Endovenous thermal ablation for varicose veins: strengths and weaknesses
The Netherlands
ABSTRACT
Endovenous ablation is a frequently used method for treating varicose veins. Endovenous laser ablation is the most frequently used technique, followed by radiofrequency ablation. Endovenous thermal treatments heat the vein, leading to thrombotic occlusion and finally fibrosis of the vein wall. Endovenous steam ablation is a new technique that has not yet been extensively studied. In this article, the procedures, strengths, and weaknesses of the currently available endovenous thermal ablation treatments are discussed.
INTRODUCTION
Endovenous treatment is currently one of the most frequently used methods for treating varicose veins. Varicose veins are manifestations of chronic venous disease (CVD), which may lead to serious complications. CVD is a common medical condition. The prevalence of varicose veins is estimated to range from 2%-40%.1-4 The prevalence of venous leg ulcers, the end-stage of CVD, is much lower. It is very difficult, if not impossible, to predict which patients with varicose veins will develop a leg ulcer. Nevertheless, it has been estimated that about half of venous leg ulcers are the result of superficial venous insufficiency.5 The cost of treating leg ulcers is very high; the treatment of varicose veins, which may reduce the incidence of leg ulcers by 50%, is therefore likely to be cost-effective.
Treatment for varicose veins can roughly be divided into four categories: compression therapy, surgical treatment, sclerotherapy, and endovenous thermal ablation. Surgical ligation of the junction with or without stripping has been the standard of care in the treatment of insufficient great and small saphenous veins for more than 100 years.
In the last decade, endovenous thermal ablation (EVTA) procedures have become the most frequently used therapy for saphenous varicose veins, especially in countries where reimbursement of the procedure has been introduced. Such minimally invasive techniques meet the demand for cosmetically superior, less invasive and more successful treatment modalities. Only introduced 10 years ago, these techniques have radically changed the treatment of varicose veins.6 The EVTA techniques currently available are: endovenous laser ablation (EVLA), radiofrequency ablation (RFA), and endovenous steam ablation. The advantage of EVTA is that it is minimally invasive and can easily be performed under local tumescent anesthesia, without the need for spinal or general anesthesia. Moreover, according to a metaanalysis of the different treatment techniques for varicose veins, recurrence rates are lower after EVTA than after classic surgery.7
The first EVTA procedures were performed by RFA with the VNUS® Closure Plus system.8 EVLA was developed soon after and soon became the most frequently used EVTA method around the world. In the last few years, two new RFA systems have been introduced: VNUS Closure Fast (segmental RFA) and radiofrequency induced thermotherapy (RFITT). The latest thermal ablation technique uses steam at a temperature of 120°C. In the following paragraphs, the different EVTA techniques will be described and their strengths and weaknesses explored.
ENDOVENOUS LASER ABLATION
EVLA can be performed under local tumescent anesthesia in an outpatient setting. Venous access is obtained by puncturing with a 16F or 18F needle or cannula under ultrasound guidance. Most commonly, the insufficient great saphenous vein (GSV) is entered at knee level and the small saphenous vein (SSV) at midcalf. After entrance to the vein has been established, a guide wire is passed through the needle into the vein up to the level of the junction with the deep venous system. If the vein is too tortuous, is small in diameter (due to spasm) and has large side branches, or contains thrombotic or sclerotic segments (after superficial vein thrombosis or prior treatment), advancing the wire can be difficult and caution is indicated because of the increased risk of perforation and embolic events. After checking the position of the guide wire with ultrasound, the needle is removed, and a small cutaneous incision of 3 mm is made. An introducer sheath is placed over the guide wire and is positioned a few centimeters below the junction. Subsequently, the laser fiber (diameter ranges from 200 to 600 μm) is introduced after removing the guide wire. In some laser sets, there is no guide wire and the sheath is directly introduced through a cannula. In other laser kits the laser fiber is already inside the sheath. A crucial step in the EVLA procedure is the positioning of the tip of the laser fiber 1 to 2 cm distally from the junction under ultrasound guidance, in longitudinal view (Figure 1). About 250 to 500 mL (depending on the length of vein treated) of tumescent anesthesia is administered into the perivenous space, again under ultrasound guidance using a syringe or mechanical infusion pump (Figure 2). Tumescent anesthesia is warranted because it reduces pain, cools perivenous tissue, and decreases the venous diameter. After activation, the protocol may use continuous laser pullback (usually at about 3-5 mm/s, depending on the power and wavelength; with the 1320-nm laser, a pullback speed of 1 mm/s is commonly used)9 or a pulsed pullback, with the objective of administering about 30 to 60 J/cm. Compressive bandages or medical elastic stockings (20-30 mm Hg at the ankle) are indicated for 1 week after treatment.
