gravity, venous insufficiency would be rare. The hypothesis underpinning the value of APG is that rapid calf expansion on dependency or a slow reduction in calf volume on leg elevation are a failure of our natural defense mechanisms against gravity. The APG parameters quantifying this are termed the venous filing index (VFI) and the venous drainage index (VDI), respectively. They are measured in mL/s for consistency and comparability and they serve to quantify the observations of Trendelenburg. The VFI and VDI may have relationships to ultrasound phenomena or imaging in individual veins, like reflux or iliac occlusion. However, APG assesses the calf as a global unit, providing the rate of volume change. In this way, it behaves as a pure test, uncorrupted by words like reflux, resistance, pressure, and wall tension, which are difficult to measure and are only part of any venous pathology. If the pathology is considered a chronic insufficiency of venous drainage of the leg, then APG is the noninvasive, objective test to quantify this whenever insufficiency is suspected.
In memory of Dr Evi Kalodiki (1956–2018) who dedicated her working life to APG research.
Introduction
Air plethysmography (APG®, ACI Medical LLC, San Marcos, CA, USA) testing has undergone many modifications in the last few years, including modernization and digitalization of the apparatus, introduction of new parameters, and changes in the interpretation of old parameters. In contrast to duplex ultrasound, the whole apparatus can fit into a paper takeaway lunch bag without requiring wheels for portability (Figure 1). Tracings are stored as file icons, which, when opened, can be displayed on a touch screen. Three taps with a pointer are all that is required for an onscreen display of the venous filling index (VFI) and the venous drainage index (VDI). Furthermore, the output is a volume versus time chart, whichrequires minimal training for interpretation. The ease and convenience of these new modifications has placed the test in an outpatient setting, which can be performed by allied health care professionals. The test and apparatus (Figure 2) can be compared (in time and simplicity) with measuring the ankle brachial pressure index (ABPI).

Figure 1. Air plethysmography apparatus.
The entire apparatus, excluding laptop, weighs 1.8 kg and fits
into a paper takeaway lunch bag.
Failings of clinical assessment
Varicose veins with reflux are a common finding in the general population1 and reflux may occur in legs without evidence of venous disease.2 Symptoms of leg pain, discomfort, heaviness, swelling, cramps, and pruritus are also common,3 and, after prolonged standing, most will develop some of these symptoms as well as edema,4 which may not be venous in origin. A relationship between lesion assessment and leg symptoms is easy to propose. However, the clinical dilemma is to determine whether there is a true relationship between the symptoms and signs of the patient or whether the reality is only a statistical association. Venous-like symptoms are also common in other diseases, which confounds clinical assessment.5 Leg symptoms are often unexplained6 and varicose vein symptoms may occur more often in people without varicose veins.7 Furthermore, there is lack of a strong relationship between symptoms and signs. Approximately 20% of patients with venous leg ulcerations have no visible varicose veins8 and an equal percentage are painless.9 Phlebologists will all have come across patients who have venous ulcers with minimal symptoms or quality of life (QOL) impairment. It is also common to see patients with extensive skin changes who present only because their partner has concerns regarding the appearance. The neuropathy of venous disease may play a role in this discord,10 which raises the hypothesis that, like the neuropathic disability of the diabetic foot, venouslike symptoms are a healthy phenomenon designed to offset venous disease progression by invoking remedial action through elevation and compression hosiery.
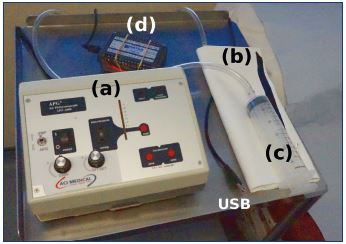
Figure 2. Components of an air plethysmography apparatus.
The APG apparatus comprises a pump (a), sensor calf cuff (b),
calibration syringe (c), and data unit (d), which connects to a
laptop via a USB cable. It has only 8 buttons, including the
on-off switch.
