Venous embryology: the key to understanding anomalous venous conditions
George Washington University School of Medicine, Washington DC, USA
ABSTRACT
Venous embryology can explain many of the defects resulting in venous anomalies in later life, yet is often overlooked. Venous malformations are vascular malformations that only affect the venous system. They are classified into two different types depending on the embryological stage when the defective development occurs. Venous malformations originating during the early stage of embryogenesis are termed extratruncular, while those originating during the late stage of embryogenesis are classified as truncular. A defect at any point in the complex development stages of the evolution and involution of multiple paired embryonic veins can result in various conditions of defective venous trunkTherefore, truncular lesions in general are associated with more serious hemodynamic consequences than extratruncular lesions due to their direct involvement with the truncal venous system.
This review provides a detailed overview of venous embryology and a number of truncular venous malformations to illustrate how a thorough knowledge of this subject can aid in their diagnosis and treatment.
INTRODUCTION
A thorough understanding of vascular system anatomy is a prerequisite for all vascular specialists. However, a knowledge of venous embryology is seldom acquired even though all mature and named vessels originate from their precursor, embryonic vessels, and vascular anomalies are closely linked to them.
Vascular anomalies are relatively rare and difficult to understand and interpret. Yet, venous embryology is one of the most neglected areas of basic science in medicine despite its critical ability to explain the many obscure conditions related to anomalous anatomy (eg, membranous occlusion of suprahepatic inferior vena cava as a cause of primary Budd-Chiari Syndrome).1,2
Such venous anomalies are a result of the defective development of embryonic veins during the vascular trunk formation period in the later stage of embryonic development.3,4 A benign narrowing (stenosis) of the jugular-azygos vein system is a good example of how defective development can cause a unique condition, in this case chronic cerebrospinal venous insufficiency (CCSVI).5,6
A basic knowledge of vascular embryology and in particular, the evolutional and involutional development of the venous system involved in the maturation of the truncal vein, is essential for the recognition and interpretation of a number of venous anomalies.7,8
DEFINITION
When the embryo starts to grow at an exponential rate in the early stage of embryogenesis, rapid growth and expansion of the embryonic vessels must follow suit to fulfill their critical role as the channels to supply essential nutrient requirements. A defect at any point in the complex development stages of evolution and involution of multiple paired embryonic veins can result in congenital vascular malformations (CVM).9,10 The prevalence of defective development in the vascular structure of the newborn is in the range of 1% to 3%.
As CVMs are birth defects that arise during the various stages of development of the vascular system,11,12 they can involve one or more components: artery, vein, lymphatics, and/or capillary vessels. Venous malformations are vascular malformations that only affect the venous system.13,14 They may exist alone as an independent lesion or combined with other CVMs as lymphatic malformations,15,16 arteriovenous malformations,17,18 and/or capillary malformations.19,20 The clinical behavior of the malformation is solely dependent on the embryonic stage at which the developmental arrest/defect occurs.
When defective development occurs in the ‘early’ stage of embryogenesis, the embryonic vessels remain in the form of reticular networks and do not evolve into the vascular trunk formation. After birth these networks can remain as independent clusters of primitive venous tissue without direct involvement of the main venous trunk itself (eg, extratruncular venous malformation) (Figure 1). These primitive vascular structures maintain the mesenchymal cell properties and its evolutional ability to proliferate when stimulated by exogenous (eg, surgery, trauma) or endogenous factors (menarche, pregnancy, female hormones).7,8,10,21,22
When defective development occurs in the vascular trunk formation period in the ‘later stage’ of embryonic development, the defects involve ‘named’ vessels (eg, iliac, femoral, and popliteal vessels) and are limited to the vessel trunk itself. Examples of such truncular venous malformations include popliteal vein aneurysm, absence/aplasia of the femoral vein, jugular vein stenosis/webs, and hypoplastic iliac vein (Figure 2). These are ‘embryologically mature’ lesions, which no longer possess the evolutionary capacity to proliferate. However, truncular lesions present with more serious hemodynamic consequences in general compared with extratruncular lesions due to their direct involvement with the truncal venous system (eg, avalvulosis, marginal veins, popliteal vein aneurysm, inferior vena cava stenosis/occlusion).23,24
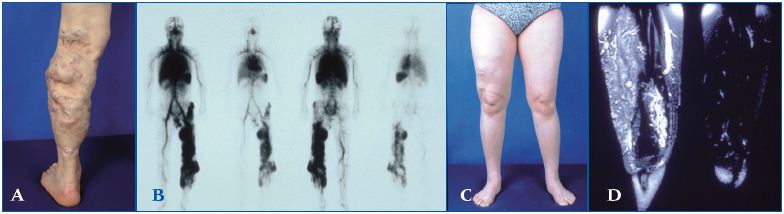
Figure 1 (A – D). Extratruncular venous malformation (VM)
1A depicts a clinical condition of extratruncular venous malformation (VM) lesions affecting the entire left lower leg, but mostly limited superficially to the soft tissue level. 1B (whole body blood pool scintigraphy) displays compatible findings to show the extent of the lesion on the same extremity. On the contrary, 1C shows a benign looking VM lesion in the right lateral upper thigh mimicking varicose veins.
However, it is the tip of the iceberg of extensive lesions infiltrating into the surrounding soft tissue as well as the muscles, shown in MRI (1D).
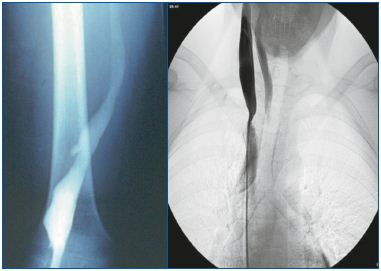
Figure 2 (A and B). Truncular venous malformation (VM) 2A demonstrates angiographic findings of a truncular VM lesion consisting of an aneurysmal dilatation of the right popliteal vein; this truncular lesion is the outcome of developmental arrest during the vascular trunk formation period in the ‘later stage’ of embryonic development.
