Sclerotherapy in the patient with diabetes: indications and results
ABSTRACT
Goal: To assess the effects of combined compression and sclerotherapy treatment on varicose veins in patients with diabetes.
Materials and methods: We evaluated 60 lower limbs with varicose veins from people with diabetes who underwent one session of sclerotherapy according to Sigg’s method. Varices were associated with reflux along the length of the great saphenous vein in 47 limbs, in the small saphenous vein in 8 limbs, and with nonsaphenous reflux in 5 limbs. Efficacy was determined by clinical and duplex scan examinations performed at 6, 8, and 12 months, and at 2, and 4 years.
Results: A total of 7 clinical failures (12%) and 14 duplex scan failures (23%) were observed. No major complications such as arterial injection, severe allergic reaction or deep vein thrombosis were reported.
Conclusions: Successful sclerotherapy for varicose veins can be performed independently of a history of diabetes, with a success rate similar to that observed in patients without diabetes, provided the patient has good glycemic control (HbA1c <6.5%).
INTRODUCTION
Varicose veins of the legs are a common condition caused by venous insufficiency as a result of valve reflux.1 Most varices are located in the great saphenous vein (GSV) and are associated with symptoms such as leg heaviness, fatigue, or throbbing pain.2 People with diabetes may be particularly prone to varicose veins as they generally present with a number of common risk factors for chronic venous disease (CVD) including poor circulation, overweight, hypertension as well as a propensity for endothelial dysfunction. Indeed, a number of studies in the literature have shown that CVD and diabetes mellitus frequently coexist.3-6 Furthermore, diabetes is associated with more severe signs in 50% of patients.7 The medical rationale for treating varicose veins in patients with diabetes is the same as those without diabetes: to reduce symptoms, improve quality of life, and prevent disease progression. Early intervention reduces the risk of ongoing vein- and skin related damage secondary to chronic venous hypertension. These changes progressively worsen with time, and earlier treatment is preferable to a wait-and see approach.
Traditionally, treatment of varicose veins involved an invasive procedure of groin to ankle stripping and ligation of the GSV. More recently, ultrasound-guided foam sclerotherapy has gained acceptance as an effective and safe alternative to surgery to treat varicose veins.1 Sclerotherapy is defined as the targeted elimination of small vessels, varicose veins, and vascular anomalies by the injection of a sclerosant (chemical irritant) into the veins with a small needle.8 The sclerosant causes inflammation, thrombosis, and finally fibrosis of the vein, which is transformed into a fibrous cord that cannot be recanalized. Sclerotherapy is a simple, cost effective, efficacious, and esthetically acceptable modality for both therapeutic and esthetic purposes.
There are few studies in the literature discussing sclerotherapy for varicose veins in patients with diabetes and the few that do exist are contradictory with some stating that diabetes is a contraindication for sclerotherapy8 and others stating that it is not.9 Most contraindications and/or special precautions relating to sclerotherapy relate to factors that either directly increase the risk of deep vein thrombosis or indirectly interfere with post treatment compression and mobilisation.9
Sclerotherapy should only be performed in people with chronic diseases if their condition is well controlled, and diabetes is no exception.10 The standard measure of longterm glycemic control is glycosylated hemoglobin (HbA1c). In a person without diabetes, normal HbA1c levels fluctuate between 4% and 6%. A person with diabetes is considered to have good glycemic control if their HbA1c level is below about 6.5%, although this may not always be achievable particularly in older patients or in those in whom hypoglycemia must be avoided.11 When levels rise to above 7% the individual is not considered to have good control. However, in the absence of a true contraindication,8 several precautions should be observed before performing sclerotherapy in an individual with diabetes.
Before the procedure, a complete clinical examination and duplex scan investigation should be performed to detect any associated arterial disease. To avoid prolonging inflammation in the vein wall, any existing edema and soft tissue changes associated with CVD should first be reduced by the application of a short stretch bandage.