Unfortunately, current clinical assessments using research questionnaires, such as the Aberdeen varicose vein questionnaire (AVVQ)11 and the venous clinical severity score (VCSS),12 are not separated into the classic symptoms and signs. Though the AVVQ QOL questionnaire is promoted as a patient-reported outcome measure (PROM), the reality is that the score for each question has already been decided by the clinician to 3 decimal places, regardless of the value/relevance of that question to the individual patient.13 Perhaps PROM should be replaced with a clinician-scored outcome measure (CSOM). Furthermore, QOL assessment includes the contralateral leg as part of the final score. Disadvantages of the VCSS include the negative impact of treatment scoring with a compression stocking, lack of responsiveness, and statistical noise regarding ulceration in study populations who are classified as C2 to C5 according to the clinical, etiological, anatomical, and pathophysiological (CEAP) classification14 who do not have ulcers.15 The Villalta scale in the diagnosis and assessment of postthrombotic syndrome also has problems.16 While it separates symptoms and signs conveniently, albeit unevenly with 5 symptoms versus 6 signs, it has been shown to be nonspecific. With this scale, many patients with nonthrombotic venous disease have a score above the threshold defining postthrombotic syndrome even though they have never had a thrombosis event17 or deep venous reflux. Surprisingly, specific common symptoms, such as venous claudication18 and diagnostic signs, such as abdominal collaterals19 are not included in the Villalta scale.
The prime function of veins is drainage. Therefore, the strategy of recommending APG for all patients is that it provides an objective test to quantify the degree of functional impairment (insufficiency) for screening, diagnosis, treatment, and follow-up. In this way, it acts as a bridge rationalizing the relationship between symptoms and signs. Furthermore, it serves to complete the Venn diagram of treatment aims to specify the domains that need improving (Figure 3), which may be a step closer toward personalized outcomes.
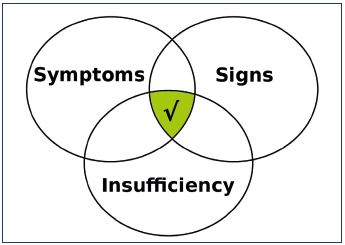
Figure 3. The Venn diagram of treatment aims that define the
domains requiring improvement.
The relative importance of incomes, such as symptoms, signs,
and functional insufficiency, should be established before their
relative importance in outcomes can be determined.
Most diseases rely on an objective assessment in addition to clinical symptoms and signs. Unfortunately, ultrasound parameters do not fill this need because they are unable to quantify venous insufficiency reliably20,21 and assessments are limited to individual veins and the experience and capability of the investigator. The high prevalence of venous reflux in the general population2 demands some form of quantification in order to assess its hemodynamic relevance. Similarly, venous obstruction is difficult to measure and not possible to quantify by ultrasound. Pelvic veins are hard to visualize and their position changes with different postures.22 Venous tone and muscle pump function, both very relevant to venous drainage, cannot be measured with ultrasound. These limitations pave the way for APG as a device with the potential to quantify all the main components of venous insufficiency.
Redefining existing parameters
The outflow fraction of venous occlusion plethysmography has been traditionally used as a way of assessing venous obstruction; it is the reduction in calf volume caused by venous discharge from a congested calf upon sudden deflation of an 80 mm Hg pneumatic thigh cuff in 1 second (Figure 4).23 This procedure is performed in the supine position and expressed as a percentage. Although the outflow fraction had promise in assessing outflow obstruction, it is not supported by evidence.24-26 Early data suggested that the outflow fraction may be an index of venous tone,27,28 and recent data have demonstrated that, if the tone of the calf is increased with a graduated elastic compression stocking, the outflow fraction increases from a median of 44% to 63%.29 Use of the outflow fraction as an obstruction parameter is not logical. On thigh cuff deflation, the discharged calf blood will fill the empty thigh veins caused by the cuff, which is irrespective of whether there is any pelvic venous obstruction. If the outflow fraction is impaired in patients following a deep vein thrombosis (DVT), the cause is usually an increase in vein stiffness with a reduction in the vein wall tone as a result of inflammatory scarring (Figure 5).
The ejection fraction parameter is valid, but it must be remembered that it is a relative parameter and that the classic test used to induce muscle pump ejection has changed. The ejection fraction is the reduction in the volume of the calf as a percentage of the working venous volume from a single tiptoe ankle flexion maneuver (Figure 4). Increases in calf venous volume and calf perforating vein incompetence are likely to reduce the ejection fraction because the same volume of ejected blood from the pumping chamber will go out of the calf or be discharged into superficial calf veins in the face of an increased working venous volume.30 Thus, the ejection fraction (EF) ratio decreases.