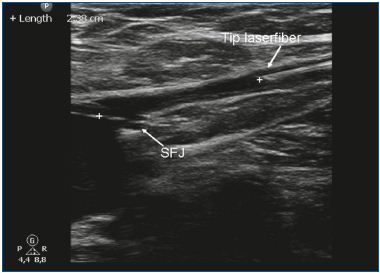
Figure 1. The tip of the laser fiber is positioned 1-2 cm below the
saphenofemoral junction (SFJ).
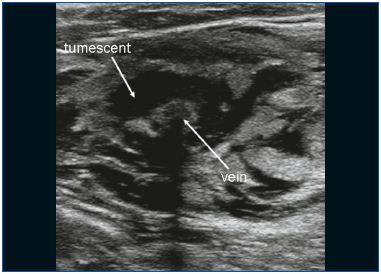
Figure 2. Tumescent anesthesia is administered around the vein.
EVLA can be used for treating insufficient GSVs and SSVs. Due to the rigidity and size of the disposables, linear saphenous veins with a diameter of 5 mm or more are ideal for EVLA (Figure 3). EVLA can also be used for ablation of the anterior accessory saphenous vein or the posterior accessory saphenous vein (often in conjunction with a Giacomini vein), and perforator veins.10-11 EVLA is the least expensive endothermal treatment. In the Netherlands, the cheapest laser disposables cost approximately 120 Euros. Another advantage of EVLA is that the amount of delivered energy can be varied. By adjusting the pullback speed, the power, or both, the total amount of delivered energy per centimeter can be altered. For small veins, only 20 J/cm is used, whereas higher energy (ie, 60 J/cm) can be used when treating large veins. Of all the thermal ablation techniques, EVLA is the most extensively studied in the medical literature. The first large case series reported high success rates12-13 and many series have followed with comparable results. In 2009, we published a meta-analysis on the different treatments for saphenous varicose veins and showed that EVLA had the highest success rate at 93% after 5 years of follow-up. EVLA did significantly better than stripping, RFA, and ultrasound-guided foam sclerotherapy.7 A recent, large randomized clinical trial performed by Rasmussen et al14 showed that EVLA, RFA, and stripping (under tumescent anesthesia) were all equally efficacious. RFA was associated with a faster recovery and less postoperative pain than EVLA and stripping.14
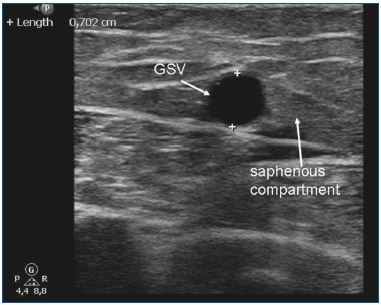
Figure 3. The great saphenous vein (GSV) in the saphenous
compartment.
Some technical difficulties may occur during an EVLA procedure, even in experienced hands. When treating recurrent varicose veins, caution is indicated because introducing the laser fiber may be difficult. In very tortuous veins, introducing the guide wire can be difficult and perforation of the vein is possible. Another disadvantage in some EVLA sets is that introduction is not a single step procedure, but requires several consecutive steps (introduction of the guide wire, the sheath, and then the laser fiber). Each additional step increases the risk of making errors. Some complications have been described that are disposable-dependant; for example, a guide wire remaining inside the body after finishing the EVLA procedure.15 Such complications are usually serious and might be prevented if the procedure could be performed with only one disposable instead of three. The side effects of EVLA are usually mild. Systematically studying all publications on EVLA showed that the most common side effects were ecchymoses and pain, with or without induration (100%). Other less common side effects included: skin burns (<1%), dysesthesia (0-22%), superficial thrombophlebitis (0- 25%), deep vein thrombosis (DVT) (0-6%), nerve injury (<1%), and hematoma. In our experience, postoperative pain may be slightly more pronounced after EVLA compared with RFA and steam ablation. Using a laser fiber with a modified tip (tulip or radial fiber) and avoiding a too high energy dose, may reduce postoperative pain;16 however, there are no good comparative studies available.