Concerning the choice of sclerosing agent, foam is not contraindicated, but agents containing a hyperconcentration of glucose should be avoided. Foam has been shown to be superior to liquid sclerotherapy in the GSV in terms of clinical and hemodynamic outcomes, with several advantages over traditional liquid sclerotherapy.12 When delivered as foam, detergent sclerosant is not diluted by blood, instead displacing it to allow direct contact of the sclerosant with the endothelium.13 This allows for the use of a smaller dose of sclerosant, which is important as the vein walls of patients with diabetes are very sensitive to these agents. Second, should extravasation occur, foam is much better tolerated than extravasated liquid. Third, foam can be readily visualized by ultrasound, which increases the accuracy with which individual varicose veins can be treated.
Due to the impaired immune response in individuals with diabetes, they are particularly susceptible to infection. Care must therefore be taken to ensure that the materials and puncture site are sterile. When injecting the sclerosing agent, the bevel of the needle should be placed into the lumen of the vein as patients with diabetic neuropathy have reduced sensitivity to pain and if the needle is not properly positioned extravasation of sclerosant might go unnoticed.
Prolonged compression therapy starting immediately after the procedure and lasting at least 30 days is imperative in all patients to achieve optimal results with sclerotherapy, but particularly in patients with diabetes because of their endothelial pathology.14 Compression minimizes the blood reentering the injected area and thrombus formation, increases fibrinolytic activity and therefore reduces scar tissue formation, decreases the incidence of postsclerotherapy hyperpigmentation, and improves venous blood flow.
To be effective, compression hosiery must exert a high pressure at calf level during calf contraction and a low pressure at rest. Compression hosiery should be tightly applied on the lower limb starting from the foot. Short stretch bandages (35%) in nylon and/or cotton are more effective than stockings or long-stretch bandages, either rubber or cotton band.
Few studies in the literature have documented the use of sclerotherapy in people with diabetes. The aim of our study was to evaluate the efficacy and safety of combined sclerotherapy and compression for varicose vein ablation in patients with well-controlled diabetes.
MATERIAL AND METHODS
Between 1982 and 2011 we performed a total of 47 000 sclerotherapy injections, of which 1400 (3%) were performed in 60 lower limbs from patients with diabetes presenting with class C2 to C6 CVD according to the Clinical, Etiological, Anatomical, and Pathophysiological (CEAP) classification.15 CVD signs were associated with a reflux along the length of the GSV in 47 cases, in the small saphenous vein (SSV) in 8 cases, and with non-saphenous reflux in 5 cases.
In 18 legs, edema associated with CVD was reduced by the use of a short-stretch bandage prior to the procedure. In 11 legs, symptomatic peripheral arterial disease (claudication) was identified, which was treated by surgery or an endovascular procedure.
All legs were treated by puncture of the saphenous trunk at the level of the thigh with a large needle (18 G, 1.2 mm) according to Sigg’s method (patient standing, open needle technique). By allowing the blood to run it is immediately possible to determine if the needle is in an artery by the color of the blood and whether the flow is synchronous with the pulse (Figure 1). In this manner, paravenous injections can be avoided. With the patient in the Trendelenburg position (Figure 2), the saphenous trunk was then injected with a 1-2% iodate solution or polidocanol 2-3% (as a foam 0.5%). It is important to use a glass syringe that slides well and to determine its position immediately, especially when using a large needle. Resistance to injection is greater if the needle is outside a vein and the liquid spreading in tissues. Strong compression is then applied (Figure 3).10 This comprises immediate spot compression (to reduce the size of the vein sclerosis) with gauze pads applied over the treated varices for 2 days (Figure 3A) and 4-cm thick cotton wool balls with a hard center over the GSV injection sites for 7 days (Figure 3B). In addition, concentric compression with a removable short-stretch bandage (35%, removed at night and replaced in the morning) is applied for 30 days. Compression needs to be modified if there is evidence of significant peripheral arterial insufficiency and low-pressure compression stockings (class I) should be worn for 30 days by patients with chronic venous insufficiency or peripheral pulses.
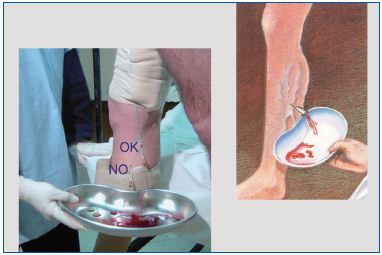
Figure 1. Puncture of the vein with Sigg’s technique « open needle »: patient standing, needle 18 G (short catheter) not connected to a syringe.