2B also presents angiographic findings of another type of truncular VM lesion this time a stenotic condition involving the right internal jugular vein trunk along its junction with the superior vena cava (Courtesy of Professors P Zamboni and R.Galeotti for 2B).
Based on the above definitions, the modified Hamburg Classification separates venous malformations into two different types: extratruncular and truncular, depending on the embryological stage when the defective development occurred (Table I).25,26 Venous malformations originating from the ‘early’ stage of embryogenesis are classified as extratruncular together with all other types of vascular malformation from the same ‘early’ stage (eg, lymphangioma). Venous malformations originating from the ‘late’ stage of embryogenesis are classified as truncular.
As all truncular lesions involve the already formed, established venous trunk to varying degrees, they present as either hypoplastic or hyperplastic vessels/lesions causing obstruction or dilatation (eg, internal jugular vein aneurysm, iliac vein stenosis), depending on the defect.27,28 It should be noted that intraluminal defects within the vein (eg, vein webs or membrane) can result in similar conditions of stenosis or obstruction (Figure 3).29,30
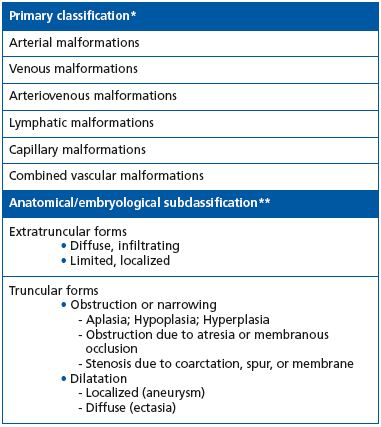
Table I. The modified Hamburg classification of congenital vascular malformations.
* Based on the predominant vascular structure in the malformation.
** Based on anatomy and developmental arrest at the different stages of embryonic life: extratruncular form from earlier stages; truncular form from late stages.
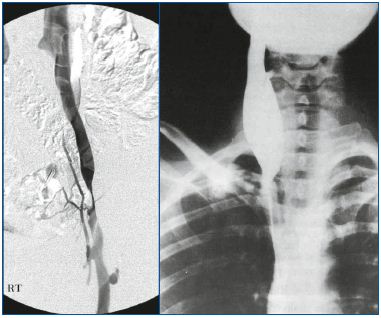
Figure 3 (A and B). Truncular venous malformation (VM) 3A shows angiographic findings for a truncular VM lesion involving a segmental stenosis of the left iliac vein. This benign looking condition precipitated a severe chronic venous insufficiency to the affected lower extremity.
3B shows another form of truncular VM involving an aneurysmal dilatation of the right internal jugular vein (Courtesy of Professors P. Zamboni and R. Galeotti for 3B).
Less frequently, truncular venous malformations may present as a persistent fetal remnant vein that has failed to involute or regress normally. This unique condition, which involves the lower extremity venous system, is known as «marginal/sciatic/lateral embryonic veins»31,32 and represents the venous malformation component of Klippel-Trenaunay Syndrome (Figure 4).3,4,21,22
As a consequence of their direct involvement with the venous system, the chronic venous congestion and hypertension due to venous reflux or occlusion caused by truncular venous malformations result in more tissue and organ damage than extratruncal lesions. Membranous, focal, or segmental lesions can cause suprahepatic stenosis of the inferior vena cava along the proximal terminal segment, a condition known as primary Budd-Chiari syndrome. This has a profound hemodynamic impact, not only on the lower extremities where it causes chronic venous hypertension, but also on the liver where it results in severe portal hypertension due to hepatic venous outlet obstruction. This congenital/developmental anomaly most frequently involves Asian and African races (Figure 5).33,34
The cerebrospinal venous circulation is not exempt from truncular venous malformations. Cerebrospinal venous malformations carry the potential risk of long-term chronic venous hypertension to the brain resulting in various clinical conditions/illnesses such as CCSVI.35,36
An example of CCSVI, internal jugular vein valve incompetence (IJVVI), has been postulated to be the cause of transient global amnesia.37,38 IJVVI is diagnosed when retrograde jugular vein flow is detected by extracranial duplex ultrasound during Valsalva maneuver. It is believed that IJVVI may produce transient mesiotemporal ischemia by venous congestion. This mechanism requires a patent venous pathway from the affected internal jugular vein through the transverse sinus, confluence, straight sinus, and vein of Galen into the basal vein of Rosenthal and into the internal cerebral veins.
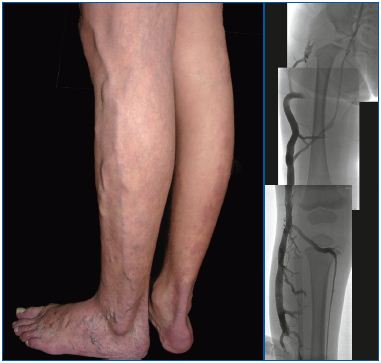
Figure 4 (A and B). Truncular venous malformation: marginal/lateral embryonic vein 4A depicts a clinical condition of the marginal/lateral embryonic vein along the lateral aspect of the left lower extremity. This unique vein structure is a persistent fetal remnant vessel following the failure of normal involution/regression and its ‘avalvulosis’ causes severe venous reflux. Marginal vein remains are a hallmark of Klippel-Trenaunay syndrome, representing its venous malformation component.
4B presents angiographic findings of this marginal vein, which is the only remaining major venous drainage route with a lack of normal development of the deep venous system. Surgical excision to control venous hypertension is therefore contraindicated.