It is of interest that the tiptoe maneuver is suboptimal as a pumping test. It should be replaced with a body weight transfer maneuver from one leg to the other. Recent work has demonstrated that here was asignificant increase in the median (inter–quartile range) EF using this maneuves in healthy subjects versus the gold standard tiptoe maneuver (60[54-64] vs 43[31-53], P<0.0005).30 Furthermore, the body weight transfer maneuver does not require ankle joint movement (Figure 6). The volume shifts in a weight transfer have been appreciated by colleagues that practice saphenous sparing surgery using the Paranà test (isometric contractions response of the leg whilst standing caused by a gentle unexpected puh or pull by the investigator) as a way of inducing reflux.31 An explanation of the success of this test could be that the isometric calf muscle contraction of the surrounding soleal venous sinuses involved in balance is more significant than the spring–like contractions of the gastrocnemius muscle involved in tiptoe activities, such as running.
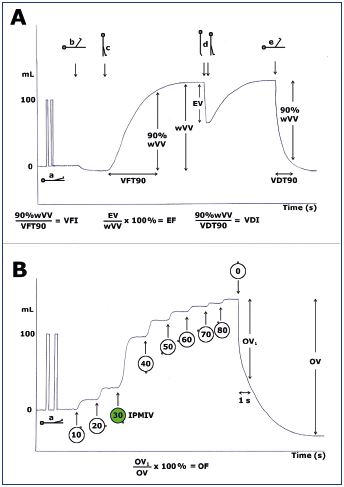
Figure 4. Schematic representation of the plethysmography
parameters.
Panel A. Standard plethysmography. Maneuvers (arrowed
events) that provoke changes in calf volume: calibration
position (a), 70 degrees drainage position (b), standing and
nonweight bearing (c), tiptoe on both legs up and down (d),
and back to the drainage position (e).
Panel B. Venous occlusion plethysmography. The thigh cuff is
inflated in 10 mm Hg increments up to 80 mm Hg and then
deflated suddenly.
Abbreviations: EF, ejection fraction; EV, ejection volume; IPMIV,
incremental pressure causing the maximum increase in calf
volume; OF, outflow fraction; OV, total outflow volume; OV1,
outflow volume in 1 second; VDI, venous drainage index; VDT,
venous drainage time; VFI, venous filling index; VFT, venous
filling time; wVV, working venous volume.
From reference 23: Lattimer CR et al. Eur J Vasc Endovasc Surg.
2016;52(1):105-112. © 2016, European Society for Vascular
Surgery.
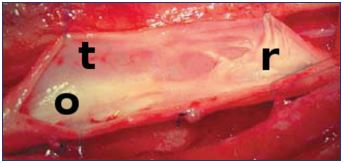
Figure 5. A femoral vein opened during surgery, demonstrating
postthrombotic fibrosis.
The valve is contracted causing reflux (r), there is an
obstruction to the flow (o), and the vein is lined with scar tissue
resulting in a reduction in tone (t).
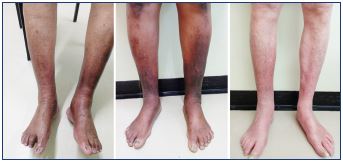
Figure 6. Three patients with discomfort, gaiter pigmentation,
some edema, and a little reflux on ultrasound.
Do any of these legs have a drainage insufficiency?
The final parameter to change meaning is the venous volume, which has been renamed as the working venous volume (Figure 4). It is the change in calf volume from a position of elevation to dependency (or vice versa). Using the original venous volume term is not accurate because the measured parameter is not the total venous volume of the calf. Patients with venous obstruction require high venous pressures for drainage, resulting in a leg that does not drain completely on elevation. It is only when the obstruction is released with a venous stent that this reserve volume is realized, and this is termed the drainage reserve volume in mL.32 The working venous volume + drainage reserve volume = venous volume. An elevated working venous volume in a nonobstructed patient quantifies venous pooling, especially when compared with the contralateral normal leg.