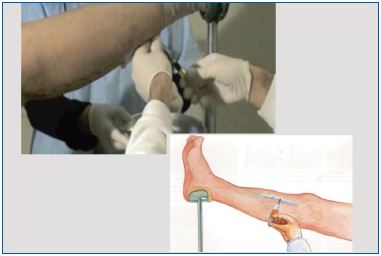
Figure 2. : Injection of sclerosing agent according to Sigg’s technique with the patient in the Trendelenburg position.
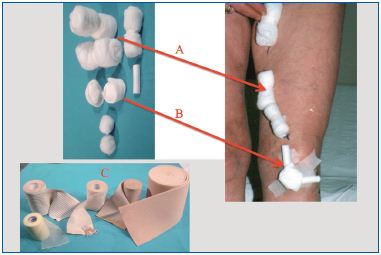
Figure 3. Application of spot and concentric therapeutic compression after the sclerotherapy procedure: spot compression comprises balls or rolls of cotton wool applied over the treated veins; concentric compression uses a short-elastic bandage from foot to groin.
Hemodynamic compression of the sapheno-femoral junction or sapheno-popliteal junction is applied for 3 days using Safeguard™ (Datascope) (Figure 4A). This device consists of an adhesive dressing and an inflatable balloon (achieved with the aid of a syringe) and is commonly used to achieve hemostasis after invasive vascular procedures. The balloon, which is transparent on Doppler ultrasound is inflated with gel (Figure 4B) until reflux at the saphenous junction is interrupted. The compressive effects of the balloon on the saphenous terminals can be verified echographically (Figure 4C and 4D). This method is used for the new sclerotherapy procedure (HCS method) that I proposed in 2010 and perform with selective suppression along the length of the GSV or SSV.16
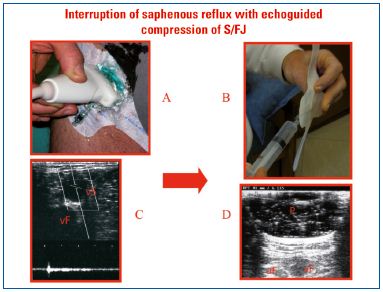
Figure 4. Hemodynamic compression according to the HCS method. (A) The SafeguardTM device comprises an inflatable balloon and adhesive dressing. (B) Application of the Safeguard device at the terminal end of the GSV. (C) Echographic view of the terminal end of the GSV (vS) and the femoral vein (vF) before and (D) after inflation of the balloon (P) to interrupt saphenous reflux with the patient in a standing position.
Efficacy was determined by both clinical and echographic criteria at 6, 8, and 12 months and after 2, and 4 years of follow-up. Using clinical criteria, treatment failures were characterized by the presence of varices in quantities exceeding 50% of the original varices, and by the presence of symptoms of chronic venous insufficiency.
Echographic failures (using a 7.5-MHz probe) were characterized by the absence of the following signs: inability to compress the vein; morphological changes to the vessel wall such as endothelial thickening, disorganized collagen bundles and fragmentation of elastic fibers; luminal changes such as hyper echogenicity of the lumen and reduction of the vessel to a fibrous cord that cannot be recanalized.17,18 On a hemodynamic level, the absence of flux, reflux or the persistence of reflux in the saphenous trunks, was determined if the reduction in size of the sapheno-femoral junction was greater than 30% of their initial diameter.19
RESULTS
Globally, after a 4-year follow-up, we documented seven clinical failures (12%) and 14 failures (23%) by Doppler ultrasound. Over time, the following recurrences occurred: 3 echographic failures at 6 months, 6 at 12 months, 4 at 2 years and 1 at 4 years; 3 clinical recurrences occurred at 12 months, and 4 at 2 years. These results are in line with those we have observed in nondiabetic patients in clinical practice.
We observed no major complications (necrosis, allergy or deep vein thrombosis). There were six cases of ascending vein wall inflammation (phlebitis) caused by a poorly applied bandage, and one case of a patient fainting (vasovagal reaction).