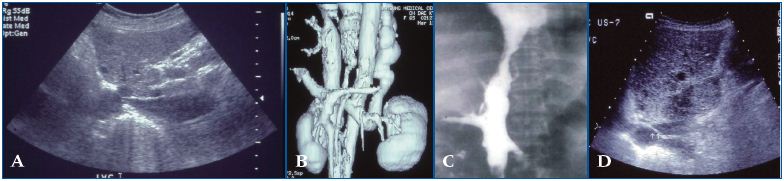
Figure 5 (A – D). Suprahepatic inferior vena cava (IVC) occlusive lesion: primary Budd-Chiari syndrome A common cause of suprahepatic IVC occlusion is focal stenosis (shown in 5A and 5B) and segmental stenosis (5C), although membranous bstruction by the web is the most common cause among Asians (5D). These are relatively simple congenital VM, which develop during the late vessel trunk formation stage. However, they have a profound hemodynamic impact on the liver with portal hypertension due to hepatic venous outlet obstruction in addition to chronic venous insufficiency affecting the lower extremities.
There are now also data supporting a role for CCVI in the development of multiple sclerosis as reported in the International Union of Phlebology Consensus on Venous Malformations – 2009.39 It is hypothesized that truncular venous malformations causing stenosis along the internal jugular, innominate, superior vena cava, and azygos vein system, may contribute to the development or exacerbation of multiple sclerosis.40,41
DEVELOPMENT OF THE PRIMITIVE VENOUS SYSTEM
The heart and blood vessels develop from the mesoderm as isolated masses and cords of mesenchymal cells as early as 15 to 16 days in order to rapidly deliver sufficient nutrients to the exponentially proliferating cells and dispose of waste products via connection with maternal blood vessels in the placenta.42-44 By the beginning of the fourth week of gestation, an extensive network of blood vessels has formed from the mesenchyme as clusters of angiogenetic cells throughout the embryonic body to establish a communication with extra-embryonic vessels and to create a ‘primitive vascular system’: the Vitelline- Umbilical-Cardinal Vein System (Figures 6 and 7).42-44
The primitive vascular structure in complex capillary and reticular plexuses in the early embryonic stage is soon replaced by the newly developed paired cardinal veins as an axial, truncal venous system. In addition, the paired vitelline vessels from the yolk sac develop into the hepatic portal system, while the paired umbilical vessels from the chorion and body stalk form the ductus venosus. The anterior and posterior cardinal veins merge to become the ‘common cardinal veins,’ draining centrally into the sinus venosus (sinus horns) and also receiving the ‘vitelline’ and ‘umbilical’ veins (Figure 6). At 4 weeks, the paired umbilical veins return blood from the placenta to capillary networks in the liver. During the fifth week of development, the right umbilical vein degenerates, involutes together with the proximal portion of the left umbilical veins, leaving only the distal part of the left umbilical vein as a single vein to return blood from the placenta to the embryo.
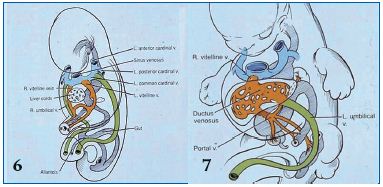
Figure 6. Embryonic veins at the fifth week of gestation: anterior/posterior/common cardinal vein and vitelline/umbilical vein developmental process
The embryo demonstrates the development of paired sets of ‘vitelline’ and ‘umbilical’ veins in its fifth week, which initially drain the yolk sac and allantois, but later drain the intestines and the placenta, respectively. Paired sets of anterior and posterior cardinal veins join to form the ‘common cardinal veins’, draining centrally into the sinus venosus. The common cardinal veins also receive ‘vitelline’ and ‘umbilical’ veins, as depicted.
Figure 7. Embryonic veins at the seventh week of gestation: vitelline/umbilical vein developmental process
At the seventh week of embryonic development, the entire right umbilical vein and proximal left umbilical vein regress. The distal left umbilical vein subsequently anastomoses with the hepatic sinuses to form the ductus venosus. The ductus venosus allows venous blood from the umbilical vein and the portal vein direct access to the inferior vena cava (IVC). The distal/upper-most segment of the right vitelline vein remains as the most proximal segment of the IVC reaching the heart via paired sinus venosus, while all other parts of the vitelline veins regress/involute completely.
At 8 weeks, the distal left umbilical vein anastomoses with the hepatic sinuses to form the ductus venosus. This newly formed structure allows venous blood from the umbilical vein and portal vein to bypass the liver and flow into the inferior vena cava and finally to reach the heart via the paired sinus venosus (Figure 7).
EVOLUTION OF THE EMBRYONIC VEIN FOR HEAD AND NECK: PRECARDINAL AND ANTERIOR CARDINAL VEINS
The part of the body distal to the developing heart (head, neck, upper torso, and upper limbs) drains through the ‘bilateral anterior cardinal veins’ also known as the precardinal veins, whereas, the caudal portion of the body (body and lower limbs) drains through the ‘bilateral posterior cardinal veins’ also known as the postcardinal veins.45,46
Numerous large tributary vessels develop from the anterior cardinal veins and converge as cerebral plexuses. Blood passes from the plexuses to the heart through the anterior cardinal and common cardinal veins. The anterior cardinal (precardinal) veins, common cardinal, and terminal/proximal posterior cardinal (postcardinal) veins go through a major evolutionary process to become the veins of the heart, the superior vena cava (SVC), and its tributaries.
Paired anterior cardinal veins anastomose to allow blood to drain from the ‘left anterior cardinal vein’ into the ‘right anterior cardinal vein’. This anastomosis grows from the left anterior cardinal vein to the right anterior cardinal vein to form the left brachiocephalic (innominate) vein.