RADIOFREQUENCY ABLATION
Several systems for radiofrequency ablation exist. The first RFA procedures were performed with the VNUS® Closure Plus system.8 In the last few years two new RFA systems have been introduced: VNUS® Closure Fast (segmental RFA) and radiofrequency induced thermotherapy (RFITT). Segmental RFA is currently the most popular method.
Procedure Access to the GSV is obtained with a 16-gauge needle under ultrasound guidance, typically at or below knee level or at the most distal point of reflux. The SSV is usually punctured at mid calf. The Closure catheter (VNUS Medical Technologies, Inc, Sunnyvale, California) is positioned 2 cm distally from the junction under longitudinal ultrasound visualization. With the Closure Plus system, a cuff or bandage can be used to express the blood from the vein. The small electrodes at the end of the “umbrella” catheter have direct contact with the venous wall and emit high radiofrequency energy (regulated by power, impedance, and time) that is generated by a radiofrequency generator (VNUS Medical Technologies, Inc). The radiofrequency heats local tissue up to 85°C to 90°C at the site of direct contact, with the heat conducted to deeper tissue planes, causing collagen shrinkage, denudation of endothelium, and obliteration of the venous lumen.17 The catheter pullback speed is 3 cm/min (total pullback time is 20 min on average for the GSV between the saphenofemoral junction and knee level, but can be faster at higher temperatures).18
Segmental RFA (Closure Fast) has a 7 cm therapeutic distal segment that heats to 120°C.19 This technique is much faster than the Closure Plus technique and can be performed under local tumescent anesthesia in an outpatient setting. Similar to EVLA, perivenous tumescent anesthesia is applied to optimize surface contact and to decrease pain and risk of dysesthesia.20 According to the methodology described in the first report on segmental RFA, external compression provided by the ultrasound probe and manual compression is recommended during the treatment to enhance contact of the catheter with the vein wall.21 The first 7 cm of vein is treated with two heat cycles (20 s each). The catheter is then repositioned to the adjacent segment guided by shaft markers in 6.5-cm steps to allow a 5 mm overlap of heated vein segments. Total treatment time is much shorter with segmental RFA than with the Closure Plus system and usually takes only 2 to 3 min. Compressive bandages or medical elastic compression stockings are indicated for 1 week after treatment.
Since 2000, several published case series have shown that RFA can be successfully used to treat saphenous varicose veins.8, 22-25 The first long-term, large, single center case series reported that RFA was effective in about 90% of 140 limbs after 2 years.20 A separate study reported success rates of 83%-88% after 5-year follow-up.26 Our meta-analysis showed that RFA (using Closure Plus) had a success rate of 88%, which was lower than the success rate of EVLA.6
Segmental RFA was not included in the analysis, as at the time no studies were available. However, there are now a few publications on segmental RFA with promising results. The first case series of 252 treated GSVs reported an occlusion rate of 99.6%,21 and two other trials demonstrated success rates >90%.27,28 The main advantage of segmental RFA is probably that it results in less postoperative pain than EVLA. This is thought to be related to the lower maximal temperature that is reached during RFA, and the absence of vein wall perforations.27 A further advantage of segmental RFA is that it is a standardized procedure and introduction of the catheter is performed in one step. This may lower the risk of disposable-related complications.
On the one hand, standardization of a procedure is an advantage. On the other hand, it may not be possible to treat certain ‘special’ cases. With segmental RFA it is impossible to treat veins with a length smaller than 7 cm, although this may change with the recent introduction of a new catheter with a 3 cm heating segment. In certain cases (ie, patients with side branches, or small tortuous parts of varicose veins), it may also be desirable to change the energy delivery, but with segmental RFA it is not possible to treat veins at other than the preset temperature. As a result of the relatively low temperature that is reached during segmental RFA, the working mechanism is collagen denaturation and shrinkage of the vein wall.17 This differs from EVLA in which carbonization and more rigorous destruction of the vein wall is also reported.29 The long-term effectiveness of segmental RFA has not yet been studied and will only become clear after a randomized study comparing EVLA and segmental RFA with long-term follow-up has been conducted.