New parameters
The venous drainage index (VDI) is the main recent advance in APG (Figure 4). It is the mirror opposite of the venous filling index (VFI) and both are measured in mL/s for comparative evaluation.33 Trendelenburg first realized that gravitational maneuvers were important in venous assessment34; JC Allan first demonstrated these on a tilt table using APG35 and now the rate of filling and drainage can be quantified digitally.36 This parameter has been validated extensively for use in the quantification of venous obstruction.37 It is responsive to stenting38 and experimental obstruction32 using an inflatable thigh cuff set at predetermined inflation pressures. It could be argued that the VFI and VDI are the most important measurements in assessing venous insufficiency in contrast to ultrasound findings in individual veins. Furthermore, the measurements are global and just record the change in calf volume. These measurements may be more relevant than measurements of reflux or diameter. Dependency maneuvers reflect that venous disease is a failure of our defense mechanisms against gravity. They are independent of the patient’s ability to contract muscles or the investigators skill at compressing the calf.
The incremental pressure causing the maximal increase in calf volume (IPMIV) is the main response of the calf to a 10 mm Hg stepwise increase in thigh cuff inflation pressures.29 It is a venous occlusion plethysmography parameter that is related to venous tone (Figure 4). This parameter has also been used to test the threshold pressure at which a graduated elastic compression stocking fails to prevent calf expansion.29
Reference values
The normal reference values for dependent venous filling (VFI) and elevation drainage (VDI) depend on the size of the sensor cuff and calf, the range of postural change and the speed of this change. For example, the venous drainage time cannot be less than the time taken to elevate the leg. In consequence, there is a grey zone where subjects may have a borderline value. Small thin patients will have a reduced VFI in comparison to tall patients with a wide calf. Furthermore, all results should be interpreted in the context of the build of the patient. Comparing the results to the normal contralateral leg is very helpful as well as standardising the results by scaling the values against an adjusted vWW of 100mL. As an indicator for a normal sized Caucasian person the VFI should be <2.0 mL/s. Regarding elevation drainage, a VDI value >16 mL/s, a drainage time < 6 seconds with a straight-line appearance of the drainage line and an abrupt termination point would exclude significant central venous obstruction. A VDI <8 mL/s is indicative of venous occlusion. Unfortunately, clinical symptoms and signs may not help to determine cutoff values defining significant disease because of the lack of significant correlations between symptoms and signs as well as the lack of their correlation with haemodynamic values.
Superficial venous disease
Diagnosis
It is often not apparent from taking a history and examining a leg with duplex ultrasound that the patient has venous insufficiency; for example:
•The patient with nonspecific leg symptoms and C1 disease, and, other than the mild pitting edema present in all adults who stand for too long (occupational edema), may have very little reflux on ultrasound. Would treatment of the mild reflux improve the patient?
•In the UK, treatments for superficial venous disease are not permitted for cosmetic reasons alone. The objectives are not lesion-based, but symptom based with the presence of any reflux. Is this reflux significant?
•Would treatment of a mildly refluxing saphenous trunk be advised in a patient with lymphedema?
•Patients with gaiter pigmentation and again little apparent reflux on ultrasound (Figure 6).
In the four examples above, if the VFI was not elevated, this would discourage interventional treatment because the diagnosis is not likely to be venous.
Quantification
Patients with comorbidities, such as diabetes, arthritis, back pain, and cardiac impairment may also have venous disease. Quantification of the amount of insufficiency with the VFI may help determine its significance. Assessments with duplex ultrasound are qualitative and usually performed using a pumping maneuver of the calf. Significant reflux may be missed; for example, a refluxing anterior accessory saphenous vein or refluxing perforating vein. Not only does the VFI serve as a quality control check on the hemodynamic significance of the ultrasound findings, but it can give an answer to how much.
Outcomes
Currently, ultrasound outcomes do not represent incomes. For example, the pre-op diagnosis is based on reflux, the treatment is based on the presence of an occlusion, and the ideal end point should be restoration of competency. If the wrong vein is ablated inadvertently, then the ultrasound success of occlusion will still be present. Quantifying the insufficiency before and after with the VFI in mL/s has the advantage of reconciling incomes with outcomes. In this way, the phlebologist will have objective feedback on how well the leg has been treated.