The portion of the left anterior cardinal vein distal to the anastomosis, becomes the ‘left internal jugular vein’ and joins the ‘left subclavian vein’ from the developing upper limb. The left anterior cardinal vein proximal to the brachiocephalic anastomosis regresses/atrophies with the terminal segment of the left posterior cardinal vein, ultimately becoming the Great Cardiac Vein. The ‘oblique vein’ of the left atrium (Vein of Marshall) at the back of the left atrium and the ‘coronary sinus’ of the heart comprise the Great Cardiac Vein. The distal portions of the bilateral anterior cardinal veins therefore become the bilateral internal jugular veins and the blood from the left internal jugular vein passes through the left brachiocephalic vein, draining directly into the SVC (Figure 8).47,48
On the right-hand side, the proximal part of the right anterior cardinal vein forms the SVC with the right common cardinal vein in conjunction with the right horn of the sinus venosus (Figure 8). The SVC therefore consists of three different segments:
1. Right anterior cardinal vein proximal to the brachiocephalic anastomosis
2. Right common cardinal vein
3. Right horn of the sinus venosus
These veins are further involved in the formation of the arch of azygos vein together with the proximal segment of the right posterior cardinal vein. The termination of the left posterior cardinal vein transforms into the Great Cardiac Vein, which drains into the left atrium. The azygos venous system is initially derived from the paired supracardinal venous systems, one of three cardinal veins that drain the caudal portion of the body together with the postcardinal (posterior cardinal) veins.49,50
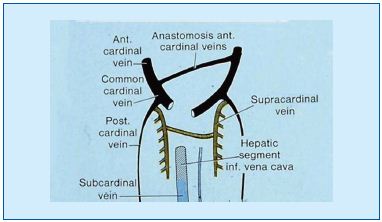
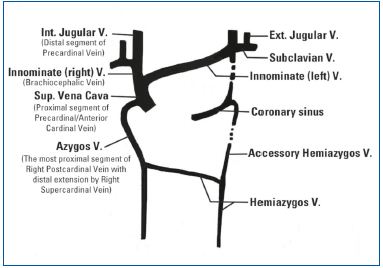
Figures 8 (top) and 9 (bottom). Precardinal/anterior cardinal vein developmental process
Paired anterior cardinal veins form common cardinal veins with paired posterior cardinal veins, draining centrally into the sinus venosus (sinus horns) as depicted. Paired anterior cardinals soon form an anastomosis between them; the connection grows from the left to the right anterior cardinal vein to form the left brachiocephalic (innominate) vein. The left anterior cardinal vein distal (cranial) to the anastomosis becomes the ‘left internal jugular vein,’ while the left anterior cardinal vein proximal to the brachiocephalic anastomosis regresses/atrophies to become the base of the ‘coronary sinus’ of the heart as displayed. The right anterior cardinal (precardinal) vein proximal to the right brachiocephalic vein forms the superior vena cava (SVC) with the common cardinal, and terminal/proximal segment of the posterior cardinal (postcardinal) vein.
Three sets/pairs of cardinal veins: precardinal, postcardinal, and supracardinal, evolve to form the azygos venous system. The azygos venous system is initially derived from the paired supracardinal vein. The proximal segment of the right supracardinal vein forms the arch of azygos vein together with the cranial part of the right posterior cardinal vein, while the cranial part of the left supracardinal vein becomes the hemiazygos and also accessory azygos veins as displayed in 9. The hemiazygos vein on the left side drains into the azygos vein located in the right side before draining into the SVC. The ‘accessory’ hemiazygos vein runs along the course of the involuted left common cardinal vein and drains into the hemiazygos vein before it crosses over the midline to the azygos vein.
The right supracardinal vein remains as the ‘azygos vein’ together with the distal portion of the right posterior cardinal vein to form the arch of azygos vein. The left supracardinal vein becomes the hemiazygos vein and accessory azygos vein. The hemiazygos vein on the left drains into the azygos vein located on the right side and subsequently into the SVC. The ‘accessory’ hemiazygos vein, which runs along the course of the involuted left common cardinal vein, drains into the hemiazygos vein before it crosses the midline to flow into the azygos vein (Figure 9).
ANOMALOUS DEVELOPMENT OF THE SUPERIOR VENA CAVA
Due to the complex nature of the various stages of evolution and involution of multiple paired embryonic veins, several anomalous conditions associated with the SVC can develop. These may affect the common cardinals, anterior and posterior cardinals, and primitive jugular veins. The likelihood of development anomalies associated with the SVC is relatively high due to the involvement of three different embryonic vein segments.
For example, a left-sided SVC may develop from ‘persistent’ left anterior and left common cardinal veins,51,52 and is often associated with the absence of the right SVC.53,54 In this condition, the right brachiocephalic vein crosses the midline to join a vertical left brachiocephalic vein, thus forming a left SVC. As a consequence of this developmental defect of the common cardinal vein, the persistent left SVC can be associated with the presence of two azygos veins. When a left SVC is present, the anatomy of the azygos veins may be reversed; the hemiazygos vein (the remnant of the proximal part of the left posterior cardinal vein) located on the left, will drain directly into the left-sided SVC, in the way that a normal azygos vein (the remnant of the proximal part of the right posterior cardinal vein) would drain into the SVC on the right side. This anomalous condition is the result of a developmental arrest/defect during the late stage of embryogenesis. The left SVC is grouped with other similar truncular venous malformations (eg, double SVC, internal jugular vein stenosis/aneurysm).
A double SVC is another well-known vein anomaly that occurs as a result of failure of degeneration/involution of the left anterior cardinal vein proximal to the brachiocephalic anastomosis.55,56 The double SVC is further subgrouped based on combined anomalous veins.