Figure 4. Steam is ejected from two areas at the tip of the catheter.
STEAM ABLATION
Endovenous steam ablation (EVSA) is a new method of thermal vein ablation that works by heating the venous structure with steam to a maximum temperature of 120°C (Figure 4). The procedure is very similar to EVLA and can be performed with the patient under local tumescent anesthesia in an outpatient setting. The vein is punctured with a 16-gauge needle or cannula under ultrasound guidance. The GSV is usually entered at the distal site of reflux, at or just above knee level because access is easy at this site and the risk of nerve injury is low. The SSV is usually punctured halfway or at a position in the distal third of the calf, depending on vein diameter and extent of reflux. After puncturing the vein, the steam catheter (1.2 mm diameter) is passed through the hollow needle into the vein and the echo-dense tip of the catheter is then carefully positioned 3 cm from the junction, under ultrasound guidance. This is again the most pivotal step in the procedure. About 250 to 500 mL (depending on the length of vein treated) of tumescent anesthesia is administered into the perivenous space under ultrasound guidance. Tumescent anesthesia is necessary to reduce pain, cool the perivenous tissue, and to decrease venous diameter. After activation, the catheter releases small “puffs” of steam and is pulled back in a stepwise fashion. At the first activation, 3 cm below the saphenofemoral or saphenopopliteal junction, four puffs of steam should be administered, while exerting gentle manual pressure on the junction. Further along the vein, two or three puffs of steam can be administered at 1 cm intervals depending on vein diameter. For the first 4 cm of treatment, manual compression of the junction should still be applied as the steam can reach several centimeters beyond the catheter tip. After the procedure, patients are advised to wear thigh-length medical elastic compression stockings (pressure range 25-35 mm Hg) for 1 week and to mobilize immediately after the treatment.
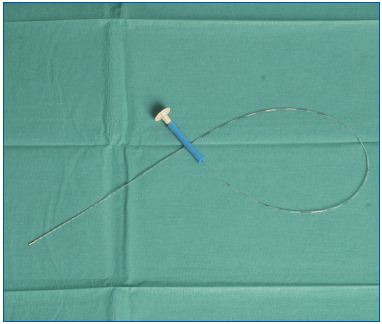
Figure 5. The flexible steam catheter has a small diameter.
Two features that might be advantageous (compared with EVLA) are that EVSA is performed with a very small volume of sterile water (approximately 2 mL per treated vein) and that the temperature is relatively constant, with a maximum of 120°C. The steam catheter is introduced directly through the puncturing needle, without the need for a guide wire or sheath, resulting in an easy and safe procedure. Only one case series on steam ablation has been published, which showed that patient-reported outcomes were favorable, the procedure was very well tolerated, pain scores were low, and patients were very satisfied with the treatment.30 An advantage of the EVSA procedure is that the catheter is minute and very flexible (Figure 5); the diameter of the SVS steam catheter (1.2 mm) is almost 50% smaller than the catheter used for segmental RFA (2.33 mm). The flexibility of the steam catheter may facilitate placement into more tortuous vessels and perforator veins, which are sometimes difficult to access with the more rigid catheters used for RFA and the stiff glass fibers used for EVLA. Even vein tributaries may, therefore, be treated with EVSA. The steam is released from two small areas at the tip of the catheter, allowing treatment of any length of vein. The steam is released under pressure and, therefore, disperses over a distance of at least 2 cm. This may be of additional benefit in the treatment of short perforator veins and short segments of meandering tributaries.
The main limitation of steam ablation is the lack of evidence; only three reports on steam ablation have been published to date.30-32 The other problem is that steam ablation is not yet reimbursed, which will limit the number of procedures performed and thus make outcome measurements even more difficult to obtain. Larger comparative studies are needed to compare the long-term efficacy and the risk-benefit ratio of steam ablation with those of existing endovenous techniques.