Discord outcomes
Treatment success could be defined as a significant improvement in three domains: QOL, clinical severity, and reduction in the insufficiency. A full house where all three improve is not always the case. A discord is defined in the other 6 (out of 7) possibilities of the Venn diagram (Figure 7). The VFI is an essential component of a discord outcome analysis.39 Typical discords include an improvement in the VCSS and the VFI, but deterioration in the AVVQ. Another discord is an improvement in the AVVQ and VCSS, but deterioration in the VFI. A discord outcome analysis interrogates the process from diagnosis to follow-up with the aim of providing transparency in clinical trials,39 which may be a first step in improving appropriateness in interventional treatments.40
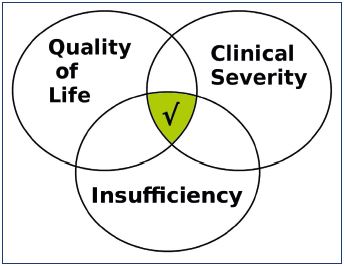
Figure 7. The discord outcome analysis comprises three
outcome domains illustrated as a Venn diagram.
A global success (central tick mark) occurs with a significant
improvement in all three domains. The other six possibilities
are discord outcomes.
From reference 39: Kalodiki E et al. Eur J Vasc Endovasc Surg.
2019;57(2):247-274. © 2018, European Society for Vascular
Surgery.
Deep venous disease
Screening test
On rapid leg elevation, the venous blood in the calf should discharge like a waterfall. The behavior should be likened to a falling column of fluid. This is a resistance free flow without a pressure–volume relationship and is apparent as a drainage line on the APG tracing (in contrast to the outflow curve seen with venous occlusion plethysmography). With increasing obstruction, this line changes to a curve with a decrease in the VDI and an increase in the drainage time (Figure 8).32 The VDI has become the gold-standard test in the assessment of obstruction. It is gravitational, fast, and it does not rely on the recoil of congested calf blood issuing into empty thigh veins caused by the occlusion cuff (as it does in venous occlusion plethysmography). The necessity of screening for deep venous disease is debatable; however, missing deep venous disease can result in great morbidity, including venous leg ulceration. Pelvic ultrasound is operator dependent and insufficient for diagnosis in most patients, contrast venography is not without radiation exposure and renal toxicity, and intravenous ultrasound is invasive. Outpatient APG may provide all the information and prevent the need for further investigations.
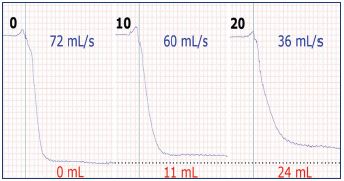
Figure 8. Volume versus time tracings demonstrating the effect
of increasing thigh cuff compression pressures (0 mm Hg to
10 mm Hg to 20 mm Hg) on obstructing elevation drainage.
The venous drainage index decreases (blue) and the
drainage reserve volume increases (red) with increasing
obstruction.
From reference 32: Lattimer CR et al. J Vasc Surg Venous
Lymphat Disord. 2017;5:88-95. © 2017, Society for Vascular
Surgery.
Significance of imaging
Imaging is anatomical and assesses individual veins. Perhaps the best imaging modality is the injection of contrast directly into both femoral thigh veins with reconstruction views. However, if collaterals are observed, then the debate arises as to whether they are sufficient to drain the leg or just a marker of severe disease (Figure 9). Furthermore, a >50% iliac vein compression occurs in 24% of the normal population40 and posture will influence the degree of collapse,36,42 as well as the size and shape of any suspected target for intervention. Functional testing with APG may help in the interpretation of images.
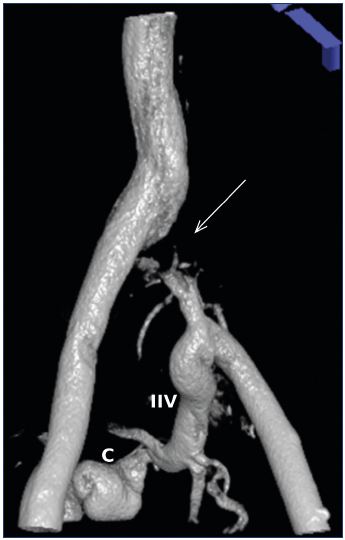
Figure 9. Surface-shaded CT venogram performed with direct
bilateral femoral vein contrast injection.