EVOLUTION OF EMBRYONIC VEINS OF THE TORSO: POSTCARDINAL, SUBCARDINAL AND SUPRACARDINAL VEINS
The posterior cardinal (postcardinal) veins are the first pair of embryonic veins to arise that drain the caudal body. They soon become integrated and taken over by the newly developing subcardinal and supracardinal veins.57-59 The shift of the systemic venous return to the right atrium in early embryonic life initiates the radical remodeling of these cardinal (embryonic) venous systems. The postcardinal, subcardinal, and supracardinal veins go through extensive evolution as well as involution for complex remodeling to form the inferior vena cava (IVC), which drains the trunk and lower extremities (Figure 10).60,61
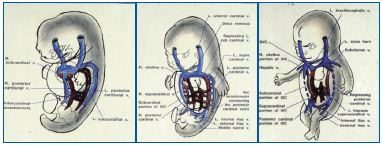
Figure 10 (A-C). Developmental process for the inferior vena cava involving postcardinal, supracardinal, and subcardinal veins Three pairs of the post-/sub-/supracardinal veins go through extensive evolution and involution to form the inferior vena cava (IVC) as well as hepatic veins, together with the bilateral vitelline and umbilical veins. The role of postcardinal (posterior cardinal) veins, the first pair of embryological veins for venous drainage of the caudal body, is soon taken over by developing pairs of subcardinal and supracardinal veins, to form the IVC as shown.
The IVC is formed in a complicated series of developmental stages from the following embryonic structures (Figure 11):
1. Suprahepatic – the most proximal segment of the IVC develops from the persistent proximal portion of the right vitelline vein, which is the precursor of the common hepatic vein.
2. A new hepatic segment develops from an anastomosis between the right vitelline vein and the right subcardinal vein distal/dorsal to the developing liver to connect this proximal-most (suprahepatic) segment to the distally located right subcardinal vein, while allowing drainage of the hepatic veins/liver.
3. The renal/mesenteric segment of the IVC is represented by a preserved segment of the right subcardinal vein.
4. The new junctional segment of the IVC is formed through an anastomosis between the right subcardinal vein and the more distally located right supracardinal vein.
5. The infrarenal segment is represented by the preserved segment of the right supracardinal vein.
6. The last segment of the IVC is formed as a new segment to connect the right supracardinal and most distal part of the bilateral posterior cardinal veins.
The IVC therefore undergoes a complicated fusion of multiple segments of different embryonic veins: vitelline, supracardinal, subcardinal, and posterior cardinal, anastomoses between these veins, as well as between sub- and supracardinal veins. As a result there is a high likelihood of developmental anomalies occurring during this complicated embryogenic process.
ANOMALOUS DEVELOPMENT OF THE INFERIOR VENA CAVA
The complex embryological development is such that variations and anomalies are common where embryological connections persist, either alone or in conjunction with aplasia or hypoplasia of normally developing channels.62,63 There are therefore many different congenital anomalies of the IVC involving its length, location, duplication, abnormal connection and draining, and residual remnants of the embryonic tissue such as webs, membranes, etc.
‘Double/duplicated’ IVC occurs as a result of the bilateral persistence of the supracardinal veins,64,65 while a ‘leftsided’ IVC is the result of caudal regression of the right supracardinal vein instead of the left supracardinal vein, which fails to involute/regress and persists (Figure 11).66,67
The absence of the suprarenal IVC arises from cava/iliac vein agenesis.68,69 When the right subcardinal vein fails to anastomose with the liver, the IVC drains into the arch of the azygos vein and the hepatic veins drain independently through the diaphragm into the right atrium (Figure 12). A posterior/retroaortic left renal vein is another example of defective regression of the anterior portion of the renal ring (1%-2%).70,71
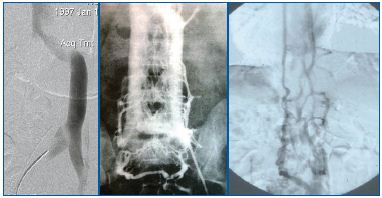
Figure 11 (A-C). ‘Left-sided’ IVC (11A) is one of the IVC anomalies that occurs as a result of failure of normal evolution and involution of the three pairs of cardinal veins. Other related anomalies are ‘Double/ duplicated’ IVC (11B) and absence of IVC development (11C).
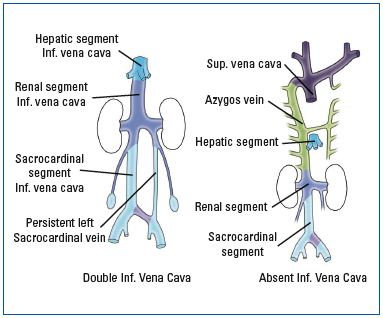
Figure 12. Failure of subcardinal vein to anastomose with the liver
When normal anastomosis of the right subcardinal vein with the liver fails due to abnormal development of the hepatic segment of the IVS, the distal part of the IVC drains directly into the arch of the azygos vein and the hepatic veins drain independently through the diaphragm into the right atrium.
Membranous, focal, segmental, and obstructive lesions in the suprahepatic IVC belong to a group of intraluminal defects of the vein wall that cause varying degrees of stenosis and obstruction, and together with venectasia and aneurysm cause venous dilatation.72,73
EVOLUTION OF THE EMBRYONIC VEIN FOR THE LIMBS: THE LATERAL EMBRYONIC VEIN SYSTEM
Truncal venous development of the lower extremities occurs in three phases to form matured veins in the later stage of embryogenesis (Figure 13).74,75
First phase: primitive fibular (peroneal) vein
Early venous outflow from the primitive lower limb occurs through a lateral/posterior fibular (peroneal) vein into the posterior cardinal vein; this is the first embryonic vein of the limb.