DISCUSSION
Few studies have examined changes to the vein wall in patients with diabetes. We demonstrate that sclerotherapy is an effective and well-tolerated procedure for varicose veins in patients with well controlled diabetes mellitus. The proportion of clinical and Duplex scan treatment failures was similar to that reported in another study from our group, which documented clinical and echographic treatment failure rates of 12% and 23%, respectively, in 1500 cases of GSV reflux treated with the Sigg method over the course of 15 years.18,19
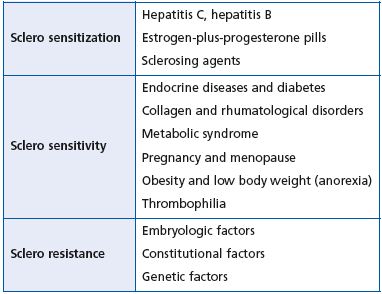
Table I. Sclerotherapy in patients with arteriopathy –
a decisional tree.
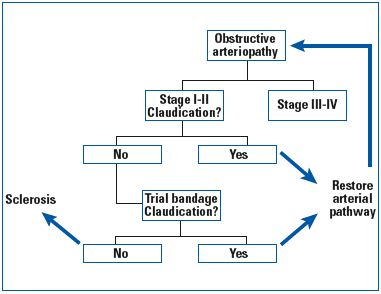
Table II. Three different treatment responses for varicose veins in patients with general pathophysiological conditions.
We have previously evaluated the results of sclerotherapy in different pathophysiological contexts (Table I).20 Diabetes is associated with vascular sensitivity, as proposed by Lanza et al who suggested the existence of a “diabetic phlebosclerosis”.21 Endothelial dysfunction in patients with diabetes, as a result of chronically elevated blood glucose levels, may influence several mediators secreted by the endothelium. For example, there is an augmentation of levels of von Willebrand factor (vWF), a glycoprotein synthesized and stored in the vascular endothelium, increased levels of which may contribute to an increased risk of thrombosis, and a decrease in production of prostacyclin, which is associated with platelet hyperaggregability. For this reason, when performing sclerotherapy in people with diabetes, concentrations of sclerosing agent must be reduced by half (whether using foam or liquid) and suitable compression must be applied.
In our center, compression is provided by a short-stretch bandage (35%), which only prevents arterial perfusion in the presence of a significant arterial obstruction, with a systolic pressure of less than 70 mm Hg at the ankle. However, the latter is a hypothetical patient and we would not propose sclerotherapy or surgical treatment in a person with significant symptoms of peripheral arterial disease. In reality, it is the patient with claudication and varices that occasionally presents for phlebological consultation (Table II). This patient is a candidate for sclerotherapy when the claudication has been resolved. We should highlight that in these patients, the re-establishment of the arterial pathway by catheter or surgery should be attempted. However, sclerotherapy may then pose a problem, as it demands compression associated with ambulation. A test bandage worn during the day for 1 week, without injections should be conducted as a feasibility assessment. A similar approach should be followed in case of suspected arterial disease. A nonelastic bandage may be tolerated in patients with a suspected subclinical arteriopathy.10 In these patients, a compression stocking delivering compression greater than 20 mm Hg should be avoided, especially in the absence of good systolic blood pressure.
Sclerotherapy, when performed appropriately, is an efficacious and safe technique, but complications can occur. The most severe include cutaneous necrosis, as a result of extravasation of sclerosant into perivascular tissues or direct injection into an arteriole, allergic reactions to sclerosants, and deep vein thrombosis. None of these adverse events were observed in our study. The persistence of significant reflux into a vein that has been treated with a sclerosing agent can lead to phlebitis and this was documented in six cases as a result of poorly applied compression. We observed one vasovagal reaction, which resolved spontaneously.
Sclerotherapy is an important tool for preventing the progression of CVD to more severe stages. In a prospective, 7-year follow-up of patients with both superficial and deep venous reflux, deterioration in clinical class was shown in most of the limbs at the end of the observation period.22 Limbs that underwent a superficial or deep venous procedure remained stable or improved over time; those that underwent elastic compression alone had worsening hemodynamic and clinical status. This may be particularly the case in patients with diabetes who are at greater risk of more severe CVD because of their already impaired endothelial function.