The left common iliac vein is occluded (arrow). Left limb
drainage is via the internal iliac vein (IIV) and collaterals (C)
and then into the right common iliac vein. Are these large
long-standing collaterals sufficient to drain the leg or are they
just an expression of the severity of the obstruction?
Inappropriate stenting
If the clinical symptoms and signs fit and a lesion is identified (usually >50% reduction in diameter43), then the patient may go on to have a venous stent, which is for life. Understandably, many patients do not improve clinically. The purpose of APG is to determine if there is a significant obstruction present in the first place and to determine if the stent has worked in its aim to improve the elevation drainage using the VDI. The interventionalist, who may never see the patient outside of the angio suite, needs to know how well the lesion has been treated and a VDI may help. Regarding a successful intervention, a discord outcome analysis may be used.39 The identification of hemodynamic improvement, but clinical deterioration or vice versa induces an interrogation about the management of that patient. It should help improve appropriateness and ethics in stenting patients for deep venous obstruction.44
Mixed venous disease
In patients with combined pelvic venous obstruction and superficial venous reflux, there is controversy as to which system to treat first. Stenting a significant obstruction reduces symptoms and venous pressure allowing the superficial system to recover. Alternatively, treating the reflux first is less invasive and technically easier. If the patient improves significantly, the obstruction may not have been as severe as originally proposed. In this case, the deep venous disease will not need treating.
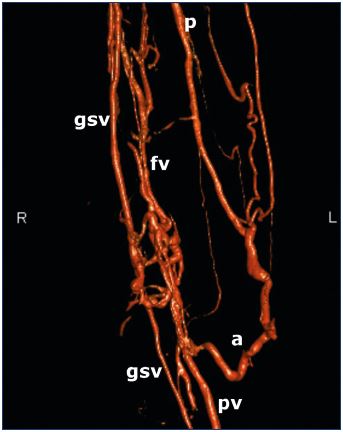
Figure 10. Lower thigh venogram reconstruction of a femoral
vein (fv) obstruction demonstrating poor recanalization and
perivenous collateralization.
The popliteal vein (pv) now drains into the profunda femoris
vein (p) from a collateral (a) (termed axialization). The great
saphenous vein (gsv) also drains the leg. It is dilated in its
upper part to accommodate the increase in drainage volume
from the popliteal vein (pv) or is it dilated from reflux? Would
great saphenous vein ablation help this patient?
A different situation is one of combined femoral vein obstruction and great saphenous vein reflux in patients with postthrombotic syndrome. The controversy here is whether the saphenous vein should be ablated, as it may be an important drainage route bypassing the femoral obstruction. If there is axial drainage of the popliteal vein via profunda vein collaterals (axial transformation), then improvement would be expected with great saphenous vein ablation. However, without APG, contrast venography is often required to establish the anatomy and predict the response to superficial ablation (Figure 10). In both situations, the VFI and the VDI are useful because they quantify filling and drainage and express them in the same units (mL/s) for comparative assessment. In the second case of femoral obstruction, the great saphenous vein can be occluded with a duplex probe to predict the hemodynamic effect of ablation (Figure 11).
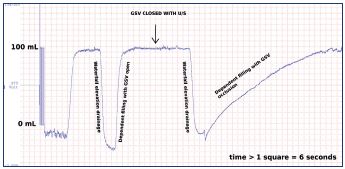
Figure 11. The digital calf volume versus time tracing of APG
on a tilt table.
Occlusion of the GSV with the ultrasound probe (arrow)
had no effect on the elevation drainage of this leg, but
it decreased the filling rate substantially. GSV ablation is
anticipated to have a beneficial effect in this patient.
Abbreviations: APG, air plethysmography; GSV, great
saphenous vein.
It has been proposed that varicose veins may be the result of iliac vein compression,45 in the same way any biological tube dilates proximal to an obstruction. The use of APG showed that this was not the case. Gravitational drainage on leg elevation was faster with a higher VDI compared with healthy controls without varicose veins.33 The explanation given was that larger draining veins contained more blood. This knowledge is useful for patients with combined pelvic obstruction and varicose veins because any real obstruction may be offset by improved elevation drainage from the varicose veins. A reduced VDI threshold in these patients would be required for a diagnosis of obstruction.