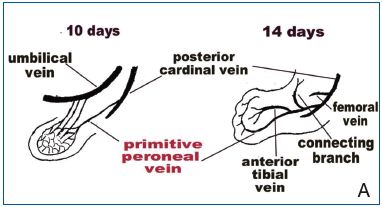
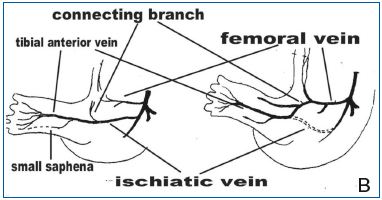
Figure 13 (A and B). Truncal venous development of the lower extremities occurs in three phases.
13A (left) depicts the first phase of truncal vein development involving evolution of the primitive fibular (peroneal) vein, which becomes the first embryonic vein of the lower limb. In the second phase (right), the ‘primitive fibular vein’ develops two branches: the ‘anterior tibial vein’ and ‘connecting branch.’ The anterior
tibial vein and primitive fibular veins together now constitute the “sciatic vein”, which is the second embryonic vein.
13B (left) illustrates the third phase in which the femoral vein is formed by ‘a connecting branch’ from the middle of the sciatic vein, to establish a new definitive deep venous system. The sciatic vein (right) regresses and the femoral vein is further evoluted, following anastomoses with sciatic veins, and passes down the leg as the ‘posterior tibial’ vein to complete the evolution of the veins along the lower limb.
Second phase: sciatic vein
The ‘primitive fibular vein’ develops two branches: the ‘anterior tibial vein’ and the ‘connecting branch’. The anterior (medial) tibial vein becomes the main deep draining vein of the calf. The anterior tibial vein and primitive fibular veins together now constitute the “sciatic vein”, which is the second embryonic vein. A part of the primitive fibular vein distal to the anterior tibial vein/branch evolutes to become the ‘short/lesser saphenous vein.’
Third phase: femoral vein with persisting sciatic vein
‘A connecting branch’ growing medially from the middle of the sciatic vein connects with a new proximal medial vessel that will become the femoral vein and the definitive deep venous system, while the sciatic vein regresses. A third embryonic vein of the leg develops to become the femoral vein, which terminates in the posterior cardinal vein, anterior to the sciatic vein. This advances toward the connecting branch of the lateral fibular/sciatic vein. The femoral vein is further evoluted with anastomoses to sciatic veins and passes down the leg as the ‘posterior tibial’ vein, to finish the evolution of the veins along the lower extremity. This third embryonic vein is also known as the precursor of the long/greater saphenous vein.
With a defect in the second stage, the lateral fibular vein will persist and become the ‘marginal vein.’ However, if a defect occurs in the passage to the third stage, a ‘sciatic vein’ will remain as the main draining vein of the limb. As an embryonic vein, a persisting marginal vein is always ‘valveless’ and can cause a severe reflux resulting in chronic venous hypertension/stasis as well as a high risk of venous thrombosis and subsequent pulmonary embolism among Klippel-Trenaunay syndrome patients.76,77
The venous development of the upper extremities is almost identical to that of the lower extremities.74,75 The ulnar portion of the border/marginal vein persists, forming the subclavian, axillary, and basilic veins at different levels. The subclavian vein eventually drains into the anterior cardinal vein, which subsequently evolutes to the internal jugular vein. The cephalic vein develops secondarily in relationship to the radial border vein, and it later anastomoses with the external jugular vein and finally opens into the axillary vein.

REFERENCES
1. Lee BB, Villavicencio L, Kim YW, et al. Primary Budd-Chiari syndrome: outcome of endovascular management for suprahepatic venous obstruction. J Vasc Surg. 2006;43:101-108.
2. Romagnoli R, Bertolani M, Saviano M, Pantusa M, Modena MG, Benassi A. Developmental interruption of the intra-hepatic segment of the inferior vena cava with azygos-hemiazygos continuation. Eur J Radiol. 1984;4:244- 247.
3. Lee BB, Laredo J, Lee TS, Huh S, Neville R. Terminology and classification of congenital vascular malformations. Phlebology. 2007;22:249-252.
4. Lee BB, Villavicencio L. General considerations. Congenital vascular malformations. Arteriovenous anomalies. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery. 7th Edition. Philadelphia, PA, USA: Saunders Elsevier;2010:1046-1064.
5. Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis J Neurol Neurosurg Psychiatry. 2009;80:392-399.
6. Lee BB, Laredo J, Neville R. Embryological background of truncular venous malformation in the extracranial venous pathways as the cause of chronic cerebrospinal venous insufficiency. Int Angiol. 2010;29:95- 108.
7. Leu HJ. Pathoanatomy of congenital vascular malformations. In: Belov S, Loose DA, Weber J, eds. Vascular Malformations. Reinbek, Germany: Einhorn-Presse Verlag;1989;16:37-46.
8. Woolard HH. The development of the principal arterial stems in the forelimb of the pig. Contrib Embryol. 1922;14:139-154.
9. Lee BB. New approaches to the treatment of congenital vascular malformations (CVMs) – single center experiences – (Editorial Review). Eur J Vasc Endovasc Surg. 2005;30:184-197.
10. Bastide G, Lefebvre D. Anatomy and organogenesis and vascular malformations. In: Belov St, Loose DA, Weber J, eds. Vascular Malformations. Reinbek: Einhorn-Presse Verlag GmbH;1989:20-22.
11. Lee BB, Bergan JJ. Advanced management of congenital vascular malformations: a multidisciplinary approach. Cardiovasc Surg. 2002;10:523-533.
12. Lee BB, Laredo J, Deaton DH, et al. Arteriovenous malformations: evaluation and treatment. In: Gloviczki P, ed. Handbook of Venous Disorders. Guidelines of the American Venous Forum. 3rd Edition. London, UK: A Hodder Arnold Ltd;2009.
13. Lee BB, Do YS, Byun HS, Choo IW, Kim DI, Huh SH. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg. 2003;37:533-538.