DISCUSSION
The “gold standard” for the treatment of insufficient saphenous veins has been ligation plus stripping for the past 100 years. This situation has changed in the last decade with the introduction of endovenous thermal ablation techniques. EVTA techniques are always performed under duplex guidance and are proving to be very effective with high success rates at short-term follow-up. As the effectiveness of current EVTA treatments is excellent (>90%), side-effects are mild, and serious complications rare, any new EVTA procedure should at least perform equally or preferably have some advantages over existing techniques. The hypothesis is that EVSA will be at least as effective as EVLA or RFA. The advantages of steam over the other ablation procedures may be better patient tolerance; a safer, faster and easier procedure; lower costs; and ease of use for perforator veins and tributaries. Future studies should compare the different endovenous treatments in terms of effectiveness and patient-reported outcomes. Further work is also required to try to answer the remaining questions about the exact working mechanism of the different EVTA treatments. In an era of health technology assessment and cost-effectiveness analyses, treatment-related costs will become increasingly important and this will remain a crucial issue in the future.
Two features that might be advantageous (compared with EVLA) are that EVSA is performed with a very small volume of sterile water (approximately 2 mL per treated vein) and that the temperature is relatively constant, with a maximum of 120°C. The steam catheter is introduced directly through the puncturing needle, without the need for a guide wire or sheath, resulting in an easy and safe procedure. Only one case series on steam ablation has been published, which showed that patient-reported outcomes were favorable, the procedure was very well tolerated, pain scores were low, and patients were very satisfied with the treatment.30 An advantage of the EVSA procedure is that the catheter is minute and very flexible (Figure 5); the diameter of the SVS steam catheter (1.2 mm) is almost 50% smaller than the catheter used for segmental RFA (2.33 mm). The flexibility of the steam catheter may facilitate placement into more tortuous vessels and perforator veins, which are sometimes difficult to access with the more rigid catheters used for RFA and the stiff glass fibers used for EVLA. Even vein tributaries may, therefore, be treated with EVSA. The steam is released from two small areas at the tip of the catheter, allowing treatment of any length of vein. The steam is released under pressure and, therefore, disperses over a distance of at least 2 cm. This may be of additional benefit in the treatment of short perforator veins and short segments of meandering tributaries.
The main limitation of steam ablation is the lack of evidence; only three reports on steam ablation have been published to date.30-32 The other problem is that steam ablation is not yet reimbursed, which will limit the number of procedures performed and thus make outcome measurements even more difficult to obtain. Larger comparative studies are needed to compare the long-term efficacy and the risk-benefit ratio of steam ablation with those of existing endovenous techniques.
DISCUSSION
The “gold standard” for the treatment of insufficient saphenous veins has been ligation plus stripping for the past 100 years. This situation has changed in the last decade with the introduction of endovenous thermal ablation techniques. EVTA techniques are always performed under duplex guidance and are proving to be very effective with high success rates at short-term follow-up. As the effectiveness of current EVTA treatments is excellent (>90%), side-effects are mild, and serious complications rare, any new EVTA procedure should at least perform equally or preferably have some advantages over existing techniques. The hypothesis is that EVSA will be at least as effective as EVLA or RFA. The advantages of steam over the other ablation procedures may be better patient tolerance; a safer, faster and easier procedure; lower costs; and ease of use for perforator veins and tributaries. Future studies should compare the different endovenous treatments in terms of effectiveness and patient-reported outcomes. Further work is also required to try to answer the remaining questions about the exact working mechanism of the different EVTA treatments. In an era of health technology assessment and cost-effectiveness analyses, treatment-related costs will become increasingly important and this will remain a crucial issue in the future.

REFERENCES
1. Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53:149-153.
2. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidencebased report of the VEINES task force. Venous Insufficiency Epidemiologic and Economic Studies. Int Angiol. 1999;18:83-102.
3. Abramson JH, Hopp C, Epstein LM. The epidemiology of varicose veins. A survey in western Jerusalem. J Epidemiol Community Health. 1981;35:213-217.
4. Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4:96-101.
5. Magnusson MB, Nelzen O, Risberg B, Sivertsson R. A colour Doppler ultrasound study of venous reflux in patients with chronic leg ulcers. Eur J Vasc Endovasc Surg. 2001;21:353-360.
6. De Maeseneer M. The endovenous revolution. Br J Surg. 2011;98:1037- 1038.
7. Van Den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T..Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
8. Goldman MP. Closure of the greater saphenous vein with endoluminal radiofrequency thermal heating of the vein wall in combination with ambulatory phlebectomy: preliminary 6-month follow-up. Dermatol Surg. 2000;26:452-456.
9. Goldman MP, Mauricio M, Rao J. Intravascular 1320-nm laser closure of the great saphenous vein: a 6- to 12- month follow-up study. Dermatol Surg. 2004;30:1380-1385.