Summary
APG offers an objective assessment of all the main causes of venous insufficiency, with VFI and VDI being the most useful parameters. The test does not require radiation exposure, contrast, or vein puncture. In contrast to the questionnaires, which are research tools, APG is a clinical instrument providing useful information to rationalize the relationship between symptoms and signs, direct treatment, and quantify outcomes. Its ease of use, portability, and value places APG out of the vascular laboratory and into the outpatient setting. In conclusion, all patients need to be investigated with APG whenever venous insufficiency is suspected.

REFERENCES
1. Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53:149-153.
2. Evans CJ, Allan PL, Lee AJ, Bradbury AW, Ruckley CV, Fowkes FG. Prevalence of venous reflux in the general population on duplex scanning: the Edinburgh vein study. J Vasc Surg. 1998;28:767-776.
3. Bradbury A, Evans C, Allan P, Lee A, Ruckley CV, Fowkes FG. What are the symptoms of varicose veins? Edinburgh vein study cross sectional population survey. Br Med J. 1999;318:353-356.
4. Blattler W, Thomae HJ, Amsler F. Venous leg symptoms in healthy subjects assessed during prolonged standing. J Vasc Surg Venous Lymphat Disord. 2016;4:455-462.
5. Van der Velden SK, Shadid NH, Nelemans PJ, Sommer A. How specific are venous symptoms for diagnosis of chronic venous disease? Phlebology. 2014;29:580-586.
6. Amsler F, Rabe E, Blattler W. Leg symptoms of somatic, psychic, and unexplained origin in the populationbased Bonn vein study. Eur J Vasc Endovasc Surg. 2013;46:255-262.
7. Blaettler W, Amsler F, Mendoza E. The relative impact on leg symptoms of fears of getting varicose veins and of great saphenous vein reflux. Phlebology. 2013;28:347-352.
8. Obermayer A, Garzon K. Identifying the source of superficial reflux in venous leg ulcers using duplex ultrasound. J Vasc Surg. 2010;52:1255-1261.
9. Hareendran A, Bradbury A, Budd J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care. 2005;14:53-57.
10. Yim E, Vivas A, Maderal A, Kirsner RS. Neuropathy and ankle mobility abnormalities in patients with chronic venous disease. JAMA Dermatol. 2014;150:385-389.
11. Smith JJ, Garratt AM, Guest M, Greenhalgh RM, Davies AH. Evaluating and improving health-related quality of life in patients with varicose veins. J Vasc Surg. 1999;30:710-719.
12. Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307-1312.
13. Aber A, Poku E, Phillips P, et al. Systematic review of patient-reported outcome measures in patients with varicose veins. Br J Surg. 2017;104:1424-1432.
14. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248- 1252.
15. Lattimer CR, Kalodiki E, Azzam M, Geroulakos G. Responsiveness of individual questions from the venous clinical severity score and the Aberdeen varicose vein questionnaire. Phlebology. 2014;29:43-51.
16. Villalta S, Bagatella P, Piccioli A, Lensing A, Prins M, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome (abstract). Haemostasis. 1994;24:158a.
17. Trinh F, Paolini D, Fish J, Kasper G, Lurie F. Use of Villalta score for defining postthrombotic disease may lead to falsepositice diagnosis in 42% of patients with primary chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2018;6:291.
18. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118- 126.
19. Kurstjens RL, van Vuuren TM, de Wolf MA, de Graaf R, Arnoldussen CW, Wittens CH. Abdominal and pubic collateral veins as indicators of deep venous obstruction. J Vasc Surg Venous Lymphat Disord. 2016;4:426-433.
20. Raju S, Ward M Jr, Jones TL. Quantifying saphenous reflux. J Vasc Surg Venous Lymphat Disord. 2015;3:8-17.
21. Lattimer CR, Azzam M, Kalodiki E, Geroulakos G. Quantifying saphenous recirculation in patients with primary lower extremity venous reflux. J Vasc Surg Venous Lymphat Disord. 2016;4:179-186.
22. Vaccaro AR, Kepler CK, Rihn JA, et al. Anatomical relationships of the anterior blood vessels to the lower lumbar intervertebral discs: analysis based on magnetic resonance imaging of patients in the prone position. J Bone Joint Surg Am. 2012;94:1088-1094.