14. Lee BB. Current concept of venous malformation (VM). Phlebolymphology. 2003;43:197-203.
15. Lee BB, Kim YW, Seo JM, et al. Current concepts in lymphatic malformation (LM). J Vasc Endovasc Surg. 2005;39:67-81.
16. Lee BB, Villavicencio JL. Primary lymphedema and lymphatic malformation: are they the two sides of the same coin? Eur J Vasc Endovasc Surg. 2010;39:646-653.
17. Lee BB, Lardeo J, Neville R. Arteriovenous malformation: how much do we know? Phlebology. 2009;24:193- 200.
18. Lee BB, Do YS, Yakes W, Kim DI, Mattassi R, Hyon WS. Management of arterial-venous shunting malformations (AVM) by surgery and embolosclerotherapy. A multidisciplinary approach. J Vasc Surg. 2004;39:590-600.
19. Goldman MP, Fitzpatrick RE, Ruiz- Esparza J. Treatment of port-wine stains (capillary malformation) with the flashlamp-pumped pulsed dye laser. J Pediatr. 1993;122:71-77.
20. Berwald C, Salazard B, Bardot J, Casanova D, Magalon G. Port wine stains or capillary malformations: surgical treatment. Ann Chir Plast Esthet. 2006;51:369-372.
21. Lee BB, Laredo J, Lee SJ, Huh SH, Joe JH, Neville R. Congenital vascular malformations: general diagnostic principles. Phlebology. 2007;22:253-257.
22. Lee BB, Laredo J, Kim YW, Neville R. Congenital vascular malformations: general treatment principles. Phlebology. 2007;22:258-263.
23. Lee BB. Changing concept on vascular malformation: no longer enigma. Ann Vasc Dis. 2008;1:11-19.
24. Lee BB. Mastery of vascular and endovascular surgery. In: Zelenock, Huber, Messina, Lumsden, Moneta (eds). Chapter 76. Arteriovenous malformation. Philadelphia: Lippincott, Williams and Wilkins publishers;2006:597-607.
25. Belov S. Classification, terminology, and nosology of congenital vascular defects. In: Belov S, Loose DA, Weber J, eds. Vascular Malformations. Reinbek, Germany: Einhorn-Presse;1989:25-30.
26. Belov ST. Anatomopathological classification of congenital vascular defects. Sem Vasc Surg. 1993;6:219-224.
27. Zamboni P, Cossu A, Carpanese L, Simonetti G, Massarelli G, Liboni A. The so-called venous aneurysms. Phlebology. 1990;5:45-50.
28. Vaket L, Poppelier G, Vermeire P. Sur un cas d’anomalie combinée de la veine cave supérieure et du système azygos. Acta Anat. 1958 ;32:235-239.
29. Croquet V, Aube C, Pilette C, et al. Syndrome due to membranous obstruction of the inferior vena cava of congenital origin. Ten-year follow-up after radiologic treatment. Gastroenterol Clin Biol. 1999;23:259-263.
30. Rao KS, Gupta BK, Banerjee A, Srivastava KK. Chronic Budd-Chiari syndrome due to congenital membranous obstruction of the inferior vena cava: clinical experience. Aust N Z J Surg. 1989;59:335-338.
31. Kim YW, Lee BB, Cho JH, Do YS, Kim DI, Kim ES. Haemodynamic and clinical assessment of lateral marginal vein excision in patients with a predominantly venous malformation of the lower extremity. Eur J Vasc Endovasc Surg. 2007;33:122-127.
32. Mattassi R. Approach to marginal vein: current issue. Phlebology. 2007;22:283- 286.
33. Lee BB, Laredo J, Deaton D, et al. Endovascular management of Budd- Chiari Syndrome – suprahepatic inferior vena cava occlusive disease. In: Heuser RR, Henry M, eds. Textbook of Peripheral Vascular Interventions. Second edition. Section XII. Chapter 83. London, UK: Informa Healthcare, Informa UK Ltd;2008:725-731
34. Zamboni P, Pisano L, Mari C, Galeotti R, Feo C, Liboni A. Membranous obstruction of the inferior vena cava and Budd-Chiari syndrome. Report of a case. J Cardiovasc Surg. 1996 (Torino); 37:583-587.
35. Abe T, Singer RJ, Marks MP, Norbash AM, Crowley RS, Steinberg GK. Coexistence of occult vascular malformations and developmental venous anomalies in the central nervous system: MR evaluation. Am J Neuroradiol. 1998;19:51-57.
36. Schaller B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Rev. 2004;46:243- 260.
37. Schreiber SJ, Doepp F, Klingebiel R, Valdueza JM. Internal jugular vein valve incompetence and intracranial venous anatomy in transient global amnesia. J Neurol Neurosurg Psychiatry. 2005;76:509-513.
38. Akkawi NM, Agosti C, Rozzini L, Anzola GP, Padovani A. Transient global amnesia and disturbance of venous flow patterns. Lancet. 2001;357:957.
39. Lee BB, Bergan J. Gloviczki P, et al; International Union of Phlebology (IUP). Diagnosis and treatment of venous malformations – Consensus Document of the International Union of Phlebology (IUP)-2009. Int Angiol. 2009;28:434-451.
40. Nedelmann M, Kaps M, Mueller-Forell W. Venous obstruction and jugular valve insufficiency in idiopathic intracranial hypertension. J Neurol. 2009;256:964-969.
41. Leriche H, Aubin ML, Aboulker J. Cavo-spinal phlebography in myelopathies. Stenoses of internal jugular and azygos veins, venous compressions and thrombosis. Acta Radiol Suppl. 1976;347:415-417.
42. Langman J. Medical Embryology. 5th ed. Baltimore, MD: Williams and Wilkins;1985:212–217.
43. Warwick R, Williams P. Gray’s Anatomy. 37th ed. Edinburgh, London, Melbourne, New York: Churchill Livingstone;1989:326-327.