10. Proebstle TM, Herdemann S. Early results and feasibility of incompetent perforator vein ablation by endovenous laser treatment. Dermatol Surg. 2007;33:162-168.
11. Bush RG, Hammond K. Treatment of incompetent vein of Giacomini (thigh extension branch). Ann Vasc Surg. 2007;21:245-248.
12. Navarro L, Min RJ, Bone C. Endovenous laser: a new minimally invasive method of treatment for varicose veins—preliminary observations using an 810 nm diode laser. Dermatol Surg. 2001;27:117-122.
13. Min RJ, Zimmet SE, Isaacs MN, Forrestal MD. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167-1171.
14. Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079-1087.
15. Kichari JR, Salomonsz R, Postema RR. [Chronic pain due to a retained guidewire following endovascular laser therapy for varicose veins]. [Article in Dutch]. Ned Tijdschr Geneeskd. 2008;152:1387-1390.
16. Doganci S, Demirkilic U. Comparison of 980 nm laser and bare-tip fibre with 1470 nm laser and radial fibre in the treatment of great saphenous vein varicosities: a prospective randomised clinical trial. Eur J Vasc Endovasc Surg. 2010;40:254-259.
17. Schmedt CG, Sroka R, Steckmeier S, et al. Investigation on radiofrequency and laser (980 nm) effects after endoluminal treatment of saphenous vein insufficiency in an ex-vivo model. Eur J Vasc Endovasc Surg. 2006;32:318- 325.
18. Zikorus AW, Mirizzi MS. Evaluation of setpoint temperature and pullback speed on vein adventitial temperature during endovenous radiofrequency energy delivery in an in-vitro model. Vasc Endovascular Surg. 2004;38:167- 174.
19. VNUS website. http://www.vnus.com (last accessed 4 August 2008).
20. Weiss RA, Weiss MA. Controlled radiofrequency endovenous occlusion using a unique radiofrequency catheter under duplex guidance to eliminate saphenous varicose vein reflux: a 2-year follow-up. Dermatol Surg. 2002;28:38-42.
21. Proebstle TM, Vago B, Alm J, Göckeritz O, Lebard C, Pichot O. Treatment of the incompetent great saphenous vein by endovenous radiofrequency powered segmental thermal ablation: first clinical experience. Vasc Surg. 2008;47:151-156.
22. Sybrandy JE, Wittens CH. Initial experiences in endovenous treatment of saphenous vein reflux. J Vasc Surg. 2002;36:1207-1212.
23. Goldman MP, Amiry S. Closure of the greater saphenous vein with endoluminal radiofrequency thermal heating of the vein wall in combination with ambulatory phlebectomy: 50 patients with more than 6-month follow-up. Dermatol Surg. 2002;28:29-31.
24. Manfrini S, Gasbarro V, Danielsson G, et al. Endovenous management of saphenous vein reflux. Endovenous Reflux Management Study Group. J Vasc Surg. 2000;32:330-342.
25. Chandler JG, Pichot O, Sessa C, Schuller-Petrovi´c S, Osse FJ, Bergan JJ. Defining the role of extended saphenofemoral junction ligation: a prospective comparative study. J Vasc Surg. 2000;32:941-953.
26. Merchant RF, Pichot O; Closure Study Group. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg. 2005;42:502- 509.
27. Shepherd AC, Gohel MS, Lim CS, Hamish M, Davies AH. Pain following 980-nm endovenous laser ablation and segmental radiofrequency ablation for varicose veins: a prospective observational study. Vasc Endovascular Surg. 2010;44:212-216.
28. Proebstle TM, Alm J, Gockeritz O, et al. Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg. 2011;54:146-152.
29. Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28:56-61.
30. van den Bos RR, Milleret R, Neumann M, Nijsten T. Proof-of-principle study of steam ablation as novel thermal therapy for saphenous varicose veins. J Vasc Surg. 2010;53:181-186.
31. Milleret R, Mehier H, Llopinet A, et al. Oblitération veineuse par vapeur à haute température. Phlebologie. 2008;61:223-226.
32. van Ruijven PW, van den Bos RR, Alazard LM, van der Geld CW, Nijsten T. Temperature measurements for dose-finding in steam ablation. J Vasc Surg. 2011;53:1454-1456.