CONCLUSIONS
The results of our study show that sclerotherapy is not contraindicated in the presence of well-controlled diabetes (HbA1c < 6.5%). On the contrary, this may be one of the best indications for sclerotherapy for a number of reasons. First, the long-term prognosis of chronic venous insufficiency can be severe with subcutaneous tissue involvement and ulcer formation. Safe and effective measures to delay or prevent this scenario are therefore beneficial, particularly in patients with diabetes. Second, excess scar tissue and aggravation of diabetic neuropathy via nerve damage are common complications following surgery, limiting the benefits of invasive procedures for removing varicose veins in patients with diabetes. Finally, there was no significant difference in sclerotherapy results between our patients with diabetes and our global population of patients undergoing sclerotherapy by the same method.

REFERENCES
1. Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1-59.
2. Labropoulos N, Kang SS, Mansour MA, Giannoukas AD, Buckman J, Baker WH. Primary superficial vein reflux with competent saphenous trunk. Eur J Vasc Endovasc Surg. 1999;18:201-206.
3. Marston WA, Carlin RE, Passman MA, Farber MA, Keagy BA. Healing rates and cost efficacy of outpatient compression treatment for leg ulcers associated with venous insufficiency. J Vasc Surg. 1999;30:491-498.
4. Lionis C, Erevnidou K, Antonakis N, Argyriadou S, Vlachonikolis I, Katsamouris A; CVI Research Group. Chronic venous insufficiency. A common health problem in general practice in Greece. Int Angiol. 2002;21:86-92.
5. Florea I, Stoica LE, Jolea I. Chronic venous insufficiency – clinicalevolutional aspects. Current Health Sciences Journal. 2011;37(1).
6. Mani R, Yarde S, Edmonds M. Prevalence of deep venous incompetence and microvascular abnormalities in patients with diabetes mellitus. Int J Low Extrem Wounds. 2011;10:75-79.
7. Agus GB, Jawien A, Carelli F. Nautilus survey on chronic venous diseases. Panminerva Med. 2010;52(2 Suppl 1):5-9.
8. Khunger N, Sacchidanand S. Standard guidelines for care: Sclerotherapy in dermatology. Indian J Dermatol Venereol Leprol. 2011;77:222-231.
9. Guex JJ. Contra indications of sclerotherapy, update 2005. J Mal Vasc. 2005;30:144-149.
10. Ferrara F. La terapia sclerosante ed elastocompressiva delle flebopatie. Piccin Padova. 2009.
11. National Institute for Health and Clinical Excellence. Type 2 diabetes newer agents. Clinical guideline. London: May 2009.
12. Yamaki T, Nozaki M, Iwasaka S. Comparative study of duplex-guided foam sclerotherapy and duplex-guided liquid sclerotherapy for the treatment of superficial venous insufficiency. Dermatol Surg. 2004;30:718-722.
13. Geroulakos G. Foam sclerotherapy for the management of varicose veins: a critical reappraisal. Phlebolymphology. 2006;13:202-206.
14. Weiss RA, Sadick NS, Goldman MP, Weiss MA. Post-scleropathy compression: controlled comparative study of duration of compression and its effects on clinical outcome. Dermatol Surg. 1999;25:105-108.
15. Eklöf B, Rutherford RB, Bergan JJ, et al; American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248-1252.
16. Ferrara F, Ferrara G. Sclerotherapy of varicose veins: my method (HCS). Minerva Cardioangiol. 2012;60:125-131.
17. Bernbach HR, Ferrara F. Compression in sclerotherapy of the saphenofemoral junction our experience (1500 cases). XVI World Meeting of the Union Internationale de Phlébologie Montecarlo 31/08-04/09, 2009.
18. Ferrara F, Bernbach HR. La sclérothérapie compressive de la petite veine saphène: contrôles par écho- Doppler et thermographie. Phlébologie. 2004;57:183-186.
19. Ferrara F, Bernbach HR. La compression écho-guidée après sclérothérapie, Phlébologie. 2009;6:36- 41.
20. Ferrara F, Bernbach HR. La sclérothérapie des varices récidives. Phlébologie. 2005;58:147-150.
21. Lanza G. Trattato di Anatomia Patologica. Ed. Piccin Padova 1974.
22. Lurie F, Makarova NP. Clinical dynamics of varicose disease in patients with high degree of venous reflux during conservative treatment and after surgery: a 7-year follow-up. Int J Angiol. 1998;7:234-237.