44. Hamilton WJ, Mossman HW. Hamilton, Boyd & Mossman’s Human Embryology. 4th ed. Cambridge: Heffer;1972:261.
45. Collins P. Embryology and development. In: Williams PL, Bannister LH, Berry MM, et al (eds). Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery. 38th ed. Edinburgh: Churchill Livingston;1995:327.
46. Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. Contrib Embryol Carneg Inst Washington. 1957;36:79-140.
47. Beattie J. The importance of anomalies of the superior vena cava in man. Canad Med Assoc J. 1931;25:281-284.
48. FitzGerald DP. The study of developmental abnormalities as an aid to that of human embryology, based on observations on a persistent left superior vena cava. Dublin J Med Sci. 1909;14-18.
49. Keyes DC, Keyes HC. A case of persistent left superior vena cava with reversed azygos system. Anat Rec. 1925;31:23-26.
50. Nandy K, Blair CB, Jr. Double superior vena cavae with completely paired azygos veins. Anat Rec. 1965;15:1-9.
51. Huffmire AP, Bower GC. A case of persistence of the left superior vena cava in an aged adult. Anat Rec. 1919- 20;17:127-129.
52. Basu BN. Persistent «left superior vena cava,» «left duct of Cuvier» and left horn of the sinus venosus. J Anat. 1932;66:628-270.
53. Greenfield WS. Persistence of the left vena cava superior, with absence of right. Trans Pathol Soc Lond. 1876;27:120-124.
54. Atwell WJ, Zoltowski P. A case of a left superior vena cava without a corresponding vessel on the right side. Anat Rec. 1938;70:525-532.
55. Howden R. Case of double superior vena cava with left -sided arrangement of the azygos vein. J Anat Physiol. 1887;21:72-75.
56. Gruber W. Duplicität der vena cava superior, mit vorkommen zweir nenae azygae und einer sufficienten valvula an der mündung der vena azygos sinistra. Arch Pathol Anat Physiol Klin Med. 1880;81:462-465.
57. Krizan Z, Herman O, Dzidrov V. Teilweiser fortbestand des supracardinalsystems neben der normalen vena cava inferior beim menschen. Acta Anat. 1958;34:312- 325.
58. Lewis FT. The development of vena cava inferior. Am J Anat. 1902;1.
59. Bailey FR, Miller AM. Development of the vascular system. In: Textbook of Embryology. 2nd edition. New York: William Woon and Company;1911:222-291.
60. Nemec J, Heifetz S. Persistence of left supracardinal vein in an adult patient with heart-hand syndrome and cardiac pacemaker. Congenit Heart Dis. 2008;3:219-222.
61. McClure CFW, Butler EG. The development of the vena cava inferior in man. Am J Anat. 1925:35:331-383.
62. Cornillie P, Van Den Broeck W, Simoens P. Origin of the infrarenal part of the caudal vena cava in the pig. Anat Histol Embryol. 2008;37:387-393.
63. Nemec J, Heifetz S. Persistence of left supracardinal vein in an adult patient with heart-hand syndrome and cardiac pacemaker. Congenit Heart Dis. 2008;3:219-222.
64. Hashmi ZA, Smaroff GG. Dual inferior vena cava: two inferior vena cava filters. Ann Thorac Surg. 2007;84:661- 663.
65. Esposito S, Mansueto G, Amodio F, et al. Duplication of the vena cava inferior with a continuation into the vena azygos. A report of a rare case. Minerva Chir. 1999;54:261-265.
66. Munechika H, Cohan RH, Baker ME, Cooper CJ, Dunnick NR. Hemiazygos continuation of a left inferior vena cava: CT appearance. J Comput Assist Tomogr. 1988;12:328-330.
67. Honma S, Tokiyoshi A, Kawai K, Koizumi M, Kodama K. Left inferior vena cava with regressed right inferior vena cava. Anat Sci Int. 2008;83:173- 178.
68. Gil RJ, Pérez AM, Arias JB, Pascual FB, Romero ES. Agenesis of the inferior vena cava associated with lower extremities and pelvic venous thrombosis. J Vasc Surg. 2006;44:1114- 1116.
69. Romagnoli R, Bertolani M, Saviano M, Pantusa M, Modena MG, Benassi A. Developmental interruption of the intra-hepatic segment of the inferior vena cava with azygos-hemiazygos continuation. Eur J Radiol. 1984;4:244- 247.
70. Trigaux JP, Vandroogenbroek S, De Wispelaere JF, Lacrosse M, Jamart J. Congenital anomalies of the inferior vena cava and left renal vein: evaluation with spiral CT. J Vasc Interv Radiol. 1998;9:339-345.
71. Royal SA, Callen PW. CT evaluation of anomalies of the inferior vena cava and left renal vein. AJR Am J Roentgenol. 1979;132:759-763.
72. Walden R, Hiss J, Morag B, Adar R. Congenital membranous obstruction of the inferior vena cava. Isr J Med Sci. 1978;14:342-346.
73. Wang ZG, Zhu Y, Wang SH, et al. Recognition and management of Budd-Chiari syndrome: report of one hundred cases. J Vasc Surg. 1989;10:149-156.
74. Lewis FT. The development of the veins in the limbs of rabbit embryo. Am J Anat. 1905;5:1-120.
75. Wyman J. On symmetry and homology in. limbs. Proc Boston Soc Nat Hist. 1867;11:246-278.
76. Gloviczki P, Stanson AW, Stickler GB, et al. Trenaunay syndrome: the risks and benefits of vascular interventions. Surgery. 1991;110:469-479.
77. Noel AA, Gloviczki P, Cherry KJ Jr, Rooke TW, Stanson AW, Driscoll DJ et al. Surgical treatment of venous malformations in Klippel-Trenaunay syndrome. J Vasc Surg. 2000;32:840- 847.