23. Lattimer CR, Kalodiki E, Azzam M, Geroulakos G. Haemodynamic performance of low strength below knee graduated elastic compression stockings in health, venous disease, and lymphoedema. Eur J Vasc Endovasc Surg. 2016;52(1):105-112.
24. Raju S, Kirk O, Davis M, Olivier J. Hemodynamics of “critical” venous stenosis and stent treatment. J Vasc Surg Venous Lymphat Disord. 2014;2:52-59.
25. Kurstjens RLM, de Wolf MAF, Alsadah SA, et al. The value of hemodynamic measurements by air plethysmography in diagnosing venous obstruction of the lower limb. J Vasc Surg Venous Lymphat Disord. 2016;4:313-319.
26. Kurstjens RL, Catarinella FS, Lam YL, de Wolf MA, Toonder IM, Wittens CH. The inability of venous occlusion air plethysmography to identify patients who will benefit from stenting of deep venous obstruction. Phlebology. 2018;33:483- 491.
27. Clarke H, Smith SR, Vasdekis SN, Hobbs JT, Nicolaides AN. Role of venous elasticity in the development of varicose veins. Br J Surg. 1989;76:577-580.
28. Geroulakos G, Nicolaides A. Venous tone evaluation by elastic modulus and therapeutic implications. Int Angiol. 1995;14:14-17.
29. Lattimer CR, Kalodiki E, Kafeza M, Azzam M, Geroulakos G. Quantifying the degree graduated elastic compression stockings enhance venous emptying. Eur J Vasc Endovasc Surg. 2014;47:75-80.
30. Lattimer CR, Franceschi C, Kalodiki E. Optimizing calf muscle pump function. Phlebology. 2018;33:353-360.
31. Franceschi C. Measures and interpretation of venous flow in stress tests. Manual compression and Parana manoeuver. Dynamic reflux index and Pstakis index [article in French]. J Mal Vasc. 1997;22:91-95.
32. Lattimer CR, Doucet S, Geroulakos G, Kalodiki E. Validation of the novel venous drainage index with stepwise increases in thigh compression pressure in the quantification of venous obstruction. J Vasc Surg Venous Lymphat Disord. 2017;5:88-95.
33. Lattimer CR, Kalodiki E, Mendoza E. Gravitational venous drainage is significantly faster in patients with varicose veins. Phlebology. 2016;31:546- 553.
34. Trendelenburg F. Ueber die unterbindung der vena saphena magna bei unterschenkelvaricen. Beitr Klinishen Chir. 1891;7:195-210.
35. Allan JC. Volume changes in the lower limb in response to postural alterations and muscular exercise. S Afr J Surg. 1964;2:75-90.
36. Lattimer CR, Mendoza E. Reappraisal of the utility of the tilt-table in the investigation of venous disease. Eur J Vasc Endovasc Surg. 2016;52:854-861.
37. Lattimer CR, Mendoza E, Kalodiki E. The current status of air-plethysmography in evaluating non-thrombotic iliac vein lesions. Phlebology. 2018;33:3-4.
38. Lattimer CR, Kalodiki E, Azzam M, Schnatterbeck P, Geroulakos G. Gravitational venous drainage improves significantly after iliac venous stenting but this may result in faster venous filling. J Vasc Surg Venous Lymphat Disord. 2016;4:137-138.
39. Kalodiki E, Azzam M, Schnatterbeck P, Geroulakos G, Lattimer CR. The discord outcome analysis (DOA) as a reporting standard at three months and five years in randomised varicose vein treatment trials. Eur J Vasc Endovasc Surg. 2019;57(2):247-274.
40. Lawrence PF. “Better” (sometimes) in vascular disease management. J Vasc Surg. 2016;63:260-269.
41. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39:937-943.
42. Kamm RD, Pedley TJ. Flow in collapsible tubes: a brief review. J Biomech Eng. 1989;111:177-179.
43. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6:48- 56 e1.
44. Passman MA. Where evidence, ethics, and professionalism converge. J Vasc Surg Venous Lymphat Disord. 2019;7:8- 16.
45. Gaweesh AS. Impeded venous drainage: novel view of chronic venous disease pathophysiology. Med Hypotheses. 2009;73:548-552.
